Abstract
Pig farms have achieved importance in the last few decades from the perspective of environment protection as a consequence of the intensive rearing systems in livestock production. Ammonia (NH3) and greenhouse gases (GHG), such as methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O), are emitted from slurry storage at farm prior to land application, but little is known about these losses under on-farm conditions in Spain. This study assessed the influence of management and environmental parameters on NH3 and GHG emissions from slurry storage in spring and autumn. Gas emissions were measured in a commercial pig-fattening farm from two lagoons (1000 and 768 m3 capacity, respectively) during 30 days by the floating dynamic chamber system in spring and autumn 2011 (average temperature 19 and 9 °C, respectively). Low NH3 and CH4 emissions were registered in spring (range 10–406 and 3–17 mg m−2 min−1, respectively) probably as a result of low pH values of stored slurry (6.5 to 7.0) and rainfall. High variability on NH3, CH4, and CO2 emissions was observed as a result of differences in temperature and rainfall. No NH3 emission and low CH4 and CO2 emissions were observed in autumn (average 1.2 ± 0.9 and 27 ± 22 mg m−2 min−1, respectively). Slurry loading operations increased NH3 losses from storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Gaseous emissions associated with slurry management represent a major concern due to the intensification of livestock production. Emissions of ammonia (NH3) from barns and slurry stores represent up to 80 % of the total NH3 emissions from agricultural activities (Anderson et al. 2003). These emissions may increase in the future in response to a greater demand for food from a more populated world (United Nations 2013). At the moment, pork is the most widely consumed meat product worldwide and production intensification has led to increase environmental concerns in relation to NH3 emissions to air. Also, pig production is the second contributor of greenhouse gases (GHG) from livestock sector (Gerber et al. 2013). In pig houses, NH3 volatilizes mainly from urine-fouled floor areas and from the surface area of slurry in the slurry pit. In these systems, slurry is first collected under partially slatted floors for temporary indoor storage. Thereafter, the slurry is transferred to an outdoor storage where gaseous emissions take place. Ammonia and GHG, such as methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O), are emitted from slurry storage at farm level prior to land application. There is previous scientific literature on these emissions from slurry land application to soil in Spain (Vallejo et al. 2005), but there is no information about gaseous losses from slurry storage under on-farm conditions in Spain. Ideally, emissions should be conducted at the farm scale in order to capture the reality of a farm to understand the effect of climate and regional management on emissions. In this context, emissions can vary substantially over time due to changing storage volumes, loading and emptying operations, etc. According to previous studies (Kaharabata et al. 1998; Chadwick et al. 2011; VanderZaag et al. 2014), CH4 emissions are governed by management and environmental factors in dairy and pig slurry storages, which makes it necessary to obtain a temporal pattern of these emissions in order to propose further mitigation strategies. As indicated by Phillipe et al. (Phillippe and Nicks 2015), these strategies should be integrated in a larger scale in order to take into account emissions associated with pre- and post-farming processes.
This study assessed the influence of management and environmental parameters on NH3 and GHG emissions from slurry storage in spring and autumn taking into account management operations, temperature, and manure chemical composition.
2 Methods
2.1 Farm Description
A farm-scale study was carried out at a commercial pig-fattening farm in the Basque Country (42° 53′ 41″ N, 2° 44′ 16″ W) in spring from 11 May 2011 to 9 June 2011. The farm was regulated by the IED Directive as 2000 pigs in total were reared during 160 days into two identically managed buildings in the farm. Slurry produced in each building was stored into a deep pit located under the partially slatted floor. Slurry was pumped outdoors every month to two impermeable lined lagoons: lagoon A with 1000 m3 capacity and 440 m2 surface area and lagoon B with 768 m3 capacity and 300 m2 surface area. In 7 June 2011, lagoon A was disturbed by loading operations in response to farmer’s management needs. After being emptied in December 2010, both lagoons were full in May, for spring study. Since they are emptied (two times a year), the lagoons start to receive slurry once every month. Despite the high air temperatures registered during the study (maximum 34 °C, with an average of 19 °C), none of the lagoons presented natural surface crust in spring.
In order to ascertain the temporal pattern of gaseous emissions from slurry storage, on-farm measurements continued in autumn from 28 November 2011 to 22 December 2011. After being fully emptied in August 2011, lagoons A and B presented two thirds of slurry volume stored in spring.
2.2 Gas Measurements
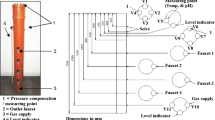
A floating dynamic chamber system described by Peu et al. (1999) was used for gaseous emission measurement. Chambers were built as rectangular polyvinyl chloride boxes (length 60 cm; width 34 cm; height 40 cm). The experimental area of chambers was 0.204 m2. Three chambers were randomly positioned on each lagoon surface by a system of ropes, and their lower end was submersed 4 cm below the surface of the slurry. Two polyvinyl chloride baffles (length 28 cm; width 1 cm; height 34 cm) were fixed perpendicularly inside the chambers in order to obtain a turbulent air stream. There was a 1-cm diameter hole on the top of the chambers in order to prevent internal overpressure. An air flow produced by a compressor (Abac FC2/24, Torino, Italy) was passed through 10 % orthophosphoric acid and distilled water to scrub NH3 and delivered through a Teflon tube into each chamber. The flow rate was 2–3 l min−1, which was fixed with a flow meter (2150/Inox, Tecfluid S.A., Barcelona, Spain) and checked by a gas meter (Gallus 2000, Itron, Washington, USA). The outlet-air stream was passed through a dryer (Envirogel, Brownell Ltd., London, UK) to avoid interferences with the high moisture content of samples. Gaseous concentrations were continuously measured by a photoacoustic analyzer (multi-gas monitor 1302, Bruel & Kjaer, Nærum, Denmark), with detection limits being 0.2 ppm for NH3, 0.4 ppm for CH4, 5 ppm for CO2, and 0.03 ppm for N2O at 20 °C and 1 atm pressure.
Gas sampling lasted 10 min in each chamber until a steady-state value was reached. A valve system was used to switch from one chamber to the other. Gaseous concentrations were sampled for 5 h each day (10:00–15:00) with a frequency of 3–4 days a week during 30 and 25 days in spring and autumn, respectively.
Gaseous emissions were calculated as follows:
where F is the gas emission (mg m−2 min−1), C out and C in the outlet and inlet gas concentration, respectively (mg m−3, at 1 atm pressure and daily temperature), Q is the air flow measured by the gas meter (m3 min−1), and A is the slurry area covered by the chamber (m2).
Emission data registered from each chamber were summed after 5 h measurement period. Daily accumulated emission was calculated as the mean of chambers for each treatment.
N2O emissions were not relevant both in spring and autumn. This is coincident with the results obtained by Harper et al. (2000) over extended periods and by Rodhe et al. (2012) and Park et al. (2006) for warm and cold conditions, respectively. To this respect, as stated in IPCC (2006), emissions from liquid slurry without natural crust cover are believed negligible due to the absence of oxidized forms of nitrogen entering systems in combination with low potential for nitrification and denitrification in the system.
2.3 Slurry Sample Collection and Analysis
Slurry was sampled from lagoons A and B at a randomly chosen location from three different points from the profile 0–40 cm depth on each pit. Three replicates of slurry samples were taken at three different moments within spring and autumn studies: beginning, middle, and end of each study. Thus, sampling times were 12 May, 23 May, and 7 June 2011 in spring study and 30 November, 12 December, and 22 December 2011 in autumn study. Samples were stored at 4 °C until analysis. Slurry samples were analyzed for dry matter (DM) and organic matter (OM) content according to AFNOR (1976) procedures. Total Kjeldahl nitrogen (TKN) was analyzed by the Kjeldalh distiller-analyzer (Kjeltec Auto 1030, Tecator, Hillerød, Denmark) after reduction with sulfur-salicylic acid, and ammonium-nitrogen (NH4 +–N) content was measured directly after distillation in oxide magnesium (official methods of the Spanish Ministry of Agriculture 1974). The ratio between carbon and nitrogen (C:N) was calculated based on OM (1.72·C) and TKN percentage content.
2.4 Lagoon Parameters and Meteorological Data
Slurry temperature data and the rainfall registered in spring and autumn studies are shown in Figs. 1a and 2a, respectively. Slurry temperature was recorded at 15 cm depth using thermocouples connected to dataloggers (FEP T type, TCdirect, Madrid, Spain), and rainfall was taken from a meteorological station situated 1.4 km away from the farm. Slurry pH was daily recorded at 15 cm depth by a pH meter (HQ40D, Hach Lange, Düsseldorf, Germany). The wind speed from stored slurry surface was recorded daily before gas measurement by a thermal anemometer (Testo 425, Testo AG, Lenzkirch, Germany). The average wind speed registered was used to fix the air flux passing the floating chamber. Daily atmospheric temperatures were registered by a datalogger (HOBO U12-013, Onset HOBO© Data Loggers, USA). Mean air temperatures for spring and autumn were 19 ± 3 and 9 ± 2 °C, respectively. Slurry temperatures for both periods are presented in Table 1.
Pattern of daily a slurry temperature (°C) in lagoons A and B and rainfall (mm), b ammonia (NH3)-accumulated emission (mg m−2), c methane (CH4)-accumulated emission (g m−2), and d carbon dioxide (CO2)-accumulated emission (g m−2) in lagoons A and B during 5 h each day in spring study. The error bars show standard deviation
2.5 Statistical Analysis
Gas emission and slurry characteristics were analyzed as repeated measures throughout spring and autumn studies using PROC MIXED procedure by the Statistical Analysis Software SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Moreover, regression analysis between gas emissions and slurry temperatures registered daily during 5 h measurement period were carried out using the Statistical Package for the Social Sciences 15.0 (SPSS Inc., Armonk, NY, USA). Significant differences are expressed at p < 0.05, unless otherwise stated.
3 Results and Discussion
3.1 Ammonia Emission
Daily NH3 emission rates measured in spring during 30 days ranged between 10 and 406 μg m−2 min−1 in the lagoons, which were in accordance with emissions reported by Aneja et al. (2001) from pig slurry lagoons under similar air temperature conditions (40–374 μg m−2 min−1). Other authors (Blunden and Aneja 2008; James et al. 2012; Lim et al. 2003) have reported higher emissions. In those studies, the free ammonia (FA) concentration in slurry was higher than in our work (Table 1). This parameter is calculated based on Anthonisen et al. (1976) equation and is a good predictor of NH3 emission from lagoons (Szögi et al. 2006). It is influenced by the joint effect of total NH4 +–N concentration, temperature, and pH. The high NH4 +–N contents presented in slurry in the current study (Table 1) suggest that this parameter did not limit NH3 volatilization rate. Blunden and Aneja (2008) reported higher NH3 emissions (>3000 μg m−2 min−1) from stored slurry with lower NH4 +–N content. Similarly, air temperatures recorded in spring study (range 14–34 °C) should warrant higher NH3 emissions according to previous studies (James et al. 2012; Lim et al. 2003). The pH values recorded in both lagoons ranged from 6.5 to 7.0 in spring study. Similar pH values have been reported for pig slurry by other authors (Aneja et al. 2001; Dai and Blanes-Vidal 2013); however, studies reporting higher NH3 emissions presented pH values above 7.0 (Blunden and Aneja 2008; James et al. 2012; Lim et al. 2003). According to Hartung and Phillips (1994), the greatest increase in NH3 release takes place between a pH of 7 and 10. Therefore, the low FA concentration in slurry in spring would suggest that slightly acidic pH values limited NH3 losses. Other factors such as rainfall could also have influenced NH3 volatilization (Aneja et al. 2001). Accumulated rainfall was 41 mm from 12 May 2011 to 6 June 2011 (Fig. 1a) and NH3 volatilization dropped considerably after each rainfall period, as observed on 12 May 2011 after 13 mm rainfall (Fig. 1b). The highest NH3 peak observed on 7 June 2011 was related to the loading operation performed by the farmer in lagoon B (Fig. 1b). As a consequence, the air-liquid interface of stored slurry would have been disturbed and NH3 emission increased seven times with respect to average emission during previous days. In the following 2 days, emission level decreased around 50 % with respect to the NH3 peak.
Contrarily to spring study, there was no NH3 emission observed in the lagoons in autumn study. Previous studies have also reported seasonal trends in NH3 emission (Blunden and Aneja 2008; James et al. 2012). This seasonal variability in NH3 losses from slurry storage was reported to be influenced by factors such as slurry temperature, NH4 +–N content and pH (Aneja et al. 2001). A regression model conducted within both studies showed a significant relationship between slurry temperature and NH3 fluxes (p < 0.05; r = 0.48). In fact, mean slurry temperature was 19 ± 3 °C in spring study and 9 ± 2 °C in autumn study, which would have affected seasonal NH3 emission. Stored slurry in autumn was more diluted compared to spring probably due to the spells of rainfall occurring in autumn (64.2 mm accumulated), and therefore, significant differences in NH4 +–N contents were observed between spring and autumn studies (Table 2). However, NH4 +–N contents in terms of % DM were not different between experimental periods, ranging 8–19 % DM.
3.2 Methane
Methane emissions in spring study ranged between 3 and 17 mg m−2 min−1 in the lagoons, which agree with results reported in previous studies (Flesch et al. 2013; Sharpe et al. 2002). However, the large variation of on-farm CH4 losses reported in the literature, ranging between 4 and 360 mg CH4 m−2 min−1, indicates that CH4 losses from the current study could be considered as low. Physicochemical parameters, such as slurry NH4 +–N, temperature, and pH, have been found to affect CH4 emission. A high FA content of slurry inhibits the anaerobic microbial consortia necessary for the production of CH4 (Nielsen and Angelidaki 2008). Inhibition has been reported at FA concentrations in excess of 124 mg NH3–N l−1 for an unadapted process (Braun et al. 1981). Despite high NH4 +–N contents in stored slurry, the low FA values estimated in spring study suggested that CH4 emission was not affected (Table 1). The warm temperatures (range 14–34 °C) in which spring study was conducted should not have minimized CH4 losses. The Intergovernmental Panel on Climate Change (2006) guidelines estimate that 74–79 % of potential CH4 is lost under temperature conditions. Methanogenesis from acetate, which mainly contributes to manure-generated CH4, is inhibited by pH lower than 7 (Attal et al. 1988). As Table 2 shows, pH values in stored slurry were below 7 throughout spring study; thus, it was concluded that pH was a significant factor limiting CH4 losses from the lagoons. Park et al. (2006) also reported low CH4 emission rates from slurry storages with pH below 7.
Methane emissions in lagoon A were not significantly different between spring and autumn studies, which averaged 6 ± 4 and 6 ± 2 mg m−2 min−1, respectively. On the contrary, significant difference on CH4 emission was observed between seasons in lagoon B (Figs. 1c and 2b), averaging 7 ± 5 mg m−2 min−1 in spring and 1.2 ± 0.9 mg m−2 min−1 in autumn. As concluded by Park et al. (2006), CH4 fluxes are related to the volume of liquid manure from which fluxes are measured, which explains that seasonal CH4 difference in lagoon B could be due to lower slurry volume stored in autumn study. Additionally, a regression model conducted between slurry temperatures and CH4 fluxes within spring and autumn studies indicates that cold slurry temperatures registered in autumn were closely related to lower CH4 emissions (p < 0.05; r = 0.46). This is in accordance with Sharpe et al. (2002), who observed a marked influence of slurry temperature on seasonal CH4 emissions.
3.3 Carbon Dioxide
Carbon dioxide emissions ranged between 5 and 248 mg m−2 min−1 in the lagoons in spring study. Results showed that CO2 emission was significantly higher than CH4 losses measured in the lagoons in spring study (Fig. 1c, d). According to Dinuccio et al. (2008), the predominant emission of carbon was as CO2 from pig slurry storage. The high CO2 emission in spring would have been influenced by the low pH in stored slurry. Methane is produced by the hydrogenotrophic methanogens which consume CO2 derived from the anaerobic decomposition of acetate (Moller et al. 2004). Nevertheless, the partial inhibition of methanogenesis by low pH values could have contributed to CO2 accumulation. This CO2 accumulation would also have contributed to acidify the slurry solution as, together with total acetic acid, both are buffer compounds that favor slurry acidification (Dai and Blanes-Vidal 2013).
Carbon dioxide emissions between seasons differed significantly in stored slurry (Figs. 1d and 2c), averaging 73 ± 47 mg m−2 min−1 in spring study and 27 ± 22 mg m−2 min−1 in autumn study. This difference was probably related to differences in seasonal slurry temperatures, coinciding with Dinuccio et al. (2008). These authors observed differences in CO2 emission from slurry stored at 25 and 5 °C in a laboratory study, being 67 and 42 mg m−2 min−1, respectively.
4 Conclusions
This study assessed NH3 and GHG emissions from slurry storage under on-farm conditions in spring and autumn. According to the results, both management and environmental parameters influenced gaseous emissions from storage. In general, low emissions of NH3 and CH4 were found from stored slurry in spring and autumn probably due to low slurry pH. Farmer’s slurry management (i.e., loading operation) increased NH3 losses from storage. High variability on NH3, CH4, and CO2 emissions were observed as a result of differences in temperature and rainfall. This study shows the importance of considering environmental factors and management practices for future evaluation of gas mitigation strategies in storage along the year.
References
AFNOR, Association Française de Normalisation (1976). Standard U44-160. Saint-Denis, France.
Anderson, N., Strader, R., & Davidson, C. (2003). Airborne reduced nitrogen: ammonia emissions from agriculture and other sources. Environment International, 29, 277–286.
Aneja, V. P., Bunton, B., Walker, J. T., & Malik, B. P. (2001). Measurement and analysis of atmospheric ammonia emissions from anaerobic lagoons. Atmospheric Environment, 35, 1949–1958.
Anthonisen, A. C., Loehr, R. C., Prakasam, T. B. S., & Srinath, E. G. (1976). Inhibition of nitrification by ammonia and nitrous-acid. Journal of Water Pollution Control Federation, 48, 835–852.
Attal, A., Ehlinger, F., Audic, J. M., & Faup, G. M. (1988). pH inhibition mechanisms of acetogenic, acetoclastic and hydrogenophilic populations. In E. R. Hall & P. N. Hobson (Eds.), Anaerobic digestion 1988: proceedings of the fifth international symposium on anaerobic digestion (pp. 71–78). Bologna: Pergammon Press.
Blunden, J., & Aneja, V. P. (2008). Characterizing ammonia and hydrogen sulfide emissions from a swine waste treatment lagoon in North Carolina. Atmospheric Environment, 42, 3277–3290.
Braun, R., Huber, P., & Meyrath, J. (1981). Ammonia toxicity in liquid piggery manure digestion. Biotechnology Letters, 3, 159–164.
Chadwick, D., Sommer, S. G., Thorman, R., Fangueiro, D., Cardenas, L., Amon, B., & Misselbrook, T. (2011). Manure management: implications for greenhouse gas emissions. Animal Feed Science and Technology, 166–67, 514–531.
Dai, X. R., & Blanes-Vidal, V. (2013). Emissions of ammonia, carbon dioxide, and hydrogen sulfide from swine wastewater during and after acidification treatment: effect of pH, mixing and aeration. Journal of Environmental Management, 115, 147–154.
Dinuccio, E., Berg, W., & Balsari, P. (2008). Gaseous emissions from the storage of untreated slurries and the fractions obtained after mechanical separation. Atmospheric Environment, 42, 2448–2459.
Flesch, T. K., Verge, X. P. C., Desjardins, R. L., & Worth, D. (2013). Methane emissions from a swine manure tank in western Canada. Canadian Journal of Animal Science, 93, 159–169.
Gerber, P.J., Steinfeld, H., Henderson, B., Mottet, A., Opio, C., Dijkman, J. et al. (2013). Tackling climate change through livestock. Technical report, FAO, Rome, Italy.
Harper, L. A., Sharpe, R. R., & Parkin, T. B. (2000). Gaseous nitrogen emissions from anaerobic swine lagoons: ammonia, nitrous oxide, and dinitrogen gas. Journal of Environmental Quality, 29(4), 1356–1365.
Hartung, J., & Phillips, V. R. (1994). Control of gaseous emissions from livestock buildings and manure stores. Journal of Agricultural Engineering Research, 57, 173–189.
Intergovernmental Panel on Climate Change (2006). IPCC Guidelines for national greenhouse gas inventories, vol.4. Agriculture, forestry and other land use. In H.S. Eggleston, L. Buendia, L. Miwa, T. Ngara, K. Tanabe (Eds.), Prepared by the National Greenhouse Gas Inventories Programme IGES, Hayama, Japan.
James, K. M., Blunden, J., Rumsey, I. C., & Aneja, V. P. (2012). Characterizing ammonia emissions from a commercial mechanically ventilated swine finishing facility and an anaerobic waste lagoon in North Carolina. Atmopheric Pollution Research, 3, 279–288.
Kaharabata, S. K., Schuepp, P. H., & Desjardins, R. L. (1998). Methane emissions from aboveground open manure slurry tanks. Global Biogeochemical Cycles, 12, 545–554.
Lim, T. T., Heber, A. J., Ni, J. Q., Sutton, A. L., & Shao, P. (2003). Odor and gas release from anaerobic treatment lagoons for swine manure. Journal of Environmental Quality, 32, 406–416.
Moller, H. B., Sommer, S. G., & Ahring, B. K. (2004). Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. Journal of Environmental Quality, 33, 27–36.
Nielsen, H. B., & Angelidaki, I. (2008). Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresource Technology, 99, 7995–8001.
Park, K. H., Thompson, A. G., Marinier, M., Clark, K., & Wagner-Riddle, C. (2006). Greenhouse gas emissions from stored liquid swine manure in a cold climate. Atmospheric Environment, 40, 618–627.
Peu, P., Beline, F., & Martinez, J. (1999). A floating chamber for estimating nitrous oxide emissions from farm scale treatment units for livestock wastes. Journal of Agricultural Engineering Research, 73, 101–104.
Phillippe, F. X., & Nicks, B. (2015). Review on greenhouse gas emissions from pig houses: production of carbon dioxide, methane and nitrous oxide by animals and manure. Agriculture, Ecosystems and Environment, 199, 10–25.
Rodhe, L. K. K., Abubaker, J., Ascue, J., Pell, M., & Nordberg, A. (2012). Greenhouse gas emissions from pig slurry during storage and after field application in northern European conditions. Biosystems Engineering, 113, 379–394.
Sharpe, R. R., Harper, L. A., & Byers, F. M. (2002). Methane emissions from swine lagoons in southeastern US. Agriculture, Ecosystems and Environment, 90, 17–24.
Spanish Ministry of Agriculture (1974). Official methodologies for soil and water analysis. Madrid, Spain.
Szögi, A. A., Vanotti, M. B., & Stansbery, A. E. (2006). Reduction of ammonia emissions from treated anaerobic swine lagoons. Transactions of the ASABE, 49, 217–225.
United Nations (2013) Demographic components of future population growth. Technical Paper No. 2013/3. 19pp.
Vallejo, A., García-Torres, L., Diez, J. A., Arce, A., & López-Fernández, S. (2005). Comparison of N losses (NO3 -, N2O, NO) from surface applied, injected or amended (DCD) pig slurry o fan irrigated soil in a Mediterranean climate. Plant and Soil, 272, 313–325.
VanderZaag, A. C., Flesch, T. K., Desjardins, R. L., Baldé, H., & Wright, T. (2014). Measuring methane emissions from two dairy farms: seasonal and manure-management effects. Agricultural and Forest Meteorology, 194, 259–267.
Acknowledgments
This work has been funded by BATFARM Interreg-Atlantic Area Project (2009-1/071) entitled “Evaluation of best available techniques to decrease air and water pollution in animal farms”. Maialen Viguria holds a grant from the Ph.D. student’s research program of the Department of Education, Universities and Research of the Basque Government. The authors are especially grateful to the farmer that provided agricultural infrastructure and to the University of the Basque Country for the computer facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viguria, M., López, D.M., Arriaga, H. et al. Ammonia and Greenhouse Gases Emission from On-Farm Stored Pig Slurry. Water Air Soil Pollut 226, 285 (2015). https://doi.org/10.1007/s11270-015-2548-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2548-6