Abstract
Advanced glycation end-products (AGEs) formation increases with metabolic disorders, leading to higher serum AGE levels in patients with progressive vascular complications. Measuring AGE levels in biological samples requires multiple pre-analytical processing steps, rendering analysis of multiple samples challenging. This study evaluated the progression of diabetic complications by analyzing AGE levels using a pre-analytical processing strategy based on a fully automated solid phase-extraction system. Serum samples from patients with diabetes, with or without macrovascular complications (Mac or non-Mac) or microvascular complications (Mic or non-Mic), were processed with the established methods. Free and total AGE levels in sera were measured using liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS). In patients with diabetes, both free and total AGE levels were elevated in those with complications compared to those without complications. In Mac and Mic groups, free and total AGE levels and z-scores (the sum of normalized AGE levels) also increased. AGE z-scores were markedly higher than those of single AGE levels in distinguishing each complication. Our study demonstrated that the free AGE z-score, measured using a new analytical method without hydrolysis, correlated with the presence of vascular complications and may serve as a marker of disease complications.
Similar content being viewed by others
Introduction
Advances in medical technology have contributed to an increase in the average life span. However, according to WHO report1, the average healthy life expectancy remains shorter than the overall average life span. Prevention of lifestyle/chronic-related diseases, which are the leading causes of death, is essential to achieve a long life while maintaining a high quality of life. Diabetes, a lifestyle-related disease, carries the risk of developing macrovascular complications, such as diabetic nephropathy, neuropathy, and retinopathy, as well as microvascular complications, including cerebrovascular disease. Duration of diabetes is related to progressive diabetic complications2,3, and these complications are difficult to manage simply through blood glucose control. The risk of developing microvascular complications increases with increasing blood glucose levels, whereas macrovascular complications are influenced by blood glucose levels, high blood pressure, and obesity4,5. Therefore, hemoglobin A1c (HbA1c) levels, as a marker of blood glucose, of approximately 5–7% may be associated with the development of macrovascular complications.
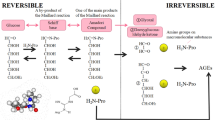
HbA1c, which is an Amadori compound generated non-enzymatically in the early stage of the Maillard reaction, is a marker of blood glucose levels in patients with diabetes. HbA1c levels are indicative of blood glucose levels at 2–3 months post-sampling6. It is difficult to predict diabetic complications using these parameters. Advanced glycation end-products (AGEs) are produced through the Maillard reaction between carbonyl and amino groups. (1) Biological components undergoing glycation include free amino acids, proteins, lipids like phosphatidylethanolamine7, nucleic acids, nucleobases8, and histone proteins9. (2) Precursors inducing glycation are not limited to glucose; they include glyoxal10, generated from sugar autoxidation and lipid oxidation; methylglyoxal11, produced through glycolysis; and glycolaldehyde12, formed via myeloperoxidase during inflammatory responses. AGEs inhibited nitric oxide synthase activity in proximal tubular epithelial cells13, suggesting that metabolic control may be impaired due to the presence of glycated proteins and free amino acids in vivo. AGEs increase with aging and the pathogenesis of diabetic complications14,15, and they could be markers for evaluating progressive diseases over the long term. AGEs can be measured using mass spectrometry in biological tissue samples, including serum, skin, and urine15,16.
The evaluation of AGE levels includes both protein-bound and free AGEs (total AGEs) or the measurement of free AGEs alone. There are reports to measure both free and total AGEs in sera in chronic kidney disease (CKD) with or without diabetes17, and total AGEs in the skin and sera in type 1 diabetes with vascular complications18. However, there are no reports comparing both free and total AGEs in sera in type 2 diabetes with macrovascular and microvascular complications. Therefore, AGEs in serum could be evaluated to assess vascular complications. Measuring free AGEs and total AGEs simultaneously is challenging due to differing pre-analytical methods. Measuring total AGEs in biological samples requires a hydrolysis step lasting over 18 h and the removal of hydrochloric acid for a further 10 h using specialized equipment, such as a speed vacuum concentrator19. This processing makes multiple sample measurements for AGEs difficult and time consuming. After the hydrolysis step, cation exchange involves a few repeated steps, such as washing and elution through a column. Our previous study has described an improved process for cation exchange to achieve more efficient AGE measurement, and we established a new assay based on a fully automated solid phase extraction system (FSPES) for total AGEs20. This assay is effective for clinical studies of AGE and for large-scale industrial applications; nonetheless, the challenge of timely hydrochloric acid removal still remains. Free AGEs, which require less hydrolysis treatment, can also be measured from samples, such as blood and saliva21,22. Free AGEs represent a useful target for the application of FSPES for multiple samples in clinical studies.
We hypothesized that AGE levels could be used to distinguish vascular complications from metabolic disorders in patients experiencing diabetes progression. Therefore, we processed serum samples from patients with diabetes using a new method without the hydrolysis step. We then evaluated the correlation between free AGE levels and vascular complications for comparison with total AGE levels.
Results
Vascular complications were correlated with AGE levels measured using serum from patients with diabetes
The clinical characteristics of patients (N = 154) with diabetes and vascular complications are summarized in Table 1 and Supplementary Fig. 1, Supplementary Tables 1 and 2. Serum samples from patients with diabetes with or without macrovascular complications (non-Mac, N = 116; or Mac, N = 38) showed no differences in HbA1c, body mass index, urine albumin-creatinine ratio (ACR), triglycerides, low-density lipoprotein cholesterol (LDL-C), γ-glutamyl transpeptidase (GGT), aspartate aminotransferase (AST), systolic blood pressure (SBP), diastolic blood pressure (DBP), or plaque score. Moreover, there were also no differences in HbA1c, body mass index, estimated glomerular filtration rate (eGFR), ACR, triglycerides, high-density lipoprotein cholesterol (HDL-C), GGT, AST, DBP, or plaque score between those with or without microvascular complications (non-Mic, N = 60; or Mic, N = 94).
To assess the relevance of AGEs as an indicator of macrovascular and microvascular complications in progressive diabetes, serum samples were treated with FSPES and free and total AGEs were analyzed using liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS). Between patients exhibiting macrovascular or microvascular complications (with: N = 103) or with nonvascular complications (without: N = 51), free Nε-(carboxymethyl)lysine (CML), free Nε-(carboxyethyl) Lysine (CEL), free Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine (MG-H1) , total CML and total CEL levels were higher in patients exhibiting vascular complications than in those with nonvascular complications (Wilcoxon rank sum test) (Fig. 1c,d,e,h,i). However, free Lys, free Arg, total Lys, total Arg and total MG-H1 levels remained unchanged (Fig. 1a,b,f,g,j). The factors that influence increased AGEs in diabetic complications were analyzed. eGFR, as a confounding factor, was correlated with variations in free CML (r = − 0.49, P < 0.001), free MG-H1 (r = − 0.5, P < 0.001), total CML (r = − 0.29, P = 0.0016) and total CEL (r = − 0.59, P < 0.001). Furthermore, free CML levels were correlated with free MG-H1 (r = 0.7, P < 0.001), and total CEL (r = 0.55, P < 0.001); in turn, free MG-H1 levels were correlated with total CEL (r = 0.55, P < 0.001), and total CML levels were correlated with total CEL (r = 0.72, P < 0.001), showing stronger positive correlation with Spearman’s correlation (Fig. 2). Each AGE showed a positive correlation with other AGEs at different concentrations.
Variations in AGEs with complications in patients with diabetes. (a) Free Lys, (b) free Arg, (c) free CML, (d) free CEL, (e) free MG-H1, (f) total Lys, (g) total Arg, (h) total CML, (i) total CEL (j) total MG-H1 levels were compared between patients without complications (without: N = 51) and with complications (with: N = 103). Data are shown with box-plot, bold horizontal bars indicating median values. Statistical analysis: Wilcoxon rank sum test; *P < 0.05; **P < 0.01; ***P < 0.001.
Correlation between increased AGEs and biochemical parameters in patients with diabetes. Statistical analysis: Spearman’ analysis; the significance level was set 0.05. Blue text indicates positive, while red text indicates negative correlation. FPG, fasting plasma glucose; BMI, body mass index; DM, duration of diabetes; ACR, urine albumin-creatinine ratio; TG, triglycerides; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; GGT, γ-glutamyl transpeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; SBP, systolic blood pressure; DBP, diastolic blood pressure; Plaque, plaque score.
In patients exhibiting macrovascular complications, free CML, free CEL, free MG-H1, total CML and total CEL increased (Wilcoxon rank sum test) (Fig. 3c,d,e,h,i, and Supplementary Table 3). However, free Lys, free Arg, total Lys, total Arg and total MG-H1 levels remained unchanged (Fig. 3a,b,f,g,j).
AGE variation in macrovascular complications. (a) Free Lys, (b) free Arg, (c) free CML, (d) free CEL, (e) free MG-H1, (f) total Lys, (g) total Arg, (h) total CML, (i) total CEL (j) total MG-H1 levels were compared between non-Mac (N = 116) and Mac (N = 38). Data are shown with box-plot, bold horizontal bars indicating median values. Statistical analysis: Wilcoxon rank sum test; *P < 0.05; **P < 0.01; ***P < 0.001.
In patients exhibiting microvascular complications, free CML , free CEL , free MG-H1 , and total CML levels increased compared to patients in the non-Mic group (Wilcoxon rank sum test) (Fig. 4c,d,e,h, and Supplementary Table 3). However, free Lys, free Arg, total Lys, total Arg , total CEL and total MG-H1 remained unchanged (Fig. 4a,b,f,g,i,j).
AGE variation in microvascular complication. (a) Free Lys, (b) free Arg, (c) free CML, (d) free CEL, (e) free MG-H1, (f) total Lys, (g) total Arg, (h) total CML, (i) total CEL (j) total MG-H1 levels were compared between non-Mic (N = 60) and Mic (N = 94). Data are shown with box-plot, bold horizontal bars indicating median values. Statistical analysis: Wilcoxon rank sum test; *P < 0.05; **P < 0.01; ***P < 0.001.
Evaluation of serum AGE levels by z-scores
AGEs showed a positive correlation with other AGEs at different concentrations, making it difficult to assess complications using a single AGE. Therefore, AGE z-scores were calculated by averaging and standardizing the AGE z-scores that increased for each complication (Supplementary Table 3). In macrovascular complications, increased levels of free CML + free CEL + , and free MG-H1 were normalized for free AGE z-scores, while increased levels of total CML + and free CEL were normalized for total AGE z-scores. In microvascular complications, increased levels of free CML + , free CEL + , and free MG-H1 were normalized for free AGE z-scores. However, total AGE z-scores were not calculated for microvascular complications because the increase in total AGEs was limited to total CML. The free and total AGE z-scores in patients exhibiting Mac were higher than those in patients with non-Mac, and the free AGE z-scores in patients exhibiting Mic were higher than those in patients with non-Mic (Fig. 5).
AGE z-scores variation in each vascular complication. (a) AGE z-score (free) between non-Mac and Mac, (b) AGE z-score (total) between non-Mac and Mac, (c) AGE z-score (free) between non-Mic and Mic were described. Data are shown with box-plot, bold horizontal bars indicating median values. Statistical analysis: Wilcoxon rank sum test; *P < 0.05; **P < 0.01; ***P < 0.001.
Next, the potential of AGE z-scores to identify each complication was analyzed with logistic regression. In patients exhibiting macrovascular complications, the z-scores of both free and total AGE levels were significantly higher than those of a single AGE level in the univariate logistic regression analysis (free CML: P = 0.107, free CEL: P = 0.144, free MG-H1: P = 0.039, total CML: P = 0.013, total CEL: P = 0.008, free AGE z-score: P = 0.002, total AGE z-score: P = 0.003) (Table 2a). However, free and total AGE z-scores were not significantly different in the multivariable logistic regression analysis (free AGE z-score: P = 0.320, OR = 1.28, 95%CI = 0.79–2.09, total AGE z-score: P = 0.267, OR = 1.31, 95%CI = 0.81–2.11, eGFR: P = 0.100, OR = 0.98, 95%CI = 0.96–1.00). In patients exhibiting microvascular complications, the free AGE z-scores were also more significant than a single AGE in univariate logistic regression (free CML: P = 0.002, free CEL: P = 0.020, free MG-H1: P ≤ 0.001, total CML: P = 0.044, free AGE z-score: P ≤ 0.001) (Table 2b). Furthermore, free AGE z-scores showed significant differences in multivariable logistic regression (free AGE z-score: P = 0.001, OR = 1.98, 95%CI = 1.32–3.09, eGFR: P = 0.883, OR = 1.00, 95%CI = 0.98–1.02). Finally, the potential of AGE z-scores as diagnostic models for each complication was analyzed using receiver operating characteristic (ROC) analysis. The area under the curve (AUC) for free AGE z-scores was 0.70, while for the total AGE z-scores, the AUC was 0.65 for patients exhibiting macrovascular complications (Fig. 6a), whereas for free AGE z-scores, the AUC was 0.68 in patients exhibiting microvascular complications (Fig. 6b). The AUC of the AGE z-scores was higher than that of single AGE in patients with Mic (free CML: AUC = 0.66, free CEL: AUC = 0.62, free MG-H1: AUC = 0.67, total CML: AUC = 0.6). Furthermore, HbA1c had lower AUC scores than that of a single AGE levels or of AGE z-scores for both complications (HbA1c in Mac: AUC = 0.57, HbA1c in Mic: AUC = 0.58). eGFR had lower AUC scores than that of a single AGE levels or of AGE z-scores in patients exhibiting Mic (eGFR in Mac: AUC = 0.7, eGFR in Mic: AUC = 0.58).
ROC curves for AGE z-scores and other parameters. (a) Free CML (orange), free CEL (yellow), free MG-H1 (lime green), total CML (green), total CEL (light green), AGE z-score (free) (light blue), AGE z-score (total) (blue), HbA1c (purple), and eGFR (pink) of ROC curves in patients with macrovascular complications were created based on the values for 154 cases. (b) Free CML (orange), free CEL (yellow), free MG-H1 (lime green), total CML (green), AGE z-score (free) (light blue), HbA1c (purple), and eGFR (pink) of ROC curve in patients with microvascular complications were created based on the values for 154 cases. AUC is presented on the graphs.
Discussion
Factors, such as obesity or smoking, are used as markers to assess risks of disease; however, new biomarkers are necessary for more precise disease prediction. We focused on AGEs as biomarkers to identify the risk of progressive disease from metabolic disorders. Measuring free AGEs without the preanalytical hydrolysis step could be more amenable for clinical applications and lead to a better understanding of metabolic disorders. Quantifying the AGEs might be difficult because of the low concentration of free AGEs in the serum. Therefore, in this study, free AGEs in serum samples were processed simultaneously using FSPES-linked error reduction to compare free and total AGEs variation in type 2 diabetes with vascular complications. The FSPES method for processing free AGEs had a shorter processing time than the manual method and did not require the 18-h hydrolysis step or the removal of hydrochloric acid, which is time-consuming and reducing. The proposed FSEPS was established as a new and more efficient analytical method. Thus, a comprehensive and stable AGE analysis method using FSPES could be established for clinical samples. If hundreds or thousands of clinical samples are processed, free AGEs could be measured more efficiently than total AGEs.
In diseases, variations in AGE levels in the blood have been reported to correlate with eGFR or creatine levels, as the deterioration of kidney function is associated with reduced excretion of AGEs23. Our data showed that AGEs were also associated with eGFR and that a single AGE had a strong positive correlation with other AGEs (Fig. 2). This suggested the presence of a metabolic domino effect, whereby disordered carbohydrate metabolism is linked to disordered lipid metabolism, and accompanying hypertension could influence the production of metabolic compounds, such as AGEs. Serum AGEs in patients vary depending on lifestyle and genetics. It was necessary to evaluate the assay while minimizing these effects. Therefore, we assessed vascular complications using a z-score normalization for several AGEs related to metabolic disorders without considering their concentration. Hanssen et al. used the AGE z-score, including CML, CEL, and pentosidine, to evaluate diseases24. In our previous reports, the detection sensitivity of pentosidine by LC–MS/MS was found to be more than 50 times lower than that of CML25. Therefore, we measured pentosidine using HPLC equipped with a fluorescence detector26,27. Due to this limitation, pentosidine was excluded from the current multiple AGEs analysis using FSPES, as it is not suitable for this method. Therefore, lysyl-AGEs28 such as CML, CEL, and arginyl-AGE, MG-H1, were used for simultaneous processing and comparative analysis in this study. The AGE z-scores could distinguish each vascular complication better than any single AGE (Fig. 5, Table 2). Because the measured AGEs were correlated with each other, we found that multivariate analysis using various AGEs was challenging. The AGE z-scores provided better discrimination of vascular complications than the individual AGEs. Therefore, we conducted the multivariate analysis using only the AGE z-scores. Furthermore, the free AGE z-scores identified patients with Mic independent of eGFR. The AGE z-scores proved to be a more effective biomarker than a single AGE or HbA1c by AUC in ROC analysis for both vascular complications occurring with progressive diabetes (Fig. 6). AGE z-scores are considered more indicative of a patient's lifestyle than absolute AGEs levels. Furthermore, free AGEs are more significant than total AGEs in evaluating changes, disease states, and diagnostic models.
Patients with long-term diabetes not only present a higher risk of diabetic complications but also have increased AGE levels. Our study showed a weak positive correlation between each AGE and the duration of diabetes, potentially might affect by effective drug treatments with biguanides, such as metformin29,30 (Fig. 2, Supplementary Table 1). The levels of free and protein bound CML, CEL, and MG-H1 have been reported to increase in the sera of patients with diabetic nephropathy with the development of chronic kidney disease17. CML levels in sera increase with the progression of retinopathy31. AGE levels in the skin are high in patients with neuropathy, and protein-bound CML in serum is elevated in patients with type 1 macroangiopathy32. In addition, skin autofluorescence (SAF) increases and is correlated with macroangiopathy and microangiopathy in patients with type 1 diabetes (T1DM)18. Comparison of our data with those previous reports demonstrated that total CML levels increased in patients with Mac, whereas single AGEs tended to increase in patients with Mic. Nonetheless, there were some differences, as some AGEs were present in very low concentrations or the formation of different AGEs varied, which could be influenced by good glycemic control through treatment or merely by the sampling method used. Free amino acids comprise only 0.5–5% of blood compared to bound amino acids. The degradation of glycated proteins was reported to be promoted at moderate glucose levels33. Our data showed that in patients with type 2 diabetes and vascular complications Mac and Mic described that free CML (nM) exhibited a more significant change than total CML. Specifically, free CML (nM) demonstrated a significant difference compared to total CML (mmol/mol Lys) in both macrovascular and microvascular complications. Similarly, for free MG-H1 versus total MG-H1, free MG-H1 (nM) exhibited a more significant change than total MG-H1 (mmol/mol Arg) in patients with vascular complications. However, for free CEL vs. total CEL, there was no significant difference between patients with or without vascular complications. Total CEL (nM) showed more significant changes than free CEL (mmol/mol Lys) in macrovascular complications, while free CEL showed more significant changes in microvascular complications (Supplementary Table 3). The significant variation in free AGEs compared to total AGEs in the serum might result from degradation of free AGEs in tissues that flow into the blood.
Numerous reports have highlighted the correlation between kidney function and AGEs. For instance, a strong negative correlation between eGFR and free MG-H1 (r = − 0.7) was reported in patients with diabetes in CKD17. We evaluated AGEs variations in nephropathy patients and confirmed that total CML and total CEL levels increased as eGFR decreased, with a particularly sharp rise observed at the G5 stage of kidney disease (eGFR < 12)20. In our study, eGFR showed a negative correlation with free CML (r = − 0.49) and free MG-H1 (r = − 0.5) in patients with vascular complications in type 2 diabetes. However, our data did not show a strong correlation, which might be due to the severe eGFR levels below 50 in the corrected samples. In the current study, each AGE was higher in patients with Mic than in those without Mic, although there were no differences in eGFR levels between these groups. This might be due to the use of well-controlled diabetic outpatients who did not exhibit significant eGFR deterioration, thus not showing the impact of eGFR on AGEs levels. Furthermore, this might suggest that the AGE generation pathway was active, and the increased levels were not due to decreased excretion resulting from a decline in eGFR levels in patients with Mic.
The limitations of this study include the collection of patients with diabetes from a single university hospital, the influence of the patients' geographic area or habits, and the small size of the study group. Clinical samples with well-controlled variables were randomly collected, resulting in only 38 samples with macrovascular complications. However, the statistical power to detect differences in microvascular and macrovascular complications was over 80% in logistic analysis. Future studies should gather more cases of macrovascular complications from various geographic areas and multiple hospitals. This will enable a comparison of similar sample sizes and better demonstrate the relation between macrovascular complications and AGEs. These studies should also include patients both before and after the onset of vascular complications.
AGEs can be measured relatively easily using fluorescent properties26 and immunochemical methods, as well as mass spectrometry. However, this approach has some issues. Autoantibodies against AGEs are present in serum34,35, which can interfere with accurate measurement by competitive ELISA. Additionally, heating serum for pretreatment36, sometimes necessary when measuring AGEs with antibodies, can generate artifact CML37 and pentosidine26 from Amadori rearrangement products. Consequently, accurately quantifying low levels of AGEs in serum using immunochemical methods is challenging. These issues should be considered when quantifying AGEs with antibodies.
In conclusion, the results of this study suggest that AGEs could be used as biomarkers for the routine evaluation of disease progression in patients with diabetes. Furthermore, a greater number of clinical samples could be analyzed more efficiently using the FSPES approach. AGEs z-scores could help identify each vascular complication in progressive diabetes. Although, it would be necessary to examine AGEs z-scores to evaluate complications in combination with other parameters, such as eGFR, more precisely. The applicability of AGE z-scores to predict complications in the early disease stage should be evaluated by cross-sectional studies in the future.
Methods
Chemical reagents
The following chemicals were used in this study: CML and CEL (Poly Peptide Laboratories, Strasbourg, France); Arg and Lys (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan); isotope-labelled internal standards (ISTDs) of [2H2]-CML, [2H4]-CEL, and [2H3]-MG-H1 (Poly Peptide Laboratories, Strasbourg, France), [13C6] lysine, and [13C6] arginine (Cambridge Isotope Laboratories, Inc., Tewksbury, USA). MG-H1 was synthesized as described previously38. Ultrapure water (H2O), acetonitrile (MeCN), and formic acid for LC–MS/MS were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Participants
This cross-sectional observational cohort study included 178 patients with type 2 diabetes mellitus (T2DM) recruited from the diabetes clinic at Kumamoto University Hospital (Kumamoto, Japan) between March 2021 and August 2021. T2DM was diagnosed according to the criteria of the World Health Organization39. Patients with T1DM, patients who tested positive for glutamic acid decarboxylase (GAD) antibodies, patients with a history of ketoacidosis, and patient’s dependent on insulin therapy for survival were excluded. Patients with severe liver disease, malignancy, or acute/chronic inflammatory disease were also excluded. Finally, data from 154 patients with T2DM (81 men and 73 women) were analyzed (Supplementary Fig. 1). Current treatment uses of hypoglycemic, antihypertensive, or antihyperlipidemic agents was recorded. Serum samples from patients with diabetes were stored at − 80 °C until use.
For all participants, age, sex, history of diabetes, height, weight, blood pressure, and plaque score were recorded. The body mass index was calculated as weight divided by height in meters squared (kg/m2).
Compliance with ethical guidelines
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Human Ethics Review Committee of Kumamoto University (Protocol Number 1737) and registered at UMIN-CTR (UMIN000015966). All participants provided their informed consent in writing.
Measurements of blood and urine parameters
To avoid the influence of external factors on glucose homeostasis, morning blood samples were collected from fasting participants. Fasting plasma glucose concentrations (FPG) HbA1c, triglycerides, high-density lipoprotein cholesterol (HDL-C), aspartate transaminase (AST), alanine aminotransferase (ALT), and γ-glutamyl transpeptidase (γ-GGP) were measured using a Hitachi 7600 analyzer (Hitachi Ltd., Tokyo, Japan). The low-density lipoprotein cholesterol (LDL-C) concentration was determined using the Friedewald formula40. The estimated glomerular filtration rate (eGFR) was calculated using the formula recommended by the Japanese Society of Nephrology41,42. The urinary albumin-to-creatinine ratio (ACR) was calculated using urinary albumin and creatinine concentrations measured in early morning fasting spot urine samples. Patients with microvascular complications were defined as those with diabetic retinopathy (DR), neuropathy (DP), or nephropathy (DN). Patients with macrovascular complications were defined as those with coronary artery disease (CAD), stroke, or peripheral artery disease (PAD).
Assessment of diabetic microvascular complications
According to the criteria of the Japan Diabetes Society43, DN was determined based on eGFR and the presence of albuminuria or proteinuria as follows: ACR ≥ 30 mg/gCr or eGFR < 30 mL/min/1.73 m2. Patients undergoing dialysis were excluded. DR was defined as the presence of characteristic changes, such as microaneurysms, hemorrhages, cotton wool spots, and new vessel formation. Patients who had previously received treatment for DR, such as laser photocoagulation or vitreous surgery, were also defined as having DR. All patients were diagnosed by ophthalmologists. The presence of DP was evaluated using the abbreviated diagnostic criteria proposed by the Diabetic Neuropathy Study Group in Japan44. The criteria comprised three items: (i) symptoms, such as tingling pain, numbness, and cramping; (ii) absence of the Achilles tendon reflex; and (iii) reduced sense of vibration. Patients with more than two positive symptoms were defined as having DP. Therefore, we defined Mic as patients with diabetes and either nephropathy and neuropathy or retinopathy. Mac were defined as patients with diabetes and either cerebrovascular disease, CAD, or PAD.
Sample preparation
Serum samples were prepared as previously described, with a minor modification, such as the addition of an internal standard20. For total AGEs, 5 μL serum samples were mixed with 20 μL H2O, 0.01 nmol of [2H2] CML, [2H4] CEL, [2H3] MG-H1, 5 nmol of [13C6] lysine, and [13C6] arginine. The samples were then reduced with 100 mM NaBH4 (in 5 mM NaOH) in 100 mM sodium borate buffer (pH 9.1) at 25 °C for 4 h. After reduction, 1 mL of 6 M HCl was added to the samples for hydrolysis at 100 °C for 18 h. The hydrolyzed samples were dried using a speed vacuum concentrator (Tokyo Rikakikai, Tokyo, Japan). Solid phase extraction was conducted with FSPES (Shimadzu Corporation, Kyoto, Japan), as described previously17. Briefly, dried samples were resuspended in 1 mL of 0.1% trifluoroacetic acid (TFA) and passed through a Strata X-C column (Phenomenex, Torrance, CA, USA), which had been prewashed with 1 mL of methanol and equilibrated with 1 mL of 0.1% TFA. The column was then washed with 3 mL of 2% formic acid (FA) and eluted with 1 mL of 7% ammonia. The eluted samples were dried.
For free AGEs, 100 μL serum samples were mixed with 52 μL H2O, 0.02 nmol of [2H2] CML, [2H4] CEL [2H3] MG-H1, 10 nmol of [13C6] lysine and [13C6] arginine, and 0.1 M aminoguanidine. The mixture was ultrafiltrated for 1 h under − 60 kPa, using 24-well Acroprep filter plates with 3 K Omega membrane (Cytiva, Tokyo, Japan) and a vacuum manifold system arranged by Shimadzu Corporation. Next, 50 μL of the filtrates was reduced with 100 mM NaBH4 (in 5 mM NaOH) in 100 mM sodium borate buffer (pH 9.1) at 25 °C for 4 h. After reduction, samples were resuspended in 900 μL of 0.1% TFA. The subsequent procedures after FSPES were the same as described above in total AGEs. The dried samples, which were used for free and total AGE, were resuspended in 0.2 mL of 20% MeCN containing 0.1% FA.
Measurement of AGEs via LC–MS/MS
Sample preparations obtained above were filtered through an ISOLUTE FILTER + (Biotage, Stockholm, Sweden) at − 20 kPa for 15 min using the vacuum manifold system (Shimadzu Corporation). The samples were analyzed using an LCMS-8060 (Shimadzu Corporation). The injection volume was 10 µL. LC was performed on a ZIC-HILIC column (150 × 2.1 mm, 5 μm) (Merck Japan, Tokyo, Japan). The mobile phase was composed of solvents A (H2O containing 0.1% FA) and B (MeCN containing 0.1% FA). The flow rate was set at 0.2 mL/min, and the column was kept at 40 °C. The retention times for these AGEs and amino acids were approximately 11–13 min. AGEs and amino acids were detected using electrospray ionization and positive ion mass spectrometric multiple reaction monitoring. Analytic information on the fragment ions and parent ions of these AGEs and amino acids is described in Supplementary Table 4.
Statistical analysis
All analyses were performed using R-4.2.1 for Windows. Patient biological parameters and AGE levels were compared for each variable using the Wilcoxon rank sum test, as the sample data did not show normality using the Shapiro–Wilk test. In Table 1 and Supplementary Table 2, data are reported as median (IQR) for each parameter and the number of patients in each group (n, %). The significance level for all data was set at 0.05. For analysis of correlations between parameters and AGEs, Spearman’s correlation was used. For the z-score, all AGE levels were logarithmically normalized to reduce the potential influence of outliers and to obtain a normal distribution24. In the macrovascular complications group, free AGE z-scores were calculated by averaging and standardizing the z-scores of free CML, free CEL, and free MG-H1. Total AGE z-scores were calculated by averaging and standardizing the z-scores of total CML and total CEL. In the microvascular complications group, free AGE z-scores were calculated by averaging and standardizing the z-scores of free CML, free CEL, and free MG-H1. The patient’s AGE z-scores were compared for each variable using the Wilcoxon rank sum test. Univariate logistic regression analysis was used to compare single AGE and AGE z-scores to identify potential complications. Multivariable logistic regression analysis was used to determine which AGE z-scores could identify each complication, including the influence of eGFR. In the ROC curve analysis, AUC determined how AGE z-scores were fit for diagnostic models to identify the complications.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
World Health Organization. World health statistics 2024: monitoring health for the SDGs, sustainable development goals. https://iris.who.int/bitstream/handle/10665/376869/9789240094703-eng.pdf?sequence=1 (2024).
Rogus, J. J., Warram, J. H. & Krolewski, A. S. Genetic studies of late diabetic complications: The overlooked importance of diabetes duration before complication onset. Diabetes 51, 1655–1662. https://doi.org/10.2337/diabetes.51.6.1655 (2002).
Poonoosamy, J. et al. Impact of intensive glycemic treatment on diabetes complications—a systematic review. Pharmaceutics. 15, 1791–1805. https://doi.org/10.3390/pharmaceutics15071791 (2023).
Fowler, M. J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 29, 116–122. https://doi.org/10.2337/diaclin.29.3.116 (2011).
Ma, C. X. et al. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc. Diabetol. 21, 74. https://doi.org/10.1186/s12933-022-01516-6 (2022).
Selvin, E. Hemoglobin A1c—using epidemiology to guide medical practice: kelly west award lecture 2020. Diabetes Care 44, 2197–2204. https://doi.org/10.2337/dci21-0035 (2021).
Suzuki, K., Nakagawa, K. & Miyazawa, T. Augmentation of blood lipid glycation and lipid oxidation in diabetic patients. Clin. Chem. Lab. Med. 52, 47–52. https://doi.org/10.1515/cclm-2012-0886 (2014).
Richarme, G. et al. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science 357, 208–211. https://doi.org/10.1126/science.aag1095 (2017).
Zheng, Q. et al. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 10, 1289. https://doi.org/10.1038/s41467-019-09192-z (2019).
Nagai, R. et al. Peroxynitrite induces formation of N-carboxymethyl lysine by the clravage of amadori product and generation of glucosone and glyoxal from glucose. Diabetes 51, 2833–2839. https://doi.org/10.2337/diabetes.51.9.2833 (2002).
Nagai, R., Araki, T., Hayashi, C. M., Hayase, F. & Horiuchi, S. Identification of N -(carboxyethyl)lysine, one of the methylglyoxal-derived AGE structures, in glucose-modified protein: mechanism for protein modification by reactive aldehydes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 788, 75–84. https://doi.org/10.1016/s1570-0232(02)01019-x (2003).
Nagai, R. et al. Glycolaldehyde, a reactive intermediate for advanced glycation end products, plays an important role in the generation of an active ligand for the macrophage scavenger receptor. Diabetes. 49, 1714–1723. https://doi.org/10.2337/diabetes.49.10.1714 (2000).
Verbeke, P., Perichon, M., Friguet, B. & Bakala, H. Inhibition of nitric oxide synthase activity by early and advanced glycation end products in cultured rabbit proximal tubular epithelial cells. Biochim. Biophys. Acta. 1502, 481–494. https://doi.org/10.1016/S0925-4439(00)00071-5 (2000).
Frye, E. B., Degenhardt, T. P., Thorpe, S. R. & Baynes, J. W. Role of the maillard reaction in aging of tissue proteins advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J. Biol. Chem. 273, 8714–18719. https://doi.org/10.1074/jbc.273.30.18714 (1998).
Yamanaka, M. et al. Non-invasive measurement of skin autofluorescence to evaluate diabetic complications. J. Clin. Biochem. Nutr. 58, 135–140. https://doi.org/10.3164/jcbn.15-132 (2016).
Jaramillo, R. et al. DNA advanced glycation end products (DNA-AGEs) are elevated in urine and tissue in an animal model of type 2 diabetes. Chem. Res. Toxicol. 30, 689–698. https://doi.org/10.1021/acs.chemrestox.6b00414 (2017).
Rabbani, N. et al. Analysis of serum advanced glycation endproducts reveals methylglyoxal-derived advanced glycation MG-H1 free adduct is a risk marker in non-diabetic and diabetic chronic kidney disease. Int. J. Mol. Sci. 24, 152. https://doi.org/10.3390/ijms24010152 (2022).
Diallo, A. M. et al. Association between the tissue and circulating advanced glycation end-products and the micro- and macrovascular complications in type 1 diabetes: The DIABAGE study. Diabetes Ther. 13, 1531–1546. https://doi.org/10.1007/s13300-022-01285-1 (2022).
Zyłka, D. S. et al. The effect of dialysis membrane type on plasma concentrations of pentosidine, a marker of carbonyl stress, in hemodialyzed patients. Pol. Arch. Med. Wewn. 119, 614–620. https://doi.org/10.20452/pamw.785 (2009).
Kato, S. et al. Rapid pretreatment for multi-sample analysis of advanced glycation end products and their role in nephropathy. J. Clin. Biochem. Nutr. 70, 256–261. https://doi.org/10.3164/jcbn.21-175 (2022).
Miyata, T. et al. Clearance of pentosidine, an advanced glycation end product, by different modalities of renal replacement therapy. Kidney Int. 51, 880–887. https://doi.org/10.1038/ki.1997.124 (1997).
Manig, F., Hellwig, M., Pietz, F. & Henle, T. Quantitation of free glycation compounds in saliva. PLoS One. 14, 9. https://doi.org/10.1371/journal.pone.0220208 (2019).
Perkins, B. A. et al. High fractional excretion of glycation adducts is associated with subsequent early decline in renal function in type 1 diabetes. Sci. Rep. 10, 12709. https://doi.org/10.1038/s41598-020-69350-y (2020).
Hanssen, N. M. J. et al. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: a case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes. 64, 257–265. https://doi.org/10.2337/db13-1864 (2014).
Arakawa, S. et al. Mass spectrometric quantitation of AGEs and enzymatic crosslinks in human cancellous bone. Sci. Rep. 10, 18774. https://doi.org/10.1038/s41598-020-75923-8 (2020).
Nakano, M., Kubota, M., Owada, S. & Nagai, R. The pentosidine concentration in human blood specimens is affected by heating. Amino Acids. 44, 1451–1456. https://doi.org/10.1007/s00726-011-1180-z (2013).
Ohno, R. et al. Mangosteen pericarp extract inhibits the formation of pentosidine and ameliorates skin elasticity. J. Clin. Biochem. Nutr. 57, 27–32. https://doi.org/10.3164/jcbn.15-13 (2015).
Bronowicka-Szydełko, A. et al. Effect of advanced glycation end-products in a wide range of medical problems including COVID-19. Adv. Med. Sci. 69, 36–50. https://doi.org/10.1016/j.advms.2024.01.003 (2024).
Adeshara, K. & Tupe, R. Antiglycation and cell protective actions of metformin and glipizide in erythrocytes and monocytes. Mol. Biol. Rep. 43, 195–205. https://doi.org/10.1007/s11033-016-3947-5 (2016).
Adeshara, K. A., Bangar, N. S., Doshi, P. R., Diwan, A. & Tupe, R. S. Action of metformin therapy against advanced glycation, oxidative stress and inflammation in type 2 diabetes patients: 3 months follow-up study. Diabetes Metab. Syndr. 14, 1449–1458. https://doi.org/10.1016/j.dsx.2020.07.036 (2020).
Endo, M. et al. Increased levels of vascular endothelial growth factor and advanced glycation end products in aqueous humor of patients with diabetic retinopathy. Horm. Metab. Res. 33, 317–322. https://doi.org/10.1055/s-2001-15122 (2001).
Papachristou, S. et al. Skin advanced glycation end products as a screening tool of neuropathy in type 2 diabetes mellitus. J. Diabetes Complicat. 36, 108356. https://doi.org/10.1016/j.jdiacomp.2022.108356 (2022).
Raupbach, J., Ott, C., Koenig, J. & Grune, T. Proteasomal degradation of glycated proteins depends on substrate unfolding: Preferred degradation of moderately modified myoglobin. Free Radic. Biol. Med. 152, 516–524. https://doi.org/10.1016/j.freeradbiomed.2019.11.024 (2020).
Shibayama, R., Araki, N., Nagai, R. & Horiuchi, S. Autoantibody against Nε-(carboxymethyl)lysine: an advanced glycation end product of the Maillard reaction. Diabetes 48, 1842–1849. https://doi.org/10.2337/diabetes.48.9.1842 (1999).
Mera, K. et al. An autoantibody against Nε-(carboxyethyl)lysine (CEL): Possible involvement in the removal of CEL-modified proteins by macrophages. Biochem. Biophys. Res. Commun. 407, 420–425. https://doi.org/10.1016/j.bbrc.2011.03.040 (2011).
Sanaka, T. et al. Plasma pentosidine levels measured by a newly developed method using ELISA in patients with chronic renal failure. Nephron 91, 64–73. https://doi.org/10.1159/000057606 (2002).
Hayashi, C. M. et al. Conversion of Amadori Products of the Maillard Reaction to Nε-(carboxymethyl)lysine by Short-Term Heating: Possible Detection of Artifacts by Immunohistochemistry. Lab. Investig. 82, 795–807. https://doi.org/10.1097/01.LAB.0000018826.59648.07 (2002).
Yamaguchi, H., Nagai, M., Sugawa, H., Yasuda, H. & Nagai, R. Development of a conventional immunochemical detection system for determination of Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine in methylglyoxal-modified proteins. Glycoconj. J. 38, 293–301. https://doi.org/10.1007/s10719-020-09957-5 (2021).
World Health Organization. Department of noncommunicable disease surveillance definition. diagnosis and classification of diabetes and its complications: report of a WHO consultation. part 1: diagnosis and classification of diabetes. World Health Org Geneva. pp. 1–59, https://iris.who.int/handle/10665/66040 (1999).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502. https://doi.org/10.1093/clinchem/18.6.499 (1972).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992. https://doi.org/10.1053/j.ajkd.2008.12.034 (2009).
Silveiro, S. P. et al. Chronic kidney disease epidemiology collaboration (CKD-EPI) equation pronouncedly underestimates glomerular filtration rate in type 2 diabetes. Diabetes Care 34, 2353–2355. https://doi.org/10.2337/dc11-1282 (2011).
Haneda, M. et al. A new classification of diabetic nephropathy 2014: a report from joint committee on diabetic nephropathy. J. Diabetes Investig. 6, 242–246. https://doi.org/10.1111/jdi.12319 (2015).
Yasuda, H. et al. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res. Clin. Pract. 77, 178–183. https://doi.org/10.1016/j.diabres.2007.01.053 (2007).
Acknowledgements
The authors thank Shimadzu corporation for technical assistance.
Funding
The study was funded by Japan Society for the Promotion of Science,23K07533, 20K05895.
Author information
Authors and Affiliations
Contributions
S.K. and R.N. designed the experiments. T.M. contributed the collection of clinical samples. R.N. acquired funding. H.S. contributed of methodology and investigation. S.K. contributed investigation, the development of methodology and analyzed data. S.K. wrote the manuscript. R.N. reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kato, S., Matsumura, T., Sugawa, H. et al. Correlation between serum advanced glycation end-products and vascular complications in patient with type 2 diabetes. Sci Rep 14, 18722 (2024). https://doi.org/10.1038/s41598-024-69822-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69822-5
- Springer Nature Limited