Abstract
Manure storage contributes significantly to greenhouse gas (GHG), NH3 and odour emissions from intensive livestock production. A pilot-scale facility with eight 6.5-m3 slurry storage units was used to quantify emissions of CH4, N2O, NH3, and odorants from pig slurry during winter and summer storage. Pig slurry was stored with or without a straw crust, and with or without interception of precipitation, i.e., four treatments, in two randomized blocks. Emissions of total reduced S (mainly H2S) and p-cresol, but not skatole, were reduced by the straw crust. Total GHG emissions were 0.01–0.02 kg CO2 eq m−3 day−1 during a 45-day winter storage, and 1.1–1.3 kg CO2 eq m−3 day−1 during a 58-day summer storage period independent of storage conditions; the GHG balance was dominated by CH4 emissions. Nitrous oxide emissions occurred only during summer storage where, apparently, emissions were related to the water balance of the surface crust. An N2O emission factor for slurry storage with a straw crust was estimated at 0.002–0.004. There was no evidence for a reduction of CH4 emissions with a crust. Current Intergovernmental Panel on Climate Change recommendations for N2O and CH4 emission factors are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Livestock manure has been estimated to contribute 17.5 Tg methane (CH4) and 3.7 Tg nitrous oxide (N2O) to the atmosphere each year (Steinfeld et al. 2006), corresponding to 31–37 % of non-CO2 greenhouse gas (GHG) emissions from agriculture (Smith et al. 2007). The magnitude of CH4 and N2O emissions differ between livestock categories; pigs are mostly produced in confined systems where manure is handled in liquid form (as a slurry) and stored prior to use.
The slurry environment is anoxic, and degradation of manure organic matter stimulates CH4 emission compared to solid manure management, but with a strong seasonal variation in emissions (Husted 1994). In some countries, regulations require slurry storage facilities to be covered in order to mitigate NH3 emissions, but often this is achieved by a naturally forming crust of manure organic matter, or crust formation can be facilitated by admixing of straw or wood chips (Sommer et al. 2006). An organic surface crust will sustain microbial activity and may thus modify emission. It is well established that surface crusts develop a potential for CH4 oxidation (e.g., Petersen et al. 2005; Ambus and Petersen 2005), and for production of N2O (e.g., Sommer et al. 2000; VanderZaag et al. 2009), during slurry storage. The Intergovernmental Panel on Climate Change (IPCC) in its latest revision of the methodology for agricultural GHG emissions (IPCC 2006) proposed that 40 % of CH4 emissions will be offset by an organic crust. Further, an emission factor for N2O, expressed as a proportion of manure N, of 0.005 was recommended for liquid manure storage in the presence of a crust. However, a crust is exposed to climatic conditions that vary seasonally, and biogenic emissions may be modified by factors such as temperature and rainfall. Therefore, more knowledge is needed on emissions as influenced by slurry storage conditions.
Besides CH4, N2O and NH3, slurry stores are a source of odour emissions that can be a nuisance to neighboring settlements (Schiffman 1998). The most important odorants include volatile sulphur-containing compounds, indoles and phenols (Blanes-Vidal et al. 2009; Eriksen et al. 2010; Hansen et al. 2012). When evaluating effects of storage conditions on GHG emissions, it is important to document also any effects on odour.
In the present study, emissions of CH4, N2O, NH3 and odorants from pig slurry during summer and winter storage were quantified, and effects of a straw crust and of rainfall were evaluated by manipulation of storage conditions. We expected a crust to modify emissions mainly during summer where storage temperature and thus microbial activity is high. Specifically, we hypothesized that a straw crust would reduce CH4 emissions from pig slurry in accordance with the observations made with cattle slurry, and that rainfall would reduce effects of a crust by shifting the balance between aerobic and anaerobic microbial activity.
Materials and methods
Pilot-scale storage facility
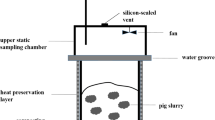
Full technical documentation of the pilot-scale storage facility was presented by Petersen et al. (2009). It includes eight cylindrical 6.5-m3 units (2 m diam. 2 m height incl. 70 cm above-ground) with a wall of 40 mm HDPE. Each unit has eight air inlets and a single outlet (all 160 mm in diameter), to enable forced ventilation in the presence of a removable cover. All openings may be capped if using the stores as static enclosures during emission measurements. Outlets are connected to a main ventilation duct; a flow restrictor on each connecting tube is connected to a differential pressure transmitter monitoring the air flow.
Storage treatments
Slurry for winter and summer storage experiments was collected at a farm with finishing pigs. Slurry was pumped from a pre-storage reservoir, to which slurry from the housing facility had been recently pumped, hence slurry age was <1 month. Portions of 4 m3 slurry were added to each experimental store; exact volumes were calculated by recording the distance to the liquid surface after filling. Slurry samples were collected from each store for analyses at the beginning, and again at the end of each storage period, after thorough mixing.
In both winter and summer storage experiments, four treatments were represented in two randomized blocks each with four storage units. The four treatments were: Admixing of 2.5 kg Mg−1 chopped wheat straw to produce a surface crust (+S), or no straw amendment (−S). This was combined with sheltering to intercept precipitation as rainfall or snow (−P), or open storage conditions except during emission measurements (+P). Hence, the four treatments were: −P−S, −P+S, +P−S and +P+S. The winter storage period lasted from 27 Nov 2007 to 11 Jan 2008 (45 days), while the summer storage period lasted from 16 Jun to 13 Aug 2008 (58 days).
Precipitation was eliminated by permanently applying the removable covers on relevant stores, but with continuous ventilation to simulate open storage conditions. Ventilation rates were 175 and 94 m3 h−1 in winter and summer experiments, respectively, in accordance with the difference in average wind speed for these periods the previous year, 5.3 and 3.7 m s−1, respectively (based on data from a nearby climate station). Inside the stores, wind velocities were always <1 m s−1 as determined by a hot-wire anemometer, and declining towards the surface of the stored slurry (data not shown). Treatments representing open storage, i.e., with precipitation reaching the stored slurry, were covered only during gas sampling (see next section).
Gas sampling
Emissions were as a rule determined weekly during 24-h periods, resulting in six and seven sampling occasions in the winter and summer experiment, respectively. During gas sampling, all eight stores were covered, and the ventilation air from each store was sub-sampled at 15 mL min−1 via a 3-mm inner diam. FEP tubing from a position immediately after the flow restrictor where the gas was well-mixed. Gas washing bottles with 20 mM H3PO4 for trapping NH3 were inserted near the point of sub-sampling. From the acid traps, the gas was transported in an insulated and heated sheath, via water traps and a peristaltic pump, to a manifold with three-way solenoid valves where sub-samples for GHG analysis were collected in 3-L Tedlar gas sampling bags during 0.5 min in every 5-min period; this gave a 2.16-L composite gas sample in 24 h. All settings and readings were recorded by LabView and stored in a log file with automated daily backup.
Selected odorants and total reduced sulphur (TRS) were monitored in six separate campaigns during the summer storage experiment. Since concentrations of odorants in the ventilation air were diluted to below the detection limits, the stores were used as static enclosures, i.e., after the 24-h period used for GHG and NH3 emission measurements, all eight air inlets and the outlet of each store were capped and a fan activated for mixing of the headspace as described by Petersen et al. (2009). After 3–3.5 h, headspace air was sampled and analyzed. Odorants were analyzed using membrane inlet mass spectrometry (MIMS) as previouosly described (Feilberg et al. 2010). Gas was pumped from each store via a multi-position valve to the instrument via a separate line of insulated tubing. Reference air for baseline correction was produced on-site by pumping outside air through an activated charcoal gas trap (Supelco, St. Louis, Missouri, USA). Tubings used were PTFE (o.d., 3.2 mm; i.d., 1.6 mm). Results for two mass-to-charge values (m/z), m/z 107 and m/z 130, are shown, i.e., 4-methylphenol (p-cresol) and 3-methylindole (skatole) (Feilberg et al. 2010; Feilberg et al. 2011), which were consistently detected above the baseline levels. At m/z 107 there is also a contribution from 4-ethylphenol, which is typically present at levels of ~10 % of p-cresol. TRS was measured with a portable Jerome Hydrogen Sulfide Analyzer (Model 631-X, Arizona Instruments; Phoenix, AZ).
Analyses
Climate data were obtained from a nearby (<1 km) meteorological station. Slurry pH was analyzed with a Sentron 3001 pH-meter (Roden, The Netherlands), and electrical conductivity (EC) with a Radiometer conductivity-meter (Copenhagen, Denmark). Slurry dry matter (DM) was determined after drying at 105 °C for 24 h, and volatile solids (VS) after an additional 3 h at 550 °C. Total and ammoniacal N was determined by Kjeldahl digestion (Kjeltec 1030, Höganäs, Sweden). Ammonia trapped in 20 mM H3PO4 was determined colorimetrically (Keeney and Nelson 1982) after adjustment to known volume.
Concentrations of CH4 were analyzed manually on a Shimadzu 14B gas chromatograph (Petersen et al. 2005), and N2O on a Chrompack 9001 gas chromatograph (Mutegi et al. 2010). MIMS measurements were carried out as described by Feilberg et al. (2010). In short, a quadrupole mass spectrometer (QMG 422, Balzers; Liechtenstein) was equipped with a custom-built membrane inlet (Mikrolab, Denmark) holding a poly-(dimethylsiloxane) (PDMS) sheet membrane (130 μm thickness, Techn. Products, Georgia). An inlet temperature of 50 °C was used. During operation a continuous flow of sampling air passes the membrane.
Data analysis
Air exchange was calculated from differential pressure readings using an algorithm given for the flow restrictor (Petersen et al. 2009). The precision of calculated air flows was ±5 %. Effects of storage conditions on changes in slurry composition were analyzed with a mixed model for the winter and the summer storage experiment separately using SAS 9.2. Treatment effects on cumulated emissions were identified by one-way ANOVA and Tukey HSD tests for each storage experiment.
The dependency of net CH4 production on slurry temperature was characterized using the Arrhenius equation:
where \( F_{{CH_{4} }} \) is the CH4 flux (g CH4 kg−1 VS h−1), A is the pre-exponential factor (g CH4 kg−1 VS h−1), E A is the apparent activation energy (J mol−1), R is the universal gas constant (8.314 J mol−1 K−1), and T is the absolute slurry temperature (K). It was assumed that 80 % of VS in the pig slurry was available for fermentative degradation (Sommer et al. 2004).
A theoretical water balance for the summer storage period was calculated from precipitation and potential evaporation data obtained from a nearby climate station as previously described (Sommer et al. 2000). Here it was assumed that evaporation from actively ventilated stores with a cover was identical to that of open stores without cover except during measurements.
Results
Changes in slurry composition
Daily mean temperature during the winter storage period ranged from −3 to 9 °C, and summer temperatures from 10 to 23 °C (Fig. 1). Precipitation during winter and summer periods were 82 and 174 mm, respectively. Considering that interception of precipitation was included as an experimental treatment, a wide range of weather conditions were represented.
Table 1 presents selected slurry characteristics for the batches of pig slurry used for winter and summer storage experiments. Although the same farm supplied the slurry for both storage periods, the material obtained for the summer experiment was clearly more concentrated, as reflected in higher EC, DM, and nitrogen concentrations. and there was a tendency for differences in DM and VS, but not in TN or TAN, between four loads of slurry collected sequentially, as indicated by standard errors. The relatively high DM content resulted in some DM accumulating at the surface during summer storage, even in the +P−S treatment.
Changes during storage, as indicated by a mixed model, are shown at the bottom of Table 1. Slurry pH declined slightly during winter, but increased in all treatments during summer storage. The increase in TN and TAN during winter without precipitation could be due to some evaporative loss of water. During summer storage, there were substantial losses of TN and TAN with open storage conditions (+P), especially without the straw crust (−S); a decline of 0.8 kg Mg−1 TN versus 0.4 kg Mg−1 TAN indicated that losses during storage were substantial. There were no overall changes in EC during storage, but significant changes at the treatment level which reflected changes in TAN (Table 1).
Emissions of CH4, N2O and NH3
Cumulated CH4 emissions were 100-fold higher during summer compared to winter storage (Table 2), presumably as a result of the different storage temperature regimes. There was an exponential relationship between slurry temperature and CH4 emissions across the two storage periods, which was similar for stores without (Fig. 2a) and with a straw crust (Fig. 2b), although emissions from stores with a crust were much more variable. The values derived for ln(A) with and without added straw were 70.5 and 83.0, respectively, whereas apparent E A were 21.5 and 25.3 kJ mol−1.
Relationships between CH4 flux and pig slurry storage temperature during winter and summer storage conditions without (a) or with a surface crust (b) were modelled with an Arrhenius relationship. Black and white symbols represent stores without and with precipitation, respectively. Inserted are the values derived for the Arrhenius constant, ln(A), and apparent activation energy, E A
No N2O emissions were observed during winter, whereas in the summer experiment significant emissions developed, but only from stores with a straw crust (Fig. 3). Emissions of N2O from both stores without precipitation, −P+S, and with precipitation, +P+S, increased following an extended lag phase, although temporal dynamics were very different. In −P+S, the emission of N2O stabilized around 1 g N2O m−2 day−1, whereas in +P+S the N2O emissions peaked after 3 weeks, followed by a sharp decline in response to decreasing temperatures and rainfall (Fig. 1).
Nitrous oxide emissions during the summer experiment with storage of pig slurry without (a) or with precipitation (b) in combination with a straw crust (black) or no straw crust (white). Also shown are water balances for stores without and with precipitation, calculated as described by Sommer et al. (2000). For emission data mean ± standard errors are shown
Also shown in Fig. 3 are theoretical water balances for −P+S and +P+S treatments. With permanent sheltering to intercept precipitation, a water deficit of −200 mm during storage was calculated, whereas the water balance dropped more slowly in +P and, following heavy rainfall in early August, returned to zero, i.e., saturation. Registrations at the end of the storage experiment suggested a water loss closer to 100 mm in stores with a permanent cover, i.e., the theoretical water balance was probably overestimated and should be taken mainly as a proxy for crust aeration status. With both −P and +P treatments, the peak in N2O emissions occurred when the theoretical water balance was −50 to −100 mm, and the dramatic drop in N2O emissions from −C + S coincided with a re-wetting of the crust. Cumulated N2O emissions during storage are shown in Table 2.
Ammonia emissions were reduced by the straw crust, especially during summer storage. For the treatment with precipitation and without straw amendment, +P−S, 75 % of the loss in TAN during summer storage (Table 1) could be accounted for by the observed NH3 loss. However, as mentioned above, the TN loss from this treatment was higher than TAN loss, suggesting that there were N losses not accounted for by the NH3 emissions.
Total GHG balances, as represented by CH4 and N2O, and indirect N2O emissions derived from NH3, were calculated assuming GWP100 year values for CH4 and N2O of 25 and 298 (Forster et al. 2007), and indirect N2O emissions corresponding to 1 % of NH3 emissions. The balance was always dominated by CH4, which accounted for 80 % or more of GHG emissions (data not shown). During winter and summer storage periods, GHG emissions were 0.01–0.02 and 1.1–1.3 kg CO2 eq m−3 day−1.
Emission of odorants
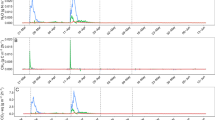
Total reduced sulphur, which is dominated by H2S (Gay et al. 2003), showed a maximum within 2 weeks of storage, possibly reflecting sulphate reduction or S mineralization from slurry organic matter (Fig. 4, top panel). Evidently, a straw crust reduced these emissions.
The three panels show emissions of TRS (top), skatole (middle) and p-cresol (bottom) during the summer storage experiment investigating four sets of storage conditions (with or without interception of precipitation (P), and with or without straw amendment (S). Due to different temporal dynamics of odorant emissions in some treatments, data from each replicate store is presented rather than averages
For skatole, the time course and concentration range was largely the same irrespective of storage conditions, i.e., there was little retention of this compound in the straw crust (Fig. 4, centre panel). In most stores a peak in skatole was observed at the second sampling, suggesting that the compound was produced during slurry organic matter decomposition.
p-Cresol exhibited a clear effect of Straw addition, i.e., a straw crust effectively reduced emissions in both −P+S and +P+S (Fig. 4, bottom panel). In most cases, a peak in p-cresol was observed at the second sampling in -S treatments, but in one replicate of −P−S this peak was apparently delayed.
Discussion
Emissions from livestock manure are difficult to quantify under practical storage conditions (Hensen et al. 2006; Sneath et al. 2006), and comparative studies are nearly impossible to conduct because of unique features with respect to feeding, management, and site-specific conditions complicating measurements. Pilot-scale storage facilities offer a possibility for side-by-side comparison of contrasting storage conditions. In the present study, pig slurry was stored summer and winter with or without admixing of straw and interception of precipitation to establish a wide range of storage conditions. Storage with a permanent cover and active ventilation was used to simulate open storage while avoiding precipitation, not as an example of practical storage conditions.
Emission of odorants
Many sulphur-containing volatile organic compounds rank high with respect to malodour (O’Neill and Phillips 1992), but in a storage experiment with 24 pig slurry materials, H2S explained 68 % of the total variability in odour emissions (Blanes-Vidal et al. 2009). Similarly, a recent study of emissions from a finishing pig production facility found that H2S and methanethiol (CH3SH) accounted for a significant part of the odour, although also p-cresol and trimethylamine were important (Hansen et al. 2012). TRS, including both organic and inorganic sulphur-compounds, thus appears to be a useful indicator of odour from pig slurry. Emissions of TRS were transient, and were clearly mitigated by straw addition. In the last part of the storage period, emissions of TRS dropped to 0 irrespective of storage conditions, probably because oxygen penetration at the slurry-air interface enabled sulfide oxidation (Millero et al. 1987) and sustained aerobic microbial degradation of organic sulphur compounds at the slurry-air interface.
Concentrations of skatole were considerably lower than those of p-cresol. The transient increase in emissions of both skatole and p-cresol from fresh pig slurry was also observed by Eriksen et al. (2010), and by Wu et al. (1999) monitoring skatole in untreated pig slurry. Both p-cresol and skatole can be degraded under aerobic or anaerobic conditions (Bossert and Young1986; Yin and Gu 2006), but a potential for retention of p-cresol apparently developed more rapidly than that for skatole retention, indicating that biodegradation was involved.
GHG emissions
Storage of pig slurry resulted in total GHG emissions of up to 1.3 kg CO2 eq m−3 day−1 during summer storage, whereas GHG emissions during winter storage were negligible. Clemens et al. (2006) stored untreated and digested cattle slurry with different cover strategies during summer and winter and found similar seasonal effects, but a maximum emission of 0.65 kg CO2 eq m−3 day−1. In their study, total GHG emissions were also dominated by CH4 emissions during summer storage.
Methanogenesis in livestock slurry is highly sensitive to temperature (Husted 1994; Sommer et al. 2007). In Table 3, apparent E A values are compiled for methanogenesis in manure and other environments, including the results from the present study. Apparent E A values from this study were low, corresponding to a moderate response to temperature, also when compared to other results for slurry materials. The proportion of readily degradable VS in pig slurry is high (Møller et al. 2004), and the most likely explanation for the moderate temperature response is therefore that degradable VS in the pig slurry was depleted at the higher temperatures, i.e., during the summer storage experiment. There was no evidence for an effect of a straw crust on CH4 emissions from the stored pig slurry, in contrast to the study of Sommer et al. (2000). Possible reasons are discussed in the next section.
Ammonia emissions were 2.0–3.1 g NH3 m−2 day−1 without straw addition (Table 2), and ten-fold lower with a straw-enforced crust during summer storage. Other pilot-scale storage experiments have reported NH3 emissions from uncovered cattle slurry of 1.6 (Clemens et al. 2006) and 2 g NH3 m−2 day−1 (VanderZaag et al. 2009). Sommer (1997), using a micrometeorological mass balance method, observed NH3 emissions from practical-scale storage tanks with digested slurry (pH 7.9–8.1) of up to 30 g NH3 m−2 day−1 (average 11 g NH3 m−2 day−1). Further, he found significant effects of incoming solar irradiance and temperature, but not wind speed, on NH3 emissions. It implies that the coverage of stores during measurement of NH3 emission, as was practiced in this study, could lead to underestimation of true fluxes during summer storage by intercepting solar irradiation. As a consequence, NH3 losses measured in treatment +P−S could have been lower than NH3 losses between measurements, possibly explaining the TN loss in this treatment (Table 1) not accounted for by NH3 emission.
As expected from previous studies, a potential for N2O emissions developed only in the presence of a crust. The highest N2O emissions observed in the present study corresponded to c. 80 mg m−2 h−1, which was higher than maximum emissions of 55 mg N2O m−2 h−1 observed by Sommer et al. (2000) and c. 30 mg N2O m−2 h−1 reported by VanderZaag et al. (2009). Straw was actively mixed with the slurry prior to storage, in accordance with local regulations, and therefore oxygen demand and water content of the developing crust was initially high. Sommer et al. (2000) found a significant relationship between N2O emissions during summer storage of cattle slurry and the water balance, as calculated from a theoretical balance between precipitation and potential evaporation. Nitrous oxide emissions in this earlier study peaked at −60 mm, which is consistent with the results of the present study (Fig. 2). As mentioned, the calculated water balance does not represent the true water loss from the store, but still is a useful proxy for temporal dynamics in crust wetness during storage. Saturation of the crust due to rainfall in the +P+S treatment coincided with an elimination of N2O emissions, indicating that gas exchange is a key factor in the regulation of microbial activity in surface crusts and hence emissions during storage.
Implications for N2O and CH4 emission factors
In the latest revision of the IPCC methodology (IPCC 2006), an N2O emission factor of 0.005 (uncertainty range: 0.0025–0.01) was proposed for liquid manure storage with an organic crust, although few measurement data have been published. The N2O emissions recorded in the present study from untreated pig slurry with a straw crust during 58 days of storage represented 0.0006–0.0012 of total N in +P+S and −P+S, respectively. Assuming this emission range is representative for the summer season (180 days) and no emissions occur during the remaining part of the year, in accordance with Fig. 2 and previous studies (Sommer et al. 2000; VanderZaag et al. 2009), then these numbers would correspond to annual N2O emission factors of 0.002–0.004, which is within the uncertainty range of the IPCC emission factor. However, as indicated by Fig. 2, N2O emissions depend on the water balance of the crust as well as storage temperature and, accordingly, is subject to inter-annual and regional differences. If a relationship with crust wetness is confirmed in future studies, then modelling of N2O emissions based on local climatic conditions should be explored.
Sommer et al. (2000) found that CH4 emissions from cattle slurry during summer storage were reduced by, on average, 38 % in the presence of floating crust materials. In a subsequent study, Clemens et al. (2006) stored digested cattle slurry with or without a crust during summer and winter storage conditions, but did not find any effect on CH4 emissions (although a reduction of 15–30 % occurred when stores were covered by a wooden lid). In the present study, simulating open storage conditions by continuous ventilation of stores amended with a cover, no effect of a straw crust on CH4 emissions was observed. It would thus appear that the proposed 40 % reduction of CH4 emissions in the presence of a crust on slurry stores (IPCC 2006) has been premature, and that the relationship between storage conditions and CH4 emissions should be better documented before effects of a crust on CH4 emissions are assumed in GHG inventories.
Can these apparent disagreements between observations be resolved? Incubation experiments have shown that an increase in CH4 availability stimulates microbial methane oxidation in surface crust materials (Petersen and Ambus 2006; Duan et al. 2013), with apparent half-saturation constants, K app , that correspond to gas phase concentrations of CH4 at least 3 orders of magnitude above atmospheric. Sommer et al. (2000) observed a reduction in CH4 emissions with crust materials while using the stores as static enclosures, and Clemens et al. (2006) when applying a wooden lid during slurry storage. In both cases, CH4 concentrations were able to build up in the air above the crust. It is thus conceivable that elevated gas phase concentrations of CH4 are required for a significant stimulation of methanotrophic activity. Supply of CH4 from the air above the slurry could be important if a large proportion of CH4 is released to the atmosphere by ebullition rather than diffusive flux.
Conclusions
This study quantified gaseous emissions from pig slurry under a wide range of storage conditions, extending previous studies on cattle slurry storage. The overall GHG balance was similar in all four experimental treatments, and was dominated by CH4 emissions during summer storage. Methane emissions were not reduced in the presence of a crust, as hypothesized, possibly because accumulation of CH4 in the air is required to stimulate methane oxidation. Peak emissions of N2O coincided with a negative water balance of −50 to −100 mm, and dropped to zero as a result of rewetting after rainfall, probably as a result of a shift towards anaerobic conditions in the crust. A better understanding of the effects of storage environment on emissions of N2O and CH4 is needed for more reliable emission estimates, and for identification of effective mitigation measures.
Abbreviations
- VS:
-
Volatile solids
- TRS:
-
Total reduced sulphur
- TN:
-
Total nitrogen
- TAN:
-
Total ammoniacal nitrogen
- GWP:
-
Global warming potential
- GHG:
-
Greenhouse gas
- −P−S:
-
No precipitation, no straw crust
- −P+S:
-
No precipitation, straw crust present
- +P−S:
-
With precipitation, no straw crust
- +P+S:
-
With precipitation, straw crust present
References
Ambus P, Petersen SO (2005) Oxidation of 13C-labeled methane in surface crusts of pig- and cattle slurry. Isot Environ Health Stud 41:125–133
Blanes-Vidal V, Hansen MN, Adamsen APS, Feilberg A, Petersen SO, Jensen BB (2009) Characterization of odor released during handling of swine slurry: Part I. Relationship between odorants and perceived odor concentrations. Atmos Environ 43:2997–3005
Bossert ID, Young LY (1986) Anaerobic oxidation of p-cresol by a denitrifying bacterium. Appl Environ Microbiol 52:1117–1122
Chin K-J, Lukow T, Stubner S, Conrad R (1999) Structure and function of the methanogenic archaeal community in stable cellulose-degrading enrichment cultures at two different temperatures (15 and 30°C). FEMS Microbiol Ecol 30:313–326
Clemens J, Trimborn M, Weiland P, Amon B (2006) Mitigation of greenhouse gas emissions by anaerobic digestion of cattle slurry. Agric Ecosyst Environ 112:171–177
Duan Y-F, Elsgaard L, Petersen SO (2013) Inhibition of methane oxidation in slurry surface crust by inorganic nitrogen. J Environ Qual 42 (in press)
Dunfield P, Knowles R, Dumont R, Moore TR (1993) Methane production and consumption in temperate and subarctic peat soils: response to temperature and pH. Soil Biol Biochem 25:321–326
Eriksen J, Adamsen APS, Nørgaard JV, Poulsen HD, Jensen BB, Petersen SO (2010) Emissions of sulphur-containing odorants, ammonia and methane from pig slurry: effects of dietary methionine and benzoic acid. J Environ Qual 39:1097–1107
Feilberg A, Adamsen APS, Lindholst S, Lyngbye M, Schäfer A (2010) Evaluation of biological air filters for livestock ventilation air by membrane inlet mass spectrometry. J Environ Qual 39:1085–1096
Feilberg A, Nyord T, Hansen MN, Lindholst S (2011) Chemical evaluation of odor reduction by soil injection of animal manure. J Environ Qual 40:1674–1682
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga G, Schulz M, Van Dorland R (2007) Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge/New York
Gay SW, Schmidt DR, Clanton CJ, Janni KA, Jacobson LD, Weisberg S (2003) Odor, total reduce sulfur, and ammonia emissions from animal housing facilities and manure storage units in Minnesota. Appl Eng Agric 19:347–360
Hansen MJ, Adamsen APS, Pedersen P, Feilberg A (2012) Prediction of odor from pig production based on chemical odorants. J Environ Qual 41:436–443
Hensen A, Groot TT, van den Bulk WCM, Vermeulen AT, Olesen JE, Schelde K (2006) Dairy farm CH4 and N2O emissions, from one square metre to the full farm scale. Agric Ecosyst Environ 112:146–152
Husted S (1994) Seasonal variation in methane emission from stored slurry and solid manures. J Environ Qual 23:585–592
IPCC (2006) Guidelines for National Greenhouse Gas Inventories. Agriculture, forestry and other land use, vol 4. Intergovernmental Panel on Climate Change, IGES, Hayama
Keeney DR, Nelson DW (1982) Nitrogen—inorganic forms. In: Page AL et al (eds) Methods of soil analysis. Part 2. Agronomy monographs 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 643–693
Khan RZ, Müller C, Sommer SG (1997) Micrometeorological mass balance technique for measuring CH4 emission from stored cattle slurry. Biol Fertil Soils 24:442–444
Millero FJ, Hubinger S, Fernandez M, Garnett S (1987) Oxidation of H2S in seawater as a function of temperature, pH, and ionic strength. Environ Sci Technol 21:439–443
Møller HB, Sommer SG, Ahring BK (2004) Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 26:485–495
Mutegi JK, Munkholm LJ, Petersen BM, Hansen EM, Petersen SO (2010) Nitrous oxide emissions and controls as influenced by tillage and crop residue management strategy. Soil Biol Biochem 42:1701–1711
O’Neill DH, Phillips VR (1992) A review of the control of odour nuisance from livestock buildings: Part 3. Properties of the odorous substances which have been identifi ed in livestock wastes or in the air around them. J Agric Eng Res 53:23–50
Petersen SO, Ambus P (2006) Methane oxidation in pig and cattle slurry storages, and effects of surface crust moisture and methane availability. Nutr Cycl Agroecosyst 74:1–11
Petersen SO, Amon B, Gattinger A (2005) Methane oxidation in slurry storage surface crusts. J Environ Qual 34:455–461
Petersen SO, Skov M, Dröscher P, Adamsen APS (2009) Pilot scale facility to determine gaseous emissions from livestock slurry during storage. J Environ Qual 38:1560–1568
Safley LM, Westerman PW (1990) Psychrophilic anaerobic digestion of animal manure: proposed design methodology. Biol Wastes 34:133–148
Schiffman SS (1998) Livestock odors: implications for human health and well-being. J Anim Sci 76:1343–1355
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O (2007) Agriculture. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA (eds) Climate change 2007: mitigation. Contribution of working group III to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge/New York
Sneath RW, Beline F, Hilhorst MA, Peu P (2006) Monitoring GHG from manure stores on organic and conventional dairy farms. Agric Ecosyst Environ 112:122–128
Sommer SG (1997) Ammonia volatilization from farm tanks containing anaerobically digested animal slurry. Atmos Environ 31:863–868
Sommer SG, Petersen SO, Søegaard HT (2000) Greenhouse gas emission from stored livestock slurry. J Environ Qual 29:744–750
Sommer SG, Petersen SO, Møller HB (2004) Algorithms for calculating methane and nitrous oxide emissions from manure management. Nutr Cycl Agroecosyst 69:143–154
Sommer SG, Zhang GQ, Bannink A, Chadwick D, Misselbrook T, Harrison R, Hutchings NJ, Menzi H, Monteny GJ, Ni JQ, Oenema O, Webb J (2006) Algorithms determining ammonia emission from buildings housing cattle and pigs and from manure stores. Adv Agron 89:261–335
Sommer SG, Petersen SO, Sørensen P, Poulsen HD, Møller HB (2007) Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr Cycl Agroecosyst 78:27–36
Steinfeld H, Gerber P, Wassenaar T, Castel V, Rosales M, de Haan C (2006) Livestock’s long shadow—environmental issues and options. Food and Agricultural Organization, Rome, Italy. http://www.fao.org/docrep/010/a0701e/a0701e00.HTM (verified 18 Nov 2011)
VanderZaag AC, Gordon RJ, Jamieson RC, Burton DL, Stratton GW (2009) Gas emissions from straw covered liquid dairy manure during summer storage and autumn agitation. Trans ASABE 52:599–608
Wu JJ, Park S, Hengemuehle SM, Yokoyama MT, Person HL, Gerrish JB, Masten SJ (1999) The use of ozone to reduce the concentration of malodorous metabolites in swine manure slurry. J Agric Eng Res 72:317–327
Yin B, Gu J-D (2006) Aerobic degradation of 3-methylindole by Pseudomonas aeruginosa Gs isolated from mangrove sediment. Hum Ecol Risk Assess 12:248–258
Acknowledgments
The skilled technical assistance of Claudia Nagy, Bodil Steensgaard, Karin Dyrberg and Morten Skov is greatly appreciated. This study was supported by the Danish Ministry for Food, Agriculture and Fisheries (VMP3-STOP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersen, S.O., Dorno, N., Lindholst, S. et al. Emissions of CH4, N2O, NH3 and odorants from pig slurry during winter and summer storage. Nutr Cycl Agroecosyst 95, 103–113 (2013). https://doi.org/10.1007/s10705-013-9551-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-013-9551-3