Abstract
Advanced glycation end-products (AGEs) of the Maillard reaction were originally measured according to their fluorescent and browning properties. A subsequent study with instrumental analyses such as high-performance liquid chromatography and gas chromatography mass spectrometry more clearly demonstrated the involvement of each AGE structure in pathological conditions. Furthermore, immunochemical methods have also been developed to clarify the localization of AGEs in tissues and measurement of AGEs in multiple clinical samples. Although the involvement of AGEs in age-related diseases has progressed due to immunochemical techniques, the relationship between AGE structure and diseases has not been clear because little was known about the epitope structure of each anti-AGE antibody. However, the development of epitope-identified antibodies against AGEs has made it possible to clarify AGE structures involved in diseases. This review discusses not only the usability of anti-AGE antibodies to evaluate AGEs and disease pathology and screen AGE inhibitors, but also describes their usage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hemoglobin A1c (HbA1c) was identified as glycated hemoglobin and has been used worldwide as a marker of blood glucose control for diabetic patients. Although there was no evidence whether the Maillard reaction progressed to advanced stage in vivo, Monnier et al. [1] demonstrated that the AGE-like fluorescent intensity in the dura mater of the human brain increased in an age-dependent manner and was increased by the pathogenesis of diabetes, strongly indicating that this reaction progressed till the formation of AGEs in vivo. This report reviews the study of glycation and importance of immunohistochemical approaches to detection of AGEs in vivo.
Localization of AGEs in vivo
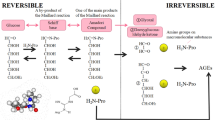
One of the advantages of immunochemical detection is to clarify the localization of antigens in tissues (Fig. 1). A monoclonal anti-AGE antibody, 6D12, is a classic antibody to detect AGE-modified proteins [2]. An enhanced accumulation of AGEs is observed in patients who have severe complications, including nephropathy [3–5] and atherosclerosis [6–8]. Although the epitope structure of 6D12 was not initially identified since it was developed by immunization with an AGE-modified protein that possess many AGEs structures, a subsequent study revealed that 6D12 recognizes not only Nε-(carboxymethyl)lysine (CML) but also Nε-(carboxyethyl)lysine (CEL) [9], demonstrating that AGEs previously detected by 6D12 in vivo may have been CML or CEL. A potential link between AGE accumulation and the aging process in neurons has also been reported. Jono et al. [10] demonstrated that the accumulation of imidazolone, pentosidine and CML in the CA4 region increased with age, suggesting that the accumulation of AGE structures in the CA4 region might be closely related to the aging process in neurons (Table 1).
Usefulness of anti-AGE antibodies. Anti-AGE antibodies are useful for evaluating the localization of AGEs in tissues, the formation pathways and in an ELISA to screen for AGE inhibitors. a Human atherosclerotic lesions were stained by monoclonal antibodies against CML (A, B) and GA-pyridine (C). Bar = 50 μm. Furthermore, a rapid ELISA has been widely used to determine the pathways of AGE formation b and in screening for AGEs inhibitors c because instrumental methods require multiple preparation steps before the analysis
An immunochemical approach in AGE research is valuable to identify new AGE structures. Glycolaldehyde (GA) is formed from serine with hypochlorous acid by the action of myeloperoxidase and reacts with proteins to form several products [11]. Therefore, antibodies have been prepared by immunization with GA-modified proteins. Human atherosclerotic lesions were stained by monoclonal antibodies against CML [8], and GA-pyridine [12]. As shown in Fig. 1, CML was noted not only in the cytoplasm of foamy macrophages (Fig. 1A), but also in the extracellular matrices (Fig 1B). On the other hand, GA-pyridine was localized exclusively in the cytoplasm of foamy macrophages (Fig. 1C). By purification with high-performance liquid chromatography (HPLC), a GA5-reactive compound was isolated and its chemical structure was characterized as 3-hydroxy-4-hydroxymethyl-1-(5-amino-5-carboxypentyl)pyridinium cation, referred to as GA-pyridine. This study demonstrated that the identified epitope structures of anti-AGE antibodies are useful to evaluate the biological distribution of AGEs.
Production of antibodies against AGE structures
AGEs are generated not only from glucose but also from carbonyl compounds such as glyoxal [13], methylglyoxal [14], glucosone [15], 3-deoxyglucosone [16] and GA [11], and those aldehydes rapidly modify proteins to form several AGE structures (Fig. 2). The preparation of AGE-specific antibodies is difficult because AGEs are modified amino acids with molecular weights of less than 500 Da. Although 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) is the most conventional coupling reagent for small molecules and produces a peptide bond between the carrier protein and hapten, EDC-conjugated hapten-carrier adducts often fail to produce immune responses against small molecule haptens [17]. Under this condition, CML, a major antigenic AGE structure, was conjugated to human serum albumin (HSA) with three different cross-linkers, EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (BS3) and glutaraldehyde, and their efficacies in the production of antibodies were compared. Although all three CML-conjugated HSAs were strongly recognized by anti-CML antibody, only CML-conjugated HSA prepared by glutaraldehyde cross-linking produced an antibody against CML [17]. Similarly, antibodies against CEL, S-(2-succinyl)cysteine (2SC) and CMC were also obtained by conjugation to carrier proteins using glutaraldehyde. In this way, the preparation of epitope-specific anti-AGE antibodies can be standardized.
Formation pathways of carbonyl compounds. Glyoxal and glucosone are generated from autooxidation of glucose. Glyoxal is also generated from autooxidation of unsaturated fatty acids. GA is formed from serine with hypochlorous acid by the action of myeloperoxidase. Methylglyoxal is generated from glycolysis pathway. 3-deoxyglucosone is formed by fructosamine-3-kinase or degradation of glycated proteins
Elucidation of formation pathways of AGEs
Although the involvement of oxidation in CML formation from Amadori products has been suggested [18], it is not known which reactive oxygen species are responsible for this process. However, our previous study demonstrated that CML is generated by the oxidative cleavage of Amadori products by hydroxyl radical and peroxynitrite, indicating that CML may be an important biological marker of oxidative stress in vivo [15, 19] (Fig. 3). CML formation was also observed when glycated-HSA, a model Amadori protein, was incubated with activated neutrophils and completely inhibited in the presence of a scavenger for hypochlorous acid (HOCl) (Fig. 3), demonstrating that HOCl-mediated CML formation from Amadori products plays a role in CML formation and tissue damage at sites of inflammation [20].
Post-translational modification is associated with glycation research
We previously demonstrated that 2SC is formed by a reaction between the thiol group of proteins and fumarate, a Krebs cycle intermediate. The level of 2SC significantly increases during the maturation of 3T3-L1 fibroblasts to adipocytes [21]. The intracellular AGE content is reported to increase when bovine endothelial cells are incubated with 30 mM glucose [22]. Thus, we previously compared the 2SC and AGE content in adipocytes. The level of 2SC increased >10-fold during adipogenesis in medium containing 30 mM glucose, whereas the levels of AGEs such as CML and Nε-(carboxyethyl)lysine (CEL) did not change [21]. This strongly demonstrates that the type of post-translational modification depends on the tissues and the metabolic abnormalities. Analysis of the 2SC proteome demonstrated that cytoskeletal proteins, enzymes, heat shock and chaperone proteins, regulatory proteins, and a fatty acid-binding protein were modified by 2SC [21]. Furthermore, Cys-39, which is involved in cross-linking of adiponectin monomers to form trimers, was identified as a key site of 2SC-modification of adiponectin in adipocytes. 2SC was found only in the intracellular, monomeric forms of adiponectin and was not detectable in polymeric forms of adiponectin in the cell culture medium or plasma. Therefore, immunochemical and instrumental analyses demonstrated new phenomena wherein succination of adiponectin blocked its incorporation into trimeric and higher molecular weight, secreted forms of adiponectin. Taken together, our study demonstrated that the increase in fumarate and 2SC is the result of mitochondrial stress in adipocytes during adipogenesis and 2SC may be a useful biomarker of mitochondrial stress in lifestyle-related diseases [23]. Although AGE structures, such as CML and pentosidine, are commonly measured in biological samples due to their stability and autofluorescent properties, 2SC is a prominent post-translational modification observed during the maturation of adipocytes. Therefore, the specific detection of each AGE component, including 2SC, is necessary in order to clarify the relationship between post-translational modifications and disease (Fig. 1).
In some situations, modified peptides are difficult to identify by an instrumental analysis, probably because of the negative or positive charges of 2SC and AGE molecules. To identify the 2SC-modified proteins, a polyclonal anti-2SC antibody was developed according to the same method that is used to produce anti-AGE antibodies. Thus, synthesized S- 2-succinylcysteamine (2SCEA) was cross-linked to keyhole limpet hemocyanin with glutaraldehyde [17], which was then immunized to rabbits. In fact, although approximately 60 spots in adipocytes were found to be positive for 2SC-proteins by immunoblotting with the anti-2SC antibody in a two-dimensional PAGE analysis, 13 types of 2SC-peptides were identified by MALDI-TOF/TOF [21]. This result demonstrated that mass spectrometry is superior for the quantification and identification of small molecules such as 2SC and AGEs, whereas antibodies are still invaluable for the detection of modified-proteins.
Inhibitors for AGE formation
Preventive medicine is the most important approach to preventing lifestyle-related diseases, and improving the daily nutritional intake is thought to prevent the pathogenesis of such diseases. Because the accumulation of AGEs is increased by the pathogenesis of lifestyle-related diseases, AGE inhibitors may be a potential strategy to prevent such diseases. Indeed, AGE inhibitors have been globally developed to prevent lifestyle-related diseases such as diabetic complications and atherosclerosis. Aminoguanidine is the first AGE inhibitor with an amino residue which traps the aldehyde group of reducing sugars [24]. Thiamine and its derivative, benfotiamine, are known to decrease the methylglyoxal level in vivo and inhibit the development of incipient nephropathy [25] and retinopathy [22] in streptozotocin-induced diabetic rats. Pyridoxamine has been shown to significantly inhibit the progress of nephropathy [26] and retinopathy [27], although the serum glucose concentration remained unchanged in a rat model of streptozotocin-induced diabetes.
Screening of AGE inhibitors by an enzyme-linked immunosorbent assay (ELISA) is useful since AGEs can be measured rapidly in a large number of samples [28] (Fig. 1). We previously reported that a high concentration (>1 mM) of catechol compounds, such as epicatechin, gallic acid and 4-MC, enhance CML formation by producing H2O2, but that, at lower (10 μM) concentrations, they inhibit CML formation due to their high antioxidative activities [29]. In concrete terms, after 10 days of incubation with 50 μM 4-methylcatechol in hyperglycemic medium, the cytoplasm of the THP-1 macrophages was diffusely positive for anti-CML antibodies. Furthermore, the oral administration of epicatechin (500 mg/kg/day) to STZ-induced diabetic mice for 45 days enhanced the accumulation of CML on the surface of gastric epithelial cells in the stomach [29]. This indicates that the effect of catechol compounds on the enhancement of the formation of CML is observed based on our in vitro studies, including a cell culture system and animal experiments. Thus, the excessive administration of catechol compounds in the form of “supplemental tablets” should increase catechol concentration more than 1 mM at viscera could be a potential enhancer of CML formation and thus result in the induction of unfavorable effects in vivo. This study provided evidence that natural compounds containing catechol structure enhance CML formation and thus high-dose flavonoid supplementation should be conducted with care to prevent any unfavorable effects of antioxidants.
Difficulty of measuring AGEs in physiological samples by immunological assays
Although the quantification of AGEs by instrumental analyses is superior to that of immunochemical analyses, anti-AGE antibodies are an easy-to-use tool for estimating the AGE content and examining the histological localization of AGEs. However, the immunochemical measurement of AGEs in physiological samples can be influenced by potential artifacts due to pretreatment steps, such as heating and alkaline treatment. For instance, the pentosidine level in physiological samples is used as a sensitive marker for the early diagnosis of renal failure. In the quantitative measurements of pentosidine reported to date, a rapid ELISA has been widely used to estimate the blood pentosidine levels in a number of clinical samples, because HPLC methods require multiple preparation steps before the analysis. However, the currently used clinical analysis of the plasma/serum pentosidine level by an ELISA requires incubation of the plasma/serum at 100 °C for 15 min to inactivate the protease [30], which is required before the anti-pentosidine antibody can bind to the pentosidine. The serum pentosidine content, as measured by HPLC, increased by heating in a temperature- and time-dependent manner [31]. A similar tendency was also observed in CML formation. Thus, CML was generated from glycated HSA by heat treatment (above 80 °C) and increased in a time-dependent manner [32]. These results demonstrated that AGEs could be generated artificially through the heating process. Furthermore, an autoantibody against AGEs [33, 34] was also shown to interfere with the detection of AGEs in biological samples by a competitive ELISA, strongly demonstrating that the measurement system of AGEs should be carefully considered. In addition, although the detection of AGEs by immunochemistry requires the blocking of antigens, conventional blocking reagents, such as animal sera, skim milk and purified serum albumin contain AGEs, and may result in artifacts. Rabbani et al. used a synthetic polypeptide, polythreonine, to prevent staining artifacts [35].
Although the plasma pentosidine concentration is relatively lower than other AGEs, such as CML and methylglyoxal-derived hydroimidazolone-1 (MG-H1) [36], its physiological concentration is frequently measured due to its autofluorescent properties and stability against acid hydrolysis. In contrast, the measurement of the MG-H1 concentration is limited to some facilities since multiple preparation steps, such as enzymatic hydrolysis, are required to measure MG-H1 by instrumental analyses. Furthermore, there is limited presently reliable and commercially available antibody against MG-H1. The measurement of AGEs is therefore expected to become more important as clinical markers if antibodies that can detect AGEs with high biological concentrations become available. The structures of AGEs in some articles are unknown because the AGE contents are estimated by fluorescent intensity and epitope-unidentified antibodies. However, since AGEs are generated from many different pathways, such as glycolysis, inflammation and lipid peroxidation, the precise detection of those structures can be markers of metabolic abnormalities. Thus, the conventional detection of AGEs by epitope-identified antibodies is important for clarifying the association between AGEs and diseases. Furthermore, we previously estimated the levels of skin AGEs by measuring the intensity of fluorophores in the fingertip. Although the structure could not be identified by the measurement of fluorescence intensity, the results are evidence that the accumulation of fluorophores in the fingertip increases with increased numbers of microvascular complications [37]. There are several detection methods such as instrumental analysis, immunochemistry and skin fluorescence intensity, those have their own advantages. Therefore, it is necessary to use them depending on purpose.
References
Monnier V.M., Kohn R.R., Cerami A.: Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. U. S. A. 81(2), 583–587 (1984)
Horiuchi S., Araki N., Morino Y.: Immunochemical approach to characterize advanced glycation end products of the Maillard reaction. Evidence for the presence of a common structure. J. Biol. Chem. 266(12), 7329–7332 (1991)
Makino H., Shikata K., Hironaka K., Kushiro M., Yamasaki Y., Sugimoto H., Ota Z., Araki N., Horiuchi S.: Ultrastructure of nonenzymatically glycated mesangial matrix in diabetic nephropathy. Kidney Int. 48(2), 517–526 (1995)
Imai N., Nishi S., Suzuki Y., Karasawa R., Ueno M., Shimada H., Kawashima S., Nakamaru T., Miyakawa Y., Araki N., Horiuchi S., Gejyo F., Arakawa M.: Histological localization of advanced glycosylation end products in the progression of diabetic nephropathy. Nephron. 76(2), 153–160 (1997)
Suzuki D., Yagame M., Jinde K., Naka R., Yano N., Endoh M., Kaneshige H., Nomoto Y., Sakai H.: Immunofluorescence staining of renal biopsy samples in patients with diabetic nephropathy in non-insulin-dependent diabetes mellitus using monoclonal antibody to reduced glycated lysine. J. Diabetes Complicat. 10(6), 314–319 (1996)
Kume S., Takeya M., Mori T., Araki N., Suzuki H., Horiuchi S., Kodama T., Miyauchi Y., Takahashi K.: Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am. J. Pathol. 147(3), 654–667 (1995)
Sakata N., Imanaga Y., Meng J., Tachikawa Y., Takebayashi S., Nagai R., Horiuchi S.: Increased advanced glycation end products in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis. 142(1), 67–77 (1999)
Sakata N., Imanaga Y., Meng J., Tachikawa Y., Takebayashi S., Nagai R., Horiuchi S., Itabe H., Takano T.: Immunohistochemical localization of different epitopes of advanced glycation end products in human atherosclerotic lesions. Atherosclerosis. 141(1), 61–75 (1998)
Koito W., Araki T., Horiuchi S., Nagai R.: Conventional antibody against Nepsilon-(carboxymethyl)lysine (CML) shows cross-reaction to Nepsilon-(carboxyethyl)lysine (CEL): immunochemical quantification of CML with a specific antibody. J. Biochem. 136(6), 831–837 (2004)
Jono T., Kimura T., Takamatsu J., Nagai R., Miyazaki K., Yuzuriha T., Kitamura T., Horiuchi S.: Accumulation of imidazolone, pentosidine and Nε-(carboxymethyl)lysine in hippocampal CA4 pyramidal neurons of aged human brain. Pathol. Int. 52(9), 563–571 (2002)
Anderson M.M., Requena J.R., Crowley J.R., Thorpe S.R., Heinecke J.W.: The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Invest. 104(1), 103–113 (1999)
Nagai, R., Hayashi, C.M, Xia, L., Takeya, M., Horiuchi, S.: Identification in human atherosclerotic lesions of GA-pyridine, a novel structure derived from glycolaldehyde-modified proteins. J. Biol. Chem. 277(50), 48905–48912 (2002)
Wells-Knecht K.J., Zyzak D.V., Litchfield J.E., Thorpe S.R., Baynes J.W.: Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 34(11), 3702–3709 (1995)
Thornalley P.J., Langborg A., Minhas H.S.: Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 344, 109–116 (1999)
Nagai R., Unno Y., Hayashi M.C., Masuda S., Hayase F., Kinae N., Horiuchi S.: Peroxynitrite induces formation of Nε-(carboxymethyl)lysine by the cleavage of Amadori product and generation of glucosone and glyoxal from glucose: novel pathways for protein modification by peroxynitrite. Diabetes. 51(9), 2833–2839 (2002)
Madson M., Feather M.S.: An improved preparation of 3-deoxy-D-erythro-hexo-2-ulose via the bis(benzoylhydrazone) and some related constitutional studies. Carbohydr. Res. 94, 183–191 (1981)
Mera K., Nagai M., Brock J.W., Fujiwara Y., Imai H., Murata T., Maruyama T., Baynes J.W., Otagiri M., Nagai R.: Glutaraldehyde is an effective cross-linker for production of antibodies against advanced glycation end products. J. Immunol. Methods. 334(1–2), 82–90 (2008)
Fu M.X., Wells-Knecht K.J., Blackledge J.A., Lyons T.J., Thorpe S.R., Baynes J.W.: Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 43(5), 676–683 (1994)
Nagai R., Ikeda K., Higashi T., Sano H., Jinnouchi Y., Araki T., Horiuchi S.: Hydroxyl radical mediates Nε-(carboxymethyl)lysine formation from Amadori product. Biochem. Biophys. Res. Commun. 234(1), 167–172 (1997)
Mera K., Nagai R., Haraguchi N., Fujiwara Y., Araki T., Sakata N., Otagiri M.: Hypochlorous acid generates Nε-(carboxymethyl)lysine from Amadori products. Free Radic. Res. 41(6), 713–718 (2007)
Nagai R., Brock J.W., Blatnik M., Baatz J.E., Bethard J., Walla M.D., Thorpe S.R., Baynes J.W., Frizzell N.: Succination of protein thiols during adipocyte maturation - a biomarker of mitochondrial stress. J. Biol. Chem. 282(47), 34219–34228 (2007)
Hammes, H.P., Du, X., Edelstein, D., Taguchi, T., Matsumura, T., Ju, Q., Lin, J., Bierhaus, A., Nawroth, P., Hannak, D., Neumaier, M., Bergfeld, R., Giardino, I., Brownlee, M.: Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med .9(3), 294–299 (2003)
Frizzell N., Rajesh M., Jepson M.J., Nagai R., Carson J.A., Thorpe S.R., Baynes J.W.: Succination of thiol groups in adipose tissue proteins in diabetes:succination inhibits polymerizaton and secretion of adiponectin. J. Biol. Chem. 284(38), 25772–25781 (2009)
Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A.: Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 232(4758), 1629–1632 (1986)
Babaei-Jadidi R., Karachalias N., Ahmed N., Battah S., Thornalley P.J.: Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 52(8), 2110–2120 (2003)
Degenhardt T.P., Alderson N.L., Arrington D.D., Beattie R.J., Basgen J.M., Steffes M.W., Thorpe S.R., Baynes J.W.: Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 61(3), 939–950 (2002)
Stitt A., Gardiner T.A., Alderson N.L., Canning P., Frizzell N., Duffy N., Boyle C., Januszewski A.S., Chachich M., Baynes J.W., Thorpe S.R.: The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 51(9), 2826–2832 (2002)
Motomura K., Fujiwara Y., Kiyota N., Tsurushima K., Takeya M., Nohara T., Nagai R., Ikeda T.: Astragalosides isolated from the root of Astragalus Radix inhibits the formation of advanced glycation end-products. J. Agric. Food Chem. 57(17), 7666–7672 (2009)
Fujiwara Y., Kiyota N., Tsurushima K., Yoshitomi M., Mera K., Sakashita N., Takeya M., Ikeda T., Araki T., Nohara T., Nagai R.: Natural compounds containing a catechol group enhance the formation of Nε-(carboxymethyl)lysine of the Maillard reaction. Free Radic. Biol. Med. 50(7), 883–891 (2011)
Sanaka T., Funaki T., Tanaka T., Hoshi S., Niwayama J., Taitoh T., Nishimura H., Higuchi C.: Plasma pentosidine levels measured by a newly developed method using ELISA in patients with chronic renal failure. Nephron. 91(1), 64–73 (2002)
Nakano M., Kubota M., Owada S., Nagai R.: The pentosidine concentration in human blood specimens is affected by heating. Amino Acids. 44(6), 1451–1456 (2013)
Miki H.: C., Nagai, R., Miyazaki, K., Hayase, F., Araki, T., Ono, T., Horiuchi, S.: conversion of Amadori products of the Maillard reaction to Nε-(carboxymethyl)lysine by short-term heating: possible detection of artifacts by immunohistochemistry. Lab. Investig. 82(6), 795–808 (2002)
Shibayama R., Araki N., Nagai R., Horiuchi S.: Autoantibody against Nε-(carboxymethyl)lysine: an advanced glycation end product of the Maillard reaction. Diabetes. 48(9), 1842–1849 (1999)
Mera K., Nagai R., Takeo K., Izumi M., Maruyama T., Otagiri M.: An autoantibody against Nε-(carboxyethyl)lysine (CEL): possible involvement in the removal of CEL-modified proteins by macrophages. Biochem. Biophys. Res. Commun. 407(2), 420–425 (2011)
Rabbani N., Godfrey L., Xue M., Shaheen F., Geoffrion M., Milne R., Thornalley P.J.: Glycation of LDL by methylglyoxal increases arterial atherogenicity: a possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes. 60(7), 1973–1980 (2011)
Thornalley P.J., Battah S., Ahmed N., Karachalias N., Agalou S., Babaei-Jadidi R., Dawnay A.: Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 375, 581–592 (2003)
Yamanaka M., Matsumura T., Ohno R., Fujiwara Y., Shinagawa M., Sugawa H., Hatano K., Shirakawa J., Kinoshita H., Ito K., Sakata N., Araki E., Nagai R.: Non-invasive measurement of skin autofluorescence to evaluate diabetic complications. J. Clin. Biochem. Nutr. 58(2), 135–140 (2016)
Oya T., Hattori N., Mizuno Y., Miyata S., Maeda S., Osawa T., Uchida K.: Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J. Biol. Chem. 274(26), 18492–18502 (1999)
Glenn J.V., Mahaffy H., Wu K., Smith G., Nagai R., Simpson D.A., Boulton M.E., Stitt A.W.: Advanced glycation end product (AGE) accumulation on Bruch's membrane: links to age-related RPE dysfunction. Invest. Ophthalmol. Vis. Sci. 50(1), 441–451 (2009)
Mera K., Takeo K., Izumi M., Maruyama T., Nagai R., Otagiri M.: Effect of reactive-aldehydes on the modification and dysfunction of human serum albumin. J. Pharm. Sci. 99(3), 1614–1625 (2010)
Nagai R., Fujiwara Y., Mera K., Yamagata K., Sakashita N., Takeya M.: Immunochemical detection of Nepsilon-(carboxyethyl)lysine using a specific antibody. J. Immunol. Methods. 332(1–2), 112–120 (2008)
Mera K., Fujiwara Y., Otagiri M., Sakata N., Nagai R.: Immunological detection of Nε-carboxymethylarginine by specific antibody. Ann. N. Y. Acad. Sci. 1126, 155–157 (2008)
Giardino I., Thornalley P.J., Edelstein D., Brownlee M.: Generation and characterisation of an antibody against AGEs that induce endothelial dysfunction. Diabetes. 47, A123 (1998)
Miyazaki K., Nagai R., Horiuchi S.: Creatine plays a direct role as a protein modifier in the formation of a novel advanced glycation end product. J. Biochem. 132(4), 543–550 (2002)
Jono T., Kimura T., Takamatsu J., Nagai R., Miyazaki K., Yuzuriha T., Kitamura T., Horiuchi S.: Accumulation of imidazolone, pentosidine and N(epsilon)-(carboxymethyl)lysine in hippocampal CA4 pyramidal neurons of aged human brain. Pathol. Int. 52(9), 563–571 (2002)
Kato S., Horiuchi S., Liu J., Cleveland D.W., Shibata N., Nakashima K., Nagai R., Hirano A., Takikawa M., Kato M., Nakano I., Ohama E.: Advanced glycation endproduct-modified superoxide dismutase-1 (SOD1)-positive inclusions are common to familial amyotrophic lateral sclerosis patients with SOD1 gene mutations and transgenic mice expressing human SOD1 with a G85R mutation. Acta Neuropathol. 100(5), 490–505 (2002)
Shibata N., Nagai R., Miyata S., Jono T., Horiuchi S., Hirano A., Kato S., Sasaki S., Asayama K., Kobayashi M.: Nonoxidative protein glycation is implicated in familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Acta Neuropathol. 100(3), 275–284 (2000)
Jono T., Nagai R., Lin X., Ahmed N., Thornalley P.J., Takeya M., Horiuchi S.: Nepsilon-(carboxymethyl)lysine and 3-DG-imidazolone are major AGE structures in protein modification by 3-deoxyglucosone. J. Biochem. 136(3), 351–358 (2004)
Matsui T., Joo H.D., Lee J.M., Ju S.M., Tao W.H., Higashimoto Y., Fukami K., Yamagishi S.: Development of a monoclonal antibody-based ELISA system for glyceraldehyde-derived advanced glycation end products. Immunol. Lett. 167(2), 141–146 (2015)
Acknowledgments
This work was supported by JSPS KAKENHI Grant No. 15H02902 and 15 K12364 to Ryoji Nagai.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagai, R., Shirakawa, Ji., Ohno, Ri. et al. Antibody-based detection of advanced glycation end-products: promises vs. limitations. Glycoconj J 33, 545–552 (2016). https://doi.org/10.1007/s10719-016-9708-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9708-9