Abstract
The cultivated peanut (Arachis hypogaea L.) is an allotetraploid, with two types of genomes, classified as AA and BB, according to cytogenetic characters. Similar genomes to those of A. hypogaea are found in the wild diploid species of section Arachis, which is one of the nine Arachis sections. The wild species have resistances to pests and diseases that affect the cultivated peanut and are a potential source of genes to increase the resistance levels in peanut. The aim of this study was to analyze the genetic variability within AA and BB genome species and to evaluate how they are related to each other and to A. hypogaea, using RAPD markers. Eighty-seven polymorphic bands amplified by ten 10-mer primers were analyzed. The species were divided into two major groups, and the AA and the BB genome species were, in general, separated from each other. The results showed that high variation is available within species that have genomes similar to the AA and the BB genomes of A. hypogaea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachis hypogaea L., the cultivated peanut, is an important crop for oil production in tropic and subtropic areas. The cultivated peanut is an allotetraploid (AABB), native to South America, where the wild diploid Arachis species are also found. The wild diploid species are a very important germplasm for the peanut breeding programs, because they have some useful traits, such as resistances to diseases and pests (Pande and Rao 2001) that are not found in A. hypogaea.
Species of the genus Arachis were assembled into nine sections, according to their morphology, geographic distribution and crossability (Krapovickas and Gregory 1994). Section Arachis is the most important, since it includes species that have genomes closely related to A. hypogaea, which is also in this section. Thus, introgression of genes from these species should be easier than from more distantly related species (Holbrook and Stalker 2003). Several studies have shown that hybrids between A. hypogaea and some species of section Arachis have a high degree of chromosome pairing, allowing the introgression of genes by genetic recombination (Singh and Moss 1984; Krapovickas and Gregory 1994; Mallikarjuna et al. 2004).
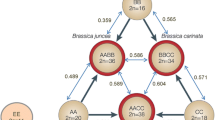
Three genomes have been identified in section Arachis, including the AA genome, found in most species, the BB genome, described for species such as A. batizocoi Krapov. et W. C. Gregory, A. ipaënsis Krapov. et W. C. Gregory, and A. magna Krapov., W. C. Gregory et C. E. Simpson, and the DD genome represented exclusively by A. glandulifera Stalker (Stalker 1991). Arachis hypogaea is believed to be an allotetraploid species, in large part because of the karyotypic differences in their chromosomes, where only one distinctively small chromosome pair (called A) is observed, and the diploid-like chromosome pairing during meiosis (Smartt and Gregory 1967). Molecular and cytogenetic evidences have indicated A. duranensis Krap. et W.C. Gregory (AA genome) and A. ipaënsis (BB genome), both from Section Arachis, as the most probable progenitors of A. hypogaea (Kochert et al. 1996; Seijo et al. 2004).
Improvement of the cultivated peanut using wild diploid species of Arachis has been focused on the AA genome, because the diploid AA genome species are well characterized and many accessions are available. The focus on the BB genome has been lower. Arachis ipaënsis has a unique known accession and the other BB genome species (lacking the small pair of chromosomes) have not been adequately studied. Thus, the analysis of genetic relationships among the BB genome species and A. hypogaea would be very useful for the selection of accessions and species to be used in the breeding programs.
Molecular markers have been used to the analysis of genetic variability and establishment of relationships in many different plant species, including Arachis (Gimenes et al. 2002; Moretzsohn et al. 2004; Tallury et al. 2005).
The aim of this study was to establish the genetic relationships among A. hypogaea and diploid species with AA and BB genomes and to analyze the genetic variability of this germplasm, using Random Amplified Polymorphic DNA (RAPD).
Material and methods
Plant material
Fifty individual plants of 28 accessions of 12 section Arachis species were analyzed (Table 1). All plants were obtained from Arachis Germplasm Bank, located at Embrapa Genetic Resources and Biotechnology–Cenargen (Brasília, Brazil). Arachis tuberosa Benth. and A. guaranitica Chodat et Hassl. of section Trierectoides were included as outgroups. Section Trierectoides is believed to be the most ancient section in the genus Arachis (Krapovickas and Gregory 1994).
DNA extraction
Total DNA was extracted from leaves as described by Ferreira and Grattapaglia (1998). The DNA concentration was estimated at 260 nm wavelength in a GENESYS 5 Spectrophotometer (Spectronic Instruments).
RAPD analysis
Each reaction was performed in a volume of 13 μl consisting of 3 μl of DNA (5 ng/μl), 1.04 μl of BSA (10 μg/μl), 1.04 μl of dNTPs (2.5 mM each), 1.3 μl of 10x Taq DNA reaction buffer, 0.4 μl of MgCl2 (50 mM), 0.2 μl of Taq DNA Polymerase (1 U), 3.0 μl of primer (1 μM) and 3.0 μl of water. Ten 10-mer primers were included in the analysis (Table 2). The amplification cycle was as following: 40 cycles of 1 min at 92°C, 1 min at 35°C, 2 min at 72°C and a final extension of 72°C for 5 min A PTC 100 thermal cycler (MJ Research) was used for the DNA amplification. The PCR products were electrophoresed in 1.5% agarose gels, stained with ethidium bromide and visualized under UV light.
Data analysis
The presence or absence of each polymorphic fragment was scored for all samples. A genetic distance matrix was obtained using Nei and Li’s coefficient (Nei and Li 1979) and submitted to cluster analysis using Neighbor Joining. The analyses were performed using the computer program Treecon (version 1.3b – Van der Peer and De Watcher 1994).
Results
The 10 primers amplified 87 polymorphic fragments. The average number of polymorphic fragments per primer was 8.7, ranging from five using primers OPN2 and OPW2 to 12 fragments using primer OPN1 (Table 2). Eight bands (9.2%) were shared between the AA and BB genome species analyzed, four bands (4.6%) were found only in the BB genome species, and ten bands (11.5%) were exclusive to the AA genome species. Thirteen bands (15%) were shared among AA, BB and AABB (A. hypogaea and A. monticola Krapov. et Rigoni) genome species, seven (8%) between AA and AABB (two of them were shared between A. duranensis and AABB) and three (3.4%) among species with BB and AABB genomes. In the BB genome group, the largest number of fragments was shared between A. batizocoi and A. magna, and the lowest between A. batizocoi and A. hoehnei Krapov. et W. C. Gregory. No bands were found to be unique to A. hypogaea.
The genetic relationships among the accessions analyzed are shown in Fig. 1. The accessions were grouped into two major clusters: the first one included accessions of A. hoehnei, A. schininii (Valls et Simpson), A. aff. kuhlmannii Krapov. et W. C. Gregory, A. cardenasii Krapov. et W. C. Gregory, A. microsperma Krapov., W. C. Gregory et Valls and A. simpsonii Krapov. et W. C. Gregory and the second included two sub-groups, being one composed by accessions of A. hypogaea, A. monticola and A. duranensis and the other subgroup composed by the accessions of three BB genome species: A. batizocoi, A. magna and A. gregoryi (Valls et Simpson). Arachis tuberosa and A. guaranitica (section Trierectoides) were used as outgroups.
Very low polymorphism was detected among the 12 accessions of A. hypogaea analyzed. On the other hand, high polymorphism was found within and among accessions of the wild diploid species analyzed (Fig. 1).
Discussion
In general, all species grouped according to their genomes. Accessions of five out of the six AA genome species included in the present study (A. cardenasii, A. microsperma, A. simpsonii, A. schininii, and A. aff. kuhlmannii) grouped together (Fig. 1). Close relationships among AA genome species were also detected in previous studies using molecular markers (Hilu and Stalker 1995; Moretzsohn et al. 2004) and crossability data (Singh and Moss 1984). Hybrids between AA genome species are usually highly fertile, with 10 bivalents (Stalker et al. 1991), also indicating they are closely related. A cross between A. cardenasii and A. hypogaea subsp. fastigiata var. fastigiata (Smartt and Gregory 1967) resulted in a tetraploid interspecific hybrid population that comprised introgression lines with many important characteristics, such as high levels of resistance to Cercospora arachidicola, moderate resistance to Cercosporidium personatum (Stalker and Beute 1993), and resistances to several insects (Stalker and Campbell 1983). These lines showed introgression of A. cardenasii chromosome segments into both genomes of A. hypogaea (Garcia et al. 1995) and were used to the identification of molecular markers linked to nematode resistance genes (Garcia et al. 1996). Arachis microsperma, A. simpsonii, A. schininii, and A. aff. kuhlmannii have not been used in crosses with A. hypogaea, but, based on our results, introgression of chromosome segments from these species into the cultivated peanut should also be possible.
Three of the four BB genome species included in this analysis (Arachis magna, A. gregoryi and A. batizocoi) also grouped together. Arachis magna is morphologically very similar to A. ipaënsis (Krapovickas and Gregory 1994), which is the most probable donor of the BB genome to A. hypogaea (Kochert et al. 1996; Seijo et al. 2004). The identification of species with high homology to the BB genome of A. hypogaea is important, because the success of breeding program that aims at the introgression of genes from diploid species depends on the choosing of species that have genomes similar to the species being improved.
Only two species did not cluster according to their genomes, A. hoehnei (BB genome) and A. duranensis (AA genome). Arachis hoehnei accessions grouped to the AA genome species and showed the smallest number of shared fragments with A. batizocoi accessions, that has a BB genome. Arachis hoehnei has been classified as a BB genome species (Fernández and Krapovickas 1994; Holbrook and Stalker 2003). Genetic relationship studies based on microsatellite (Moretzsohn et al. 2004) and AFLP markers (Tallury et al. 2005) corroborated this classification. In contrast, phylogeny analysis of genus Arachis based on the sequence variation of internal transcribed spacers (ITS) of rDNA genes (Bechara 2001) and on sequence data of the trnT-F region (Tallury et al. 2005) placed A. hoehnei in the AA genome clade. Cytogenetic studies are currently underway and should elucidate the genomic classification of A. hoehnei (Andrea Peñaloza, personal communication).
Arachis duranensis was placed very closely to A. hypogaea and distantly from the other AA genome species analyzed. Arachis duranensis is considered to be the most probable donor of the AA genome to A. hypogaea (Kochert et al. 1996; Seijo et al. 2004). Thus, this clustering result could be explained by the strict relation among the genomes of A. duranensis and A. hypogaea.
The tetraploid species (A. monticola and A. hypogaea) were very close related, as expected. Hybrids between A. monticola and A. hypogaea showed high fertility levels (Kaprovickas and Gregory 1994). The close relationship between these two species has been reported by many authors, who proposed different hypothesis to explain this tight relation. Krapovickas et al. (1974) suggested that A. monticola could have originated through a crossing between A. hypogaea and a wild diploid species. Pickersgill (1986) and Lu and Pickersgill (1993) suggested that A. monticola and A. hypogaea are probably not distinct species. Lanham et al. (1994) pointed out that A. monticola may have arisen by the hybridization between two subspecies of A. hypogaea. However, the most accepted hypothesis is that A. monticola is the allotetraploid ancestral progenitor of A. hypogaea (Kochert et al. 1996; Moretzsohn et al. 2004).
The low polymorphism observed in A. hypogaea accessions in the present study is in agreement with previous data (Kochert et al. 1996; Gimenes et al. 2002). The general low level of variability in the cultivated peanut has been attributed to its origin, through a single and recent polyplodization event followed by successive selection during breeding efforts––thus resulting in a highly conserved genome (Young et al. 1996). The morphological variation observed in this species is most probable due to the variation in few genes, as proposed by Kochert et al. (1991).
RAPD markers characterized all accessions and individuals of the wild species. This is very important for the maintenance of this material, since mislabeling and duplicates can be identified. Molecular markers have shown to be useful to the identification of mislabeling in Arachis species (Kochert et al. 1996). The diploid species analyzed showed high polymorphism within and among accessions. High polymorphism was also detected among accessions of wild diploid species using different molecular markers (Kochert et al. 1991; Moretzsohn et al. 2004; Tallury et al. 2005).
The present work has been useful to show the genetic relationships among the species of section Arachis. Furthermore, it has showed that RAPD markers can provide an easy, low cost and quick way for the identification of accessions maintained in germplasm collections.
References
Bechara MD (2001) Relações filogenéticas no gênero Arachis, utilizando espaçadores transcritos internos (ITS) de rDNA nuclear. PhD Thesis, Universidade Estadual Paulista (UNESP), Botucatu
Fernández A, Krapovickas A (1994) Cromosomas y evolución en Arachis (Leguminosae). Bonplandia 8:187–220
Ferreira ME, Grattapaglia D (1998) Introdução ao uso de marcadores moleculares em análise genética. EMBRAPA-CENARGEN, Brasília, pp 220
Garcia GM, Stalker HT, Kochert G (1995) Introgression analysis of an interspecific hybrid population in peanuts (A. hypogaea) using RFLP and RAPD markers. Genome 39:166–176
Garcia GM, Stalker HT, Shroeder E, Kochert G (1996) Identification of RAPD, SCAR and RFLP markers tightly linked to nematode resistance genes introgressed from A. cardenasii to A. hypogaea. Genome 39:836–845
Gimenes MA, Lopes CR, Valls JFM (2002) AFLP analysis of genetic relationships among. Arachis species. Genet Mol Biol 25:349–353
Hilu KW, Stalker HT (1995) Genetic relationships between peanut and wild species of Arachis sect. Arachis (Fabaceae): evidence from RAPDs. Plant Syst Evol 198:167–178
Holbrook CC, Stalker HT (2003) Peanut breeding and genetic resources. In: Janick J (ed) Plant breeding reviews. John Wiles & Sons Inc., Hoboken, pp 297–356
Kochert G, Halward T, Branch WD, Simpson CE (1991) RFLP variability in peanut (Arachis hypogaea L.) cultivars and wild species. Theor Appl Genet 81:565–570
Kochert G, Stalker HT, Gimenes M, Galgaro L, Lopes CR, Moore K (1996) RFLP and cytogenetic evidence on the origin and evolution of allotetraploid domesticated peanut Arachis hypogaea (Leguminosae). Am J Bot 83:1282–1291
Krapovickas A, Fernández A, Seeligman P (1974) Recuperacion de la fertilidad en un hibrido interspecifico esteril de Arachis (Leguminosae). Bonplandia 8:1–186
Krapovickas A, Gregory WC (1994) Taxonomia del genero Arachis (Leguminosae). Bonplandia 8:1–186
Lanham PG, Foster BP, McNicol P, Moss JP, Powell W (1994) Seed storage protein variation in Arachis species. Genome 37:487–496
Lu J, Pickersgill B (1993) Isozyme variation and species relationships in peanut and its wild relatives (Arachis L. - Leguminosae). Theor Appl Genet 85:550–560
Mallikarjuna N, Pande S, Jadhav DR, Sastri DC, Rao JN (2004) Introgression of disease resistance genes from Arachis kempff-mercadoi into cultivated groundnut. Plant Breed 123:573–576
Moretzsohn MC, Hopkins MS, Mitchell SE, Kresovich S, Valls JFM, Ferreira ME (2004) Genetic diversity of peanut (Arachis hypogaea L.) and its wild relatives based on the analysis of hypervariable regions of the genome. BMC Plant Biol 4:11
Nei M, Li W-H (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Pande S, Rao NJ (2001) Resistance of wild Arachis species to late leaf spot and rust in greenhouse trials. Plant Dis 85:851–855
Pickersgill B (1986) Evolution of hierarchical variation patterns under domestication and their taxonomic treatment. In: Styles BT (ed) Infraspecific classification of wild and cultivated plants. Clarendon Press, Oxford, pp 191–209
Seijo JG, Lavia GI, Fernández A, Krapovickas A, Ducasse D, Moscone EA (2004) Physical mapping of the 5s and 18s–25s rRNA genes by FISH as evidence that Arachis duranensis and A. ipaënsis are the wild diploid progenitors of A. hypogaea (Leguminosae). Am J Bot 91:1294–1303
Singh AK, Moss JP (1984) Utilization of wild relatives in the genetic improvement of Arachis hypogaea L. 5. Genome analysis in section Arachis and its implications in gene transfer. Theor Appl Genet 68:355–364
Smartt J, Gregory WC (1967) Interspecific cross-compatibility between the cultivated peanut Arachis hypogaea and members of the genus Arachis. Oléagineux 22:455–459
Stalker HT (1991) A new species in section Arachis of peanuts with a D genome. Am J Bot 78:630–637
Stalker HT, Beute MK (1993) Registration of four interspecific peanut germplasm lines resistant to Cercospora arachidicola. Crop Sci 33:1117
Stalker HT, Campbell WV (1983) Resistance of wild species of peanuts to an insect complex. Peanut Sci 10:30–33
Stalker HT, Dhesi JS, Parry D, Hahn J (1991) Cytological and interfertility relationships of Arachis section Arachis. Am J Bot 78:238–246
Tallury SP, Hilu KW, Milla SR, Friend SA, Alsaghir M, Stalker HT, Quandt D (2005) Genomic affinities in Arachis section Arachis (Fabaceae): molecular and cytogenetic evidence. Theor Appl Genet 111:1229–1237
Valls JFM, Simpson CE (2005) New species of Arachis L. (Leguminosae) from Brazil, Paraguay and Bolivia. Bonplandia 14:35–64
Van der Peer Y, De Watcher R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the microsoft windows environment. Comput Appl Biosci 10:569–570
Young ND, Weeden NF, Kochert G (1996) Genome mapping in legumes (Family Fabaceae). In: Paterson AH (ed) Genome mapping in plants. Landes, pp 211–277
Acknowledgments
We thank Dr. José F.M. Valls for the valuable contribution and supply plant material. This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Cunha, F.B., Nobile, P.M., Hoshino, A.A. et al. Genetic relationships among Arachis hypogaea L. (AABB) and diploid Arachis species with AA and BB genomes. Genet Resour Crop Evol 55, 15–20 (2008). https://doi.org/10.1007/s10722-007-9209-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-007-9209-6