Abstract
Phylogenetic analysis, based on nuclear rDNA internal transcribed spacers (ITS) of Arachis species, corroborated a broad sub-classification of the genus. Three clustering algorithms were used to generate dendrograms which showed that Arachis Sections Extranervosae, Heteranthae and Triseminata were most primitive, and Section Arachis was most advanced, with Sections Caulorrhizae, Erectoides, Procumbentes, Rhizomatosae, and Trierectoides intermediate in evolutionary terms, in relation to the genus Stylosanthes, when it was used as the outgroup.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Native to South America, the cultivated groundnut (peanut), Arachis hypogaea L., is an important cash crop worldwide, ranking fifth in vegetable oil production among nine major oilseed crops in the world (Tillman and Stalker 2009). In the developing countries like China and India, 50% or more groundnut is crushed for oil, whereas in the western world including USA, Japan and European Union, most groundnut goes to food (Yu et al. 2008).
Characterized by its narrow gene base, the cultivated groundnut is susceptible to many biotic and abiotic stresses, while resistance can be found in some of its wild relatives (Mallikarjuna 2003). Wild Arachis species harbor PCV (Peanut Clump Virus) and PeMoV (Peanut Mottle Virus) resistance not yet identified in the cultigen. Other desirable traits in wild groundnut include high yield factors (Nigam et al. 1991), high oil and high protein (Yu et al. 2008). In addition to their potential use in the genetic improvement of the cultivated groundnut, several wild groundnut species (A. glabrata, A. pintoi, A. repens, A. stenosperma and A. kretschmeri) are planted as forage/ground cover in USA, Columbia, Brazil, Australia and China (Miavitiz 2002; Yu et al. 2008).
The genus Arachis is placed in the tribe Aechynomeneae and subtribe Stylosanthinae together with Stylosanthes, Chapmannia, Arthrocarpum and Pachecoa (Taubert 1884; Smartt 1990). It has a number of characters in common with Stylosanthes, more so than any other related taxa. It was supposed that Arachis evolved from Stylosanthes (Singh and Simpson 1994). Understanding of genetic relationships among Arachis species is helpful for germplasm conservation and utilization of genetic diversity present in wild groundnut relatives (Koppolu et al. 2010).
Lying between the 18S and 26S nuclear ribosomal DNA (rDNA), the internal transcribed spacer (ITS) has a high copy number and can be easily amplified with universal PCR primers designed from highly conserved flanking sequences. The length and sequences of ITS regions of rDNA repeats are believed to be fast evolving. ITS sequence data is therefore considered most valuable for reconstructing phylogenetic relationships both at a lower level (e.g., between closely related species) and at a higher level, and has been widely used in phylogenetic studies of angiosperms (Wang et al. 1999; Simpson 2006). Information on the systematics of Arachis, however, has been largely inferred from research results based on comparative morphology, crossability (Gregory and Gregory 1979), and molecular markers, including isozymes (Lu and Pickersgill 1993), seed-storage proteins (Javaid et al. 2004; Singh et al. 1994), Restriction Fragment Length Polymorphisms (RFLPs) (Kochert et al. 1991), Randomly Amplified Polymorphic DNAs (RAPDs) (Hilu and Stalker 1995; Santos et al. 2003; Creste et al. 2005; Cunha et al. 2008), Simple Sequence Repeats (SSRs) (Yu et al. 2008), and Amplified Fragment Length Polymorphisms (AFLPs) (He and Prakash 2001; Tallury et al. 2005). The proposed relationships between Sections, especially those involving Section Triseminatae, are controversial (Gregory and Gregory 1979; Krapovickas and Gregory 1994; Pattee and Stalker 1995). In cultivated groundnut and its wild relatives, rDNA repeat unit polymorphisms have been reported (Singh et al. 2002), as has the cloning and sequencing of an rDNA gene repeat unit (Bhagwat et al. 2001), but to the best of our knowledge, no phylogenetic tree of the Arachis genus based on rDNA sequences is currently available.
The objectives of the present study were to sequence the nuclear rDNA ITS from selected Arachis species, analyze these sequences along with related ITS sequences already deposited in GenBank, and provide insight into groundnut taxonomy and evolution.
Materials and methods
Groundnut materials
We obtained 4 groundnut landraces and 4 accessions of wild Arachis species (Table 1) from Professor Yong Shui Chen (Dapigu, Shitouqi), Quanzhou Agricultural Research Institute, Fujian, China, Professor Zu Ming Zhang, Xuzhou Agricultural Research Institute, Jiangsu, China (Suiningerwo, Wulianchengpodun), Professor Rong Hua Tang, China National Wild Arachis Germplasm Nursery at Naning, Guangxi, China (wild species). The taxonomic identity of the groundnut materials were verified by the providers. In addition, the identity of the 4 wild Arachis species was further confirmed by their PI (Plant Introduction) numbers. Seeds were germinated to produce 6 seedlings of each entry, which were then grown in field nurseries. A minimal distance of 5 m separated adjacent plots (wild species) in all directions to restrict outcrossing and pegging into other plots. For each groundnut genotype, two leaflet samples per plant were collected from 6 different plants in 10 June, 2009.
Preparation of PCR templates
PCR template was prepared from leaflets by using a simple protocol for groundnut developed recently in our laboratory with minor modification (Wang et al. 2009). Briefly, small leaflet discs (6.5 mm2 diam.) were smashed with a thin-walled PCR tube mounted with a 1 ml pipette tip as a pestle, in 40 μl of 0.25 M sodium hydroxide (NaOH) in an Eppendorf microcentrifuge tube. The mixture was boiled for 30 s. Then 160 μl of 100 mM Tris–HCl (pH 7.6) with 5 mg/ml of polyvinylpyrrolidone (PVP) was added, followed by boiling for 2 min. After centrifugation at 10,000 RPM for 5 min, 90 μl of the supernatant was collected and placed into an Eppendorf tube with 450 μl of TE buffer, and stored at 4°C for use within a week, or stored at −20°C for several months.
PCR
To amplify nuclear rDNA ITS of groundnut, 2 μl DNA template was included in a 25 μl reaction mixture by using Tiangen 2 × Taq PCR Master Mix (Tiangen Biotech, Bejing, China) and primers a and b, as recommended by Wang et al. (1999). The amplification was performed on a Biometra™ thermocycler (Biometra, Göttingen, Germany) under the following conditions: 94°C for 3 min, 35 cycles of 50 s at 94°C, 1 min at 55°C, and 1.5 min at 72°C, followed by a final extension at 72°C for 7 min.
DNA analysis
PCR products were recovered by using the E.Z.N.A. Cycle Pure Kit (Omega Bio-Tek, Norcross, USA), and sequenced on an ABI 3730XL sequencer (Foster City, USA). Additional ITS sequences for diverse Arachis taxa representing all nine sections (a total of 43 species, including 24,2,4,3,1,4,2,2 and 1 from Sections Arachis, Caulorrhizae, Erectoides, Extranervosae, Heteranthae, Procumbentes, Rhizomatosae, Trierectoides and Triseminatae, respectively) (Table 1) were downloaded from GenBank.
Phylogenetic trees were constructed with MEGA version 4 (Tamura et al. 2007) and ITS sequences of Stylosanthes leiocarpa and S. montevidensis serving as the outgroup. The robustness of the phylogenetic trees was evaluated by comparing dendrograms obtained from three clustering algorithms, neighbor-joining (NJ), minimal evolution (ME), and maximum parsimony (MP), and bootstrap analysis with 1,000 replicates.
Results and discussion
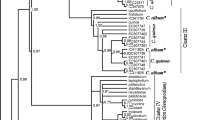
Bootstrap consensus rooted phylogenic trees were constructed based on neighbor-joining (NJ), minimal evolution (ME), and maximum parsimony (MP) clustering algorithms. These trees displayed no significant differences in the arrangement of groups or major subgroups among the 3 methods; thus, only the NJ tree is illustrated (Figs. 1 and 2).
Partial bootstrap NJ tree based on nuclear rDNA ITS sequences of section Arachis species, corresponding to Arachis Subgroup I-1 in Fig. 1
Three species of Section Extranervosae, A. burchellii, A. villosulicarpa and A. lutescens, grouped together (P = 99%). These three species, along with A. pusilla of Section Heteranthae and A. triseminata of Section Triseminatae, were distantly related to other Arachis taxa, and formed the most primitive groups (Fig. 1), most closely related to Stylosanthes. Other accessions were clustered into 2 major subgroups, with Section Caulorrhizae and A. burkartii of Series Prorhizomatosae of Section Rhizomatosae basal to all other species (Subgroup II of Fig. 1). The species of Section Arachis, including the tetraploid cultivated groundnut, A. hypogaea L., and the tetraploid species, A. monticola, formed the most phylogenetically advanced group (Subgroup I-1 of Figs. 1 and 2), with species representing Sections Erectoides, Trierectoides, Eurhizomatosae and Procumbentes and Series Rhizomatosae of Section Rhizomatosae located in an intermediate position in evolutionary terms (Subgroup I-2 of Fig. 1).
Section Arachis can be further divided into 2 subgroups (Subgroup I-1-1 and Subgroup I-1-2) (P = 62%) (Fig. 2). In this study, the 3 Arachis species with 2n = 18 (A. palustris, A. praecox and A. decora) formed a robust subgroup (P = 99%) (Fig. 2). Previous cytogenetic studies confirmed that they lack the pair “A” Chromosome (Creste et al. 2005). These 3 species, along with 1 D-genome species (A. glandulifera) (Stalker 1991) and 6 B-genome species (Cunha et al. 2008) together constituted a less advanced subgroup (Subgroup I-1-2) directly under Subgroup I-1 (Fig. 2). Earlier workers have noted the B- and D- genomes were more closely related to each other than to A-genome (Tallury et al. 2005). In contrast to an earlier report by Tallury et al. (2005), where all species of Section Arachis tested generally grouped according to their genomes, in the present study, here subgroup I-1-1 was composed of a combination of A-, B- and AABB-genome species, which represented a recently evolved group. Cunha et al. (2008) also found the B-genome species, A. hoehnei (Fernandez and Krapovickas 1994; Holbrook and Stalker 2003) grouped to A-genome species and shared the smallest number of bands with A. batizocoi (Also a B-genome species). Sequence data of the trnT-F region (Tallury et al. 2005) also placed A. hoehnei in the A-genome clade, so did SRAP (Sequence-related amplification polymorphism) analysis by Ren et al. (2010). Further cytogenetic studies may reveal the genomic classification of A. hoehnei (Cunha et al. 2008).
Two Trierectoides species (A. guarantica and A. tuberosa) and 3 Erectoides species (A. brevipetiolata, A. hermannii and A. major) were placed together in a well-defined cluster (P = 85%), suggesting that they are closely related (Fig. 1). Arachis paraguariensis subsp. capibarensis and A. paraguariensis subsp. paraguariensis, both of section Erectoides, however, could be found in close relationship with rhizomatous groundnut A. glabrata (series Rhizomatosae) and members of Section Procumbentes (Fig. 1), raising questions about the taxonomic placement of A. paraguariensis, and even about the two subspecies themselves, necessitating check of the real identification of the materials used to generate the GenBank sequences, and where applicable, nomination of the species and subspecies may be reconsidered. Our study supported the primitive status of Series Prorhizomatosae relative to Series Rhizomatosae of Section Rhizomatosae as proposed by Gregory and Gregory (1979).
In conclusion, we constructed a phylogenetic tree of the Arachis genus for the first time by using Stylosanthes as the outgroup, which provided information essentially supporting the current sub-generic classification of the Arachis genus (Krapovickas and Gregory 1994) with some exceptions. Within subgroup I-2, species from Section Erectoides scattered in more than one subgroup, so did the 2 series of Section Rhizomatosae. Taken from a practical standpoint, for the genetic improvement of the cultivated groundnut, wild relatives from Section Arachis, especially the A-genome species, are readily accessible; Other species are from the tertiary gene pool (Smartt 1990), among which the species within Subgroup I-2, i.e., those from Sections Procumbentes, Erectoides and Trierectoides and Series Rhizomatosae of Section Rhizomatosae may be more easily accessible than species from Subgroup II and the most primitive Sections, Triseminatae, Heteranthae and Extranervosae (Fig. 1).
The evolutionary relationships suggested by our study partially differed from previous reports. Gregory and Gregory (1979) and Krapovickas and Gregory (1994) proposed that Sections Extranervosae and Erectoides and Series Prorhizomatosae were primitive, and Sections Arachis, Triseminatae, Caulorrhizae, Heteranthae and Procumbentes and Series Rhizomatosae were advanced. Studies of pachytene chromosomes indicated that species in Sections Erectoides, Extranervosae and Triseminatae were more ancient than were species in Sections Arachis or Rhizomatosae (Pattee and Stalker 1995). Creste et al. (2005) postulated that the 3 species with 2n = 18 might suggest they represented a new branch with a recent origin while using RAPD (random amplified ploymorphism) to study the relationship between annual species from Sections Arachis and Heteranthae. In our study, Sections Extranervosae, Heteranthae and Triseminatae were basal, and Section Arachis was the most advanced, whereas the other Sections can be viewed as intermediate in evolutionary terms. The 3 species with 2n = 18 were advanced relative to D-genome and most B-genome species, and basal to all A-genome and AABB genome species. There were still problems with resolution within Section Arachis and confusion in Subgroup I-2, suggesting that at least a few other conservative genes or gene regions should be analyzed. Although several chloroplast DNA genes, atpB, rbcL, matK and ndhF, along with some nuclear genes such as Adh, have proven to be of high utility in plant systematic (Simpson 2006), they have not yet been used in Arachis. Analysis with these genes is absolutely necessary, since it may provide us with additional information on phylogeny of the genus.
References
Bhagwat AS, Krishna TG, Jawali N, Mitra RK (2001) Cloning and characterisation of a ribosomal RNA gene repeat unit from groundnut. Plant Cell Rep 20:193–197
Creste S, Tsai SM, Valls JFM, Gimenes MA, Lopes CR (2005) Genetic characterization of Brazilian annual Arachis species from sections Arachis and Heteranthae using RAPD markers. Genet Resour Crop Evol 52:1079–1086
Cunha FB, Nobile PM, Hoshino AA, Moretzsohn MC, Lopes CR, Gimens MA (2008) Genetic relationships among Arachis hypogaea L. (AABB) and dipoid Arachis species with AA and BB genomes. Genet Resour Crop Evol 55:15–20
Fernandez A, Krapovickas A (1994) Chromosomas y evolucion en Arachis (Leguminosae). Bonplandia 8:187–190
Gregory MP, Gregory WC (1979) Exotic germ plasm of Arachis L. interspecific hybrids. J Heredity 70:185–193
He G, Prakash C (2001) Evaluation of genetic relationships among botanical varieties of cultivated peanut (Arachis hypogaea L.) using AFLP markers. Genet Resour Crop Evol 48:347–352
Hilu KW, Stalker HT (1995) Genetic relationships between peanut and wild species of Arachis sect. Arachis (Fabaceae): evidence from RAPDs. Plant Syst Evol 198:167–178
Holbrook CC, Stalker HT (2003) Peanut breeding and genetic resources. In: Janick J (ed) Plant breeding reviews. Wiles, Hoboken, pp 297–356
Javaid A, Ghafoor A, Anwar R (2004) Seed storage protein electrophoresis in groundnut for evaluating genetic diversity. Pak J Bot 36(1):25–29
Kochert G, Halward T, Branch WD, Simpson CE (1991) RFLP variety in peanut (Arachis hypogaea L.) cultivars and wild species. Theor Appl Genet 81:565–570
Koppolu R, Upadhyaya HD, Dwivedi S, Hiosington DA, Varshney RK (2010) Genetic relationships among seven sections of genus Arachis studied by using SSR markers. BMC Plant Biol 10:15
Krapovickas A, Gregory WC (1994) Taxonomia del genero Arachis (Leguminosae). Bonplandia 8:1–86
Lu J, Pickersgill B (1993) Isozyme variation and species relationships in peanut and its wild relatives (Arachis L. - Leguminosae). Theor Appl Genet 85:550–560
Mallikarjuna N (2003) Wide hybridization in important food legumes. In: Jaiwal PK, Singh RP (eds) Improvement strategies of Leguminosae biotechnology. Kluwer, Great Britain, pp 155–171
Miavitiz E (2002) Rhizomal perennial peanut in the urban landscape. Proc Florida State Hort Soc 115:136–138
Nigam SN, Dwivedi SL, Gibbons RW (1991) Groundnut breeding: constraints, achievements and future possibilities. Plant Breed Abs 61:1128–1136
Pattee HE, Stalker HT (1995) Advances in peanut science. Am Peanut Res Edu Soc, Stillwater, OK
Ren X, Huang J, Liao B, Zhang X, Jiang H (2010) Genomic affinities of Arachis genus and interspecific hybrids were revealed by SRAP markers. Genet Resour Crop Evol DOI: 10.1007/s10722-010-9532-1
Santos VSED, Gimenes MA, Valls JFM, Lopes CR (2003) Genetic variation within and among species of five sections of the genus Arachis L. (Leguminosae) using RAPDs. Genet Resour Crop Evol 50:841–848
Simpson MG (2006) Plant systematics. Elsevier Academic Press, Burlington, MA, pp 477–491
Singh AK, Simpson CE (1994) Biosystematics and genetic resources. In: Smartt J (ed) The groundnut crop-a scientific basis for improvement. Chapman & Hall, London, pp 97–137
Singh AK, Gurtu S, Jambunathan R (1994) Phylogenetic relationships in the genus Arachis based on seed protein profiles. Euphytica 74:219–225
Singh KP, Sing A, Raina SN, Singh AK, Ogihara Y (2002) Ribosomal DNA repeat unit polymorphism and heritability in peanut (Arachis hypogaea L.) accessions and related wild species. Euphytica 123:211–220
Smartt J (1990) Grain legumes: evolution and genetic resources. Cambridge University Press, Cambridge, New York, pp 30–84
Stalker HT (1991) A new species in section Arachis of peanuts with a D genome. Am J Bot 78:630–637
Tallury SP, Hilu KW, Milla SR, Friend SA, Alsaghir M, Stalker HT, Quandt D (2005) Genomic affinities in Arachis (Fabaceae): molecular and cytogenetic evidence. Theor Appl Genetic 111:1229–1237
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599 (Publication PDF at http://www.kumarlab.net/publications)
Taubert P (1884) Leguminosae. In: Engler A, Prantl R (eds) Die natürlichen Pflanzenfamilien. Teil: Abt. 3:70–388. Verlag von Wilhelm Engelmann, Leipzig
Tillman BL, Stalker HT (2009) Peanut. In: Vollmann J, Rajcan I (eds) Handbook of plant breeding. Vol. 4. Oil crops. Springer, New York, pp 287–316
Wang JB, Zhang WJ, Chen JK (1999) Application of ITS sequences of nuclear rDNA in phylogenetic and evolutionary studies of angiosperms. Acta Phytotaxon Sinica 37:407–416
Wang CT, Wang XZ, Tang YY, Zhang JC, Yu SL, Xu JZ, Bao ZM (2009) A rapid and cheap protocol for preparation of PCR templates in peanut. Elec J Biotechnol 12(2). DOI: 10.2225/vol12-issue2-fulltext-11
Yu SL, Wang CT, Zhang JM, Zhang XY, Hu WG, Cao YL, Liang XQ, Liao BS (eds) (2008) Groundnut varieties in China and their genealogy. Shanghai Science and Technology Press, Shanghai 852 pp
Acknowledgments
We thank the earmarked fund for Modern Agro-industry Technology Research System (MATRS) Peanut Program, Ministry of Agriculture, China, China Natural Science Foundation (Grant No. 30300224), 863 New and High Technology Project (Grant No. 2002CCC03200, 2006AA10A114), New and High Technology Innovation Foundation of Shandong Academy of Agricultural Sciences (Grant No. 2006 YCX013) for supporting the study. We are most indebted to Professor Rong Hua Tang, who maintains a live collection of wild groundnut species at Naning, Guangxi Academy of Agricultural Sciences (GXAAS), China, for his generosity in providing wild groundnut species.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C.T., Wang, X.Z., Tang, Y.Y. et al. Phylogeny of Arachis based on internal transcribed spacer sequences. Genet Resour Crop Evol 58, 311–319 (2011). https://doi.org/10.1007/s10722-010-9576-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-010-9576-2