Abstract
Previous studies have demonstrated that sharks, perhaps more so than any other fishes, are capable of bioaccumulating the non-essential toxic metal mercury (Hg) to levels that threaten the health of human seafood consumers. However, few studies have explored the potential effects of Hg accumulation in sharks themselves. Therefore, the goal of this study was to examine if physiological effects occur in sharks in response to environmentally relevant levels of Hg exposure. To address this goal, the relationship between muscle Hg concentrations and muscle/hepatic levels of metallothionein (MT), a widely used protein biomarker of toxic metal exposure in fish, was examined in bonnetheads, Sphyrna tiburo, from three Florida estuaries. Total Hg concentrations in bonnethead muscle, as determined using thermal decomposition and atomic absorption spectrometry, ranged from 0.22 to 1.78 μg/g wet weight and were correlated with animal size. These observations were consistent with earlier studies on Florida bonnetheads, illustrating that they experience bioaccumulation of Hg, often to levels that threaten the health of these animals or consumers of their meat. However, despite this, MT concentrations measured using Western blot analysis were not correlated with muscle Hg concentrations. These results suggest that either environmentally relevant levels of Hg exposure and uptake are below the physiological threshold for inducing effects in sharks or MT is a poor biomarker of Hg exposure in these fishes. Of these two explanations, the latter is favored based on a growing body of evidence that questions the use of MTs as specific indicators of Hg exposure and effects in fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a highly toxic prevalent non-essential metal that is commonly found in aquatic environments (see reviews by Chen et al. 2008; Kim and Zoh 2012; Driscoll et al. 2013). It is deposited in its inorganic form into the environment primarily by anthropogenic activities such as mining, waste incineration, and the combustion of Hg-rich fossil fuels. Once Hg is deposited into an aquatic system, it often undergoes bacterial methylation to produce the organic compound, monomethylmercury (MeHg). MeHg is more readily absorbed by aquatic organisms than inorganic forms of Hg; in fact, up to or greater than 90 % of the total Hg content in some aquatic species can be in the form of MeHg (Scheuhammer et al. 2007). It is also known to bioaccumulate and biomagnify in aquatic organisms and, as a result, MeHg levels in some aquatic taxa, particularly large terminal consumers such as marine mammals, sharks, and large teleosts can range from one to ten million times greater than MeHg levels present in the ambient environment (Chen et al. 2008). Because of its high rate of uptake, distribution, and accumulation in exposed organisms, its slow excretion and its tendency to bind to and disrupt the normal function of sulfhydryl-containing proteins, MeHg is considered to be the most toxic form of Hg, capable of eliciting adverse effects such as neurotoxicity, behavioral abnormalities, reproductive impairment, immunosuppression, and death (Scheuhammer et al. 2007). Therefore, it is critical to examine potential health effects of MeHg and other forms of Hg in aquatic species, particularly those that have been found to accumulate substantial quantities of this metal.
Based on a 2010 review by Gelsleichter and Walker and more recent studies (De Boeck et al. 2010; Escobar-Sánchez et al. 2010, 2011; Pethybridge et al. 2010; Nam et al. 2011; Barrera-García et al. 2012; Hurtado-Banda et al. 2012; Maz-Courrau et al. 2012; Bosch et al. 2013; Vélez-Alavez et al. 2013), Hg is the most widely studied toxicant in elasmobranchs (sharks and rays), with levels having been examined in close to 100 species. A sizeable number of these studies observed muscle Hg concentrations that were near to or above the maximum recommended limit for human consumption in most countries (i.e., 0.3 ppm, US. Environmental Protection Agency [EPA] 2001). Furthermore, as in some other taxa, MeHg can often make up a sizeable proportion of the total Hg burden in certain elasmobranchs (i.e., >95 %), particularly largely piscivorous species. However, despite the multitude of studies that have shown the presence of elevated Hg levels in sharks and their relatives, few studies to date have considered the possible biological effects of Hg exposure in these animals as opposed to human consumers of their meat/fins. In contrast, the effects of Hg on bony fish have been studied more extensively in both past and recent years, showing impaired reproduction, growth, and health as well as neurological alterations that could result in behavioral abnormalities in species exposed to elevated, but still ecologically relevant, Hg levels (Scheuhammer et al. 2007; Adams et al. 2010; Cambier et al. 2012; Batchelar et al. 2013a, b; Gehringer et al. 2013; Ho et al. 2013; Rhea et al. 2013; Stefansson et al. 2013). Given that Hg concentrations in elasmobranchs generally rival, if not exceed, those observed in even the largest bony fish species, it is important to determine if similar effects or physiological responses of any kind occur in these fish as a result of Hg exposure.
Previous studies have shown that metallothioneins (MTs), a group of metal-binding proteins, can serve as useful indicators for detecting physiological responses to metal exposure in fish and other aquatic organisms (see reviews by Nordberg and Nordberg 2009; Shariati and Shariati 2011). MTs are low molecular weight (6–7 kDa), cysteine-rich, intracellular proteins that are present in many invertebrate and vertebrate species and appear to play roles in the homeostasis and detoxification of metal ions by binding to and sequestering several divalent transition metals such as copper (Cu), zinc (Zn), cadmium (Cd), and Hg. MTs also appear to function in other important physiological processes including the scavenging of reactive oxygen species and the regulation of cell proliferation and apoptosis (Chiaverini and De Ley 2010). In general, MT expression increases in response to elevated metal exposure, a property that has led to its widespread use as a biomarker for detecting toxic metal effects in both human and wildlife populations. Since Hg has been shown to induce MT gene transcription and protein synthesis in various fish species (e.g., spotted scat Scatophagus argus, Sinaie et al. 2010; barbel Barbus graellsii, Quirós et al. 2007), it is a potentially useful biomarker for exploring whether ecologically relevant levels of Hg uptake in sharks are associated with physiological alterations. A few laboratory-based studies have confirmed that MT is present in elasmobranchs and can be induced by exposure to some toxic metals in certain species (Hidalgo et al. 1985; Hidalgo and Flos 1986a, b; Betka and Callard 1999; Cho et al. 2005; De Boeck et al. 2010); however, they did not focus on Hg and MT has not been extensively put to use in field ecotoxicology studies on elasmobranchs.
Therefore, the goal of this study was to evaluate the use of MT as a biomarker for detecting physiological responses to toxic metal exposure in sharks in a field setting. In particular, this study examined the reliability of using MT as an indicator of Hg exposure and accumulation in the bonnethead, Sphyrna tiburo. The bonnethead was selected as the target species for this study based on the available evidence for high Hg uptake in this species (Adams and McMichael 1999; Adams et al. 2003), as well as the extensive body of knowledge on the biology of this shark from the areas sampled (Carlson and Parsons 1997; Cortes et al. 1996; Lombardi-Carlson et al. 2003). The suitability of MT as a biomarker for Hg exposure was assessed by determining if muscle or liver MT levels were correlated with S. tiburo muscle Hg concentrations. Hg and MT levels were also compared between populations of S. tiburo from three estuaries on Florida’s Gulf coast. It was hypothesized that based on the evidence for MT induction in response to toxic metal exposure in sharks and other fish, MT levels would increase proportionally along with increased Hg uptake. Furthermore, we anticipated that S. tiburo would exhibit a site-associated difference in MT levels with bonnetheads from more Hg-contaminated sites having higher MT levels than their counterparts from less Hg-contaminated locations.
Methods
Animal collection
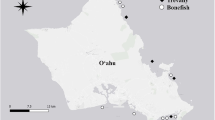
Bonnetheads (n = 50) were collected using set gill nets between 1998 and 2001 from three Florida Gulf coast locations (Fig. 1): Anclote Key (n = 15), Florida Bay (n = 20), and Apalachicola Bay (n = 15). Sharks were weighed to the nearest 0.1 kg, measured in total length (i.e., measuring from the tip of the snout to the tip of the upper lobe of the caudal fin in a natural position; TL) to the nearest 1.0 cm, and examined in order to determine sex.
Biological sample collection
Following capture, sharks were rinsed with ambient seawater and packed in ice until arrival at the laboratory. Once at the laboratory, each shark was rinsed with running local tap water and a 1-g sub-sample of muscle was removed from the lateral musculature just below the dorsal fin and placed in a cryovial, which was subsequently placed in liquid nitrogen in order to prevent moisture loss during the freezing process. Samples were stored at −80 °C until time of analysis in 2009. Sub-samples of liver were also collected (~1 g) from the ventral edge of the right lobe of the liver, placed in cryovials, frozen with liquid nitrogen, and stored at −80 °C.
Mercury analysis
Muscle samples were dried for 48 h at 60 °C in an oven using aluminum weight boats to minimize cross-contamination, homogenized using a glass mortar and pestle, and stored at 4 °C until analysis. Samples were weighed before and after drying in order to monitor water content and reduction throughout the process. Percent moisture was determined using the formula,
where W d = weight of dry sample (g) and W w = weight of wet sample (g).
Total Hg (THg) was measured in μg/g dry weight (d.w.) in S. tiburo muscle samples by the Florida Fish and Wildlife Conservation Commission’s Indian River Field Laboratory (Melbourne, FL, USA) using thermal decomposition (combustion), amalgamation, and atomic absorption spectrometry [EPA Method 7473] (EPA 1998). The analysis was completed with a calibrated DMA-80 Direct Mercury Analyzer (Milestone Inc., Shelton, Connecticut) according to Tremain and Adams (2012). Quality control procedures included analysis of laboratory method blanks, duplicate or triplicate tissue samples, and certified reference material (TORT-2 and DOLT-4 obtained from the National Research Council of Canada) for each group of 10 samples analyzed. In addition, a duplicate matrix spike was completed during the analytical run.
THg was presented in μg/g d.w. in all figures. However, because the majority of previous studies on THg in elasmobranchs have reported concentrations in μg/g wet weight (w.w.) (Gelsleichter and Walker 2010), d.w. measurements have been converted to their w.w. equivalents and are reported for comparative purposes.
Metallothionein analysis
Muscle and liver samples were homogenized in 3 volumes of buffer (100 mM Tris–HCl with 5 mM β-mercaptoethanol, pH 8.1, Hylland et al. 1995) using the FastPrep-24 bead homogenizer (MP Biomedicals, Inc., Santa Ana, CA, USA). Homogenates were centrifuged at 18,000g at 4 °C for 1 h, and the resulting supernatant was stored at −80 °C in three 200-μL aliquots until the time of analysis. A standard Bradford protein assay, using 1/50 sample dilutions, was used to determine protein concentrations (mg/mL) of each sample (Bio-Rad Laboratories, Hercules, CA, USA). Several samples were centrifuged a second time at 100,000g to attempt to reduce the occurrence of immunoreactive high molecular weight proteins, which have been observed in earlier studies on elasmobranchs in which they were assumed to represent MT associated with membrane components (Hylland et al. 1995) or oligomeric forms (Hidalgo et al. 1988).
Proteins (90 μg/sample/well) were separated via SDS-PAGE gel electrophoresis under denaturing and reducing conditions using 15 % polyacrylamide gels and the Laemmli buffer system. Gels used for the visualization of protein content were fixed in a standard fixation solution (40 % methanol, 10 % glacial acetic acid) and stained with a 0.03 % Coomassie blue in fixation solution.
For immunoblotting, proteins were electrotransferred to PVDF membranes (Bio-Rad Laboratories), which were afterward blocked in 10 % non-fat dried milk (NFDM) in Tris-buffered saline (TBS) overnight at 4ºC to prevent non-specific binding. Immunoreactive MT was detected using a polyclonal rabbit anti-cod MT antibody (KH-1, Cayman Chemical Co., Ann Arbor, MI, USA) diluted 1:500 in 1 % NFDM in TBS containing 0.05 % Tween 20 via overnight incubation at 4 °C. This antibody has been previously shown to cross-react with putative MT from several elasmobranchs (Hylland et al. 1995), including S. tiburo in preliminary studies (Gelsleichter, unpublished data). Goat anti-rabbit IgG (whole molecule)-alkaline phosphatase conjugate (Sigma-Aldrich Corporation, St. Louis, MO, USA) was used as secondary antibody (1:5,000 in 1 % NFDM in TBS containing 0.05 % Tween-20, 1 h incubation at room temperature), and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt/nitro-blue tetrazolium chloride (BCIP/NBT) (Vector Laboratories, Burlingame, CA, USA) was used as chromogen. Membranes were washed five times for 5 min each in TBS containing 0.05 % Tween 20 between each incubation. Following color reaction, membranes were rinsed in deionized water and air-dried. Band intensity for each sample was acquired and calculated using the Gel Logic Imaging System and Kodak Molecular Imaging Software (Carestream Heath, Inc., Rochester, NY, USA).
Statistical analysis
Correlations between THg and TL and MT band intensity in liver (MTliver) and muscle (MTmuscle) were analyzed using Spearman’s rank order correlation. Pearson’s product–moment correlation was used to determine if MTliver and MTmuscle were significantly correlated. Differences in mean THg levels associated with animal gender and location of capture were analyzed using Student’s t test and one-way ANOVA followed by Tukey’s post hoc test, respectively. Last, differences in MTliver and MTmuscle associated with site of capture were also analyzed via one-way ANOVA followed by Tukey’s post hoc test.
Results
Total Hg analysis
Specimens ranged in TL from 56 to 103 cm (range of free-swimming S. tiburo in these locations was 34–119 cm based on an n = 423, Manire, unpublished data). THg concentrations in S. tiburo muscle ranged from 0.86 to 6.58 μg/g d.w. (Mean ± SD = 3.00 ± 1.82 μg/g d.w.), which corresponded to w.w. values of 0.22–1.78 μg/g (Mean ± SD = 0.79 ± 0.49 μg/g w.w.) (Table 1). Of all samples analyzed, 86 % were found to have THg concentrations above 0.3 ppm, the US. EPA’s fish tissue residue criterion (US. EPA, 2001). All results of quality assurance procedures were found to be within EPA standards.
There were no significant differences in mean THg levels associated with animal gender (Student’s t test, p > 0.05). Because of this, sexes were combined for comparisons of THg levels between sites, but no significant differences were observed (one-way ANOVA, p = 0.603) (Table 1). A significant positive correlation between THg and TL was observed (Spearman’s rank order correlation, ρ = 0.628, p < 0.001) (Fig. 2).
Total mercury concentrations (THg; dry weight mg/kg) measured in Sphyrna tiburo muscle compared with the total length (TL) for each specimen (n = 50). A significant positive correlation between length and THg was observed (Spearman’s rank order correlation; ρ = 0.628, p < 0.001). AK Anclote Key, FB Florida Bay, AB Apalachicola Bay
Metallothionein analysis
Western blot analysis of S. tiburo muscle and liver consistently resulted in the observation of two immunoreactive protein bands, corresponding to molecular weights of ~34–39 and ~14 kDa (Fig. 3). The latter of these two bands was equivalent to the expected molecular weight of MT, as reported in earlier studies (~12–14 kDa, Hidalgo and Flos 1986b; Hylland et al. 1995). Despite multiple attempts at separating this band from the larger band using ultracentrifugation as successfully employed by Hylland et al. (1995), the high molecular weight band remained present. Although there is strong evidence to suggest that the high molecular weight band represented polymerized forms of MT based on previous studies (e.g., Hidalgo and Flos, 1986b; Hylland et al. 1995), optical intensity of only the low molecular weight band was analyzed for comparative purposes.
Western blot analysis of Sphyrna tiburo metallothionein (MT) in a muscle and b liver tissue. Arrows indicate size of molecular weight markers in kDa. The low molecular weight bands (black arrowhead) represent a protein of the expected size for MT, whereas high molecular weight bands (open arrowhead) likely represent MT associated with membrane components or oligomeric forms. Due to the inability to reduce the high molecular weight bands by ultracentrifugation, only the lower weight bands were used for MT analysis
Relative levels of MT in muscle and liver were not significantly correlated (Pearson’s product–moment correlation coefficient, r = −0.119, p = 0.438). There was no significant correlation between THg and either MTmuscle (Spearman’s rank order correlation, ρ = −0.18, p = 0.899) or MTliver (Spearman’s rank order correlation, ρ = 0.055, p = 0.721) (Fig. 4). There were no statistically significant differences in MTliver associated with site of collection (one-way ANOVA, p = 0.256), but MTmuscle varied significantly between S. tiburo collected from Anclote Key and Apalachicola Bay (One-way ANOVA with Tukey’s post hoc test, p < 0.05).
Levels of Sphyrna tiburo metallothionein (MT) in a muscle (n = 50) and b liver (n = 45) compared with total mercury (THg; μg/g dry weight) measured in muscle. Relative MT levels were calculated by subtracting the mean background intensity from the mean intensity of 14-kDa immunoreactive bands observed using Western blot. No significant relationship was found between THg and relative MT levels in muscle (Spearman’s rank order correlation; ρ = − 0.018, p = 0.899) or liver (Spearman’s rank order correlation; ρ = 0.055, p = 0.721)
Discussion
Muscle THg concentrations measured in S. tiburo examined in this study (0.22–1.78 μg/g w.w. with a mean of 0.79 ± 0.49 μg/g w.w.) were consistent with those observed in Florida bonnetheads in prior investigations. Adams and McMichael (1999) reported mean muscle THg concentrations of 0.50 ± 0.36 μg/g w.w. in S. tiburo from nearshore areas on the southeast Florida coast. More recently, Adams et al. (2003) reported muscle THg concentrations ranging from 0.03 to 1.60 μg/g w.w. in bonnetheads collected throughout Florida with site-specific levels in sharks from the Florida Bay (0.28–1.60 μg/g w.w.) and Tampa Bay (0.03–1.60 μg/g w.w.) regions that were similar to those observed in individuals collected from the same locations in the current study (0.25–1.78 and 0.31–1.53 μg/g w.w. for S. tiburo from Florida Bay and Anclote Key, respectively). Like Adams and McMichael (1999) and Adams et al. (2003), we also found a significant positive relationship between muscle THg concentrations and shark length, demonstrating that bioaccumulation of Hg occurs in this species. Last, like both previous studies, we observed that a sizeable proportion (86 %) of our samples had muscle THg levels that exceeded the federal fish tissue residue criterion in the USA. The occurrence of such high levels of Hg in this and other species of sharks, of course, formed the basis for our investigation on the physiological responses to Hg exposure in this group.
Despite the high levels of THg commonly observed in Florida S. tiburo, there was no evidence for elevated quantities of MT in such individuals and, in general, muscle THg concentrations and MT levels in muscle and liver were not positively correlated. Therefore, these results suggest that MTs is not likely to be a useful biomarker for Hg exposure in this species and perhaps other sharks. While these results seemed surprising based on longstanding and widespread use of MT as a specific indicator of metal exposure in aquatic organisms, they agree with a growing body of evidence that challenges its suitability as a biomarker for Hg exposure due to lack of positive correlations between endogenous Hg levels and MT content in several species (Rotchell et al. 2001; Monserrat et al. 2007; Miero et al. 2011; Gehringer et al. 2013; Sevcikova et al. 2013). As suggested in these studies, this may be due to the fact that MeHg, the most ecologically relevant and abundant Hg species found in wildlife, appears to be less capable of inducing MT than inorganic forms of Hg. It is also possible that differences in exposure to other metals or factors such as animal gender, stage of maturity, time of capture, and environmental conditions (e.g., salinity) at the time of capture influence MT production in ways that obscure possible relationships between the levels of this protein and Hg exposure. It is also feasible to consider that, while occasionally high, Hg levels in S. tiburo and some other elasmobranchs may still fall below the physiological threshold for inducing MT in these fishes. Last, it is possible that the semi-quantitative nature of MT analysis used in this study was not sensitive enough to detect fine differences in MT expression that may have been associated with THg.
The premise that MT content may not accurately reflect a MeHg-dominated Hg burden in some species because MeHg is generally less capable of inducing MT expression than inorganic forms of Hg is supported by previous research. For example, MeHg has been shown to be ineffective or less effective than inorganic Hg at inducing MT expression in both in vitro (e.g., cultured mouse neurons and astrocytes, Kramer et al. 1996a, b) and in vivo (e.g., mouse brain, liver, and kidney, Saijoh et al. 1989; Yasutake et al. 1998; Yasutake and Nakamura 2011; zebrafish (Danio rerio) liver, skeletal muscle, and brain, Gonzalez et al. 2005) animal models. Furthermore, in the few cases in which MeHg has been shown to successfully induce MT expression in vertebrate tissues (e.g., mouse brain, liver, kidney, and testis, Dufresne and Cyr 1999; Yasutake and Nakamura 2011), this effect has been largely attributed to increased presence of inorganic mercury following demethylation of MeHg (i.e., which occurs in mammals and birds, but is not thought to occur in fish) or increased production of reactive oxygen species (Aschner et al. 2006). This may explain species-specific differences in the relationship between MT content and THg concentrations in various vertebrates as both the contribution of MeHg to total Hg burden and the ability to demethylate MeHg vary considerably among taxa. Therefore, it is possible that MT is a suitable biomarker for Hg in cases when inorganic Hg represents a large contribution to total Hg uptake in a species and/or when the species is capable of demethylating MeHg and that prior evidence for positive relationships between MT content and THg concentrations (e.g., Sinaie et al. 2010) reflects such instances and/or perhaps evidence for oxidative stress. This could explain why Hg in water, but not sediment was positively correlated with hepatic MT concentrations in golden gray mullet (Liza aurata) from a metal-contaminated site on the Portugal coast, as inorganic forms of Hg would clearly represent a greater contribution to water-borne levels of this metal compared with those in sediment (Oliveira et al. 2010). This could also explain the positive association between THg and MT concentrations in L. aurata but lack of such a relationship in European sea bass (Dicentrarchus labrax) from the same area on the northwest Portugal coast, as inorganic Hg is believed to represent a greater contribution to THg in the pelagic detritivore L. aurata in comparison to the demersal benthivore D. labrax (Miero et al. 2011). However, data on the proportion that MeHg comprises of THg in S. tiburo is needed to confirm or refute this hypothesis.
The possibility that MT content may reflect exposure to other metals to a greater extent than Hg is also well supported by previous research. Numerous studies have demonstrated that metals vary in their effectiveness at inducing MT expression in many vertebrates, including sharks. For example, Cho et al. (2005) found that zinc (Zn) was more effective than copper (Cu) and cadmium (Cd) at inducing hepatic and renal MT expression in the cloudy catshark, Scyliorhinus torazame, whereas De Boeck et al. (2010) observed positive MT induction in gill and liver of spotted dogfish, Scyliorhinus canicula, in response to exposure with Cu, but not Cd, lead (Pb), or silver (Ag). Furthermore, positive relationships between concentrations of some of these metals (i.e., Cd, Cu, Zn) and MT levels in fish tissues have been observed more often than those between MT and THg. As a recent example, Gehringer et al. (2013) observed significant positive correlations between hepatic MT expression and muscle concentrations of Cu, Zn, manganese (Mn), aluminum (Al), and nickel (Ni) in largemouth bass, Micropterus salmoides, but only a weak negative relationship between MT content and THg. However, it is also possible that physiological differences between individuals and/or environmental variables other than metal uptake may influence MT production to a greater extent than Hg, as they have been hypothesized to obscure relationships between MT content and even some of the most effective MT-inducing metals in prior studies (Creti et al. 2010). For example, previous studies have reported that factors such as nutritional status, temperature, salinity, dissolved oxygen, metabolic rate, stress, immune function, gender, stage of maturity, and reproductive stage can often alter MT expression in some species, resulting in high natural variability in levels of these proteins (Baer and Thomas 1990; Monserrat et al. 2007; Dragun et al. 2009).
Given that S. tiburo is a relatively small shark species with a low trophic position, compared with many other sharks (Bethea et al. 2007), it is logical to also consider that Hg accumulation in this species may fall below the levels necessary to induce MT expression in elasmobranchs and that larger, higher trophic-level sharks may be better subjects for this research. However, while it is true that some higher trophic-level shark species have been shown to accumulate fivefold to 10-fold higher levels of Hg than S. tiburo (e.g., smooth hammerhead, Sphyrna zygaena, gulper shark, Centrophorus granulosus, kitefin shark, Dalatias licha, Storelli et al. 2002, 2003), it is also important to note that the range in THg levels observed in S. tiburo in both this study and prior investigations overlap with those observed in many other shark species in which Hg accumulation has been surveyed including a number of larger species (Gelsleichter and Walker 2010). Therefore, if induction of MT is unlikely to occur in response to typical levels of Hg accumulation experienced by most sharks, it holds minimal value as a biomarker for Hg effects in this group.
As a final point, it is possible that the use of a semi-quantitative approach such as Western blot rather than more quantitative methods more commonly used in MT studies such as electrochemical techniques (e.g., Dragun et al. 2009; Oliveira et al. 2010; Sevcikova et al. 2013) or enzyme-linked immunosorbent assays (ELISA) (e.g., Sinaie et al. 2010) may have limited our ability to detect subtle differences in MT content that could have been correlated with THg concentrations. This was an initially unplanned component of the present study made necessary by our inability to eliminate high molecular weight immunoreactive bands (i.e., presumed to be polymers), which could have resulted in overestimation of MT content using ELISA (Shariati and Shariati 2011). However, as reported in the surprisingly large number of reviews describing methodology for MT determination (Dabrio et al. 2002; Nordberg and Nordberg 2009; Ryvolova et al. 2011; Shariati and Shariati 2011), immunological approaches including Western blot are generally considered to provide the highest sensitivity and specificity for detecting MTs, provided that antibody probes successfully cross-react with target molecules. Furthermore, prior studies have successfully used comparable approaches to detect differences in MT content associated with factors that would induce its expression. For example, Ronco et al. (2005) used Western blot to demonstrate that MT levels are increased in human placenta from pregnant female smokers compared with that of non-smokers.
In conclusion, while Hg does accumulate in S. tiburo muscle to levels that exceed the maximum recommended limits for monthly human consumption, these levels do not appear to correlate with MT levels in either muscle or liver. Therefore, although other explanations for these findings have been considered, the results of this study suggest that there may be limitations for the use of MT as a biomarker for Hg exposure and effects in this species and perhaps other elasmobranchs. As mentioned, this conclusion is consistent with a sizeable and growing body of literature that question the use of MT as a specific biomarker for Hg exposure in vertebrates. Notwithstanding these results, future studies should continue to explore the relationship between Hg exposure and MT expression in sharks, particularly in species that have been shown to accumulate sizeable quantities of inorganic as well as organic forms of Hg. Future work should also focus on developing alternative biomarkers for detecting Hg effects in sharks, such as oxidative stress indicators, some of which have been shown to be potentially useful in recent studies (i.e., protein carbonyl concentrations, Barrera-García et al. 2012).
References
Adams DH, McMichael RH Jr (1999) Mercury levels in four species of sharks from the Atlantic coast of Florida. Fish Bull 379:372–379
Adams DH, McMichael Jr RH, Henderson GE (2003) Mercury levels in marine and estuarine fishes of Florida 1989–2001. Florida Fish and Wildlife Conservation Commission FMRI technical report TR-9
Adams DH, Sonne C, Basu N, Dietz R, Nam D-H, Leifsson PS, Jensen AL (2010) Mercury contamination in spotted seatrout, Cynoscion nebulosus: an assessment of liver, kidney, blood, and nervous system health. Sci Total Environ 408:5808–5816
Aschner M, Syversen T, Souza DO, Rocha JB (2006) Metallothioneins: mercury species-specific induction and their potential role in attenuating neurotoxicity. Exp Biol 231:1468–1473
Baer KN, Thomas P (1990) Influence of capture stress, salinity and reproductive status on zinc associated with metallothionein-like proteins in the livers of three marine teleost species. Mar Environ Res 29:277–287
Barrera-García A, O’Hara T, Galván-Magaña F, Méndez-Rodríguez LC, Castellini JM, Zenteno-Savín T (2012) Oxidative stress indicators and trace elements in the blue shark (Prionace glauca) off the east coast of the Mexican Pacific Ocean. Comp Biochem Physiol C: Toxicol Pharmacol 156:59–66. doi:10.1016/j.cbpc.2012.04.003
Batchelar KL, Kidd KA, Munkittrick KR, Drevnick PE, Burgess NM (2013a) Reproductive health of yellow perch (Perca flavescens) from a biological mercury hotspot in Nova Scotia, Canada. Sci Total Environ 454–455:319–327. doi:10.1016/j.scitotenv.2013.03.020
Batchelar KL, Kidd KA, Drevnick PE, Munkittrick KR, Burgess NM, Roberts AP, Smith JD (2013b) Evidence of impaired health in yellow perch (Perca flavescens) from a biological mercury hotspot in northeastern North America. Environ Toxicol Chem 32:627–637. doi:10.1002/etc.2099
Bethea DM, Hale L, Carlson JK, Cortés E, Manire CA, Gelsleichter J (2007) Latitudinal variation in the diet and daily ration of the bonnethead shark, Sphyrna tiburo, from the eastern Gulf of Mexico. Mar Biol 152:1009–1020
Betka M, Callard GV (1999) Stage-dependent accumulation of cadmium and induction of metallothionein-like binding activity in the testis of the Dogfish shark, Squalus acanthias. Biol Reprod 60:14–22
Bosch AC, Sigge GO, Kerwath SE, Cawthorn DM, Hoffman LC (2013) The effects of gender, size and life-cycle stage on the chemical composition of smoothhound shark (Mustelus mustelus) meat. J Sci Food Agric 93:2384–2392. doi:10.1002/jsfa.6100
Cambier S, Gonzalez P, Mesmer-Dudons N, Brèthes D, Fujimura M, Bourdineaud JP (2012) Effects of dietary methylmercury on the zebrafish brain: histological, mitochondrial, and gene transcription analyses. Biometals 25:165–180. doi:10.1007/s10534-011-9494-6
Carlson JK, Parsons GR (1997) Age and growth of the bonnethead shark, Sphyrna tiburo, from northwest Florida, with comments on clinal variation. Environ Biol Fish 50:331–341
Chen C, Amirbahman A, Fisher N, Harding G, Lamborg C, Nacci D, Taylor D (2008) Methylmercury in marine ecosystems: spatial patterns and processes of production, bioaccumulation, and biomagnification. EcoHealth 5:399–408. doi:10.1007/s10393-008-0201-1
Chiaverini N, De Ley M (2010) Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic Res 44:605–613
Cho YS, Choi BN, Ha E-M, Kim KH, Kim SK, Kim DS, Nam YK (2005) Shark (Scyliorhinus torazame) metallothionein: cDNA cloning, genomic sequence, and expression analysis. Mar Biotech 7:350–362
Cortes E, Manire CA, Hueter RE (1996) Diet, feeding habits, and the diel feeding chronology of the bonnethead shark, Sphyrna tiburo, in southwest Florida. Bull Mar Sci 28:353–367
Creti P, Trinchella F, Scudiero R (2010) Heavy metal bioaccumulation and metallothionein content in tissues of the sea bream Sparus aurata from three different fish farming systems. Environ Monit Assess 165:321–329
Dabrio M, Rodríguez AR, Bordin G, Bebianno MJ, De Ley M, Sestáková I, Vasák M, Nordberg M (2002) Recent developments in quantification methods for metallothionein. J Inorg Biochem 88:123–134
De Boeck G, Eyckmans M, Lardon I, Bobbaers R, Sinha AK, Blust (2010) Metal accumulation and metallothionein induction in the spotted dogfish Scyliorhinus canicula. Comp Biochem Physiol A 155:503–508. doi:10.1016/j.cbpa.2009.12.014
Dragun Z, Podrug M, Raspor B (2009) The assessment of natural causes of metallothionein variability in the gills of European chub (Squalius cephalus L.). Comp Biochem Physiol Toxicol Pharmacol 150:209–217. doi:10.1016/j.cbpc.2009.04.011
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983. doi:10.1021/es305071v
Dufresne J, Cyr DG (1999) Effects of short-term methylmercury exposure on metallothionein I, II and III mRNA levels in the testis and epididymis of the rat. J Androl 20:769–778
Escobar-Sánchez O, Galván-Magaña F, Rosíles-Martínez R (2010) Mercury and selenium bioaccumulation in the smooth hammerhead shark, Sphyrna zygaena Linnaeus, from the Mexican Pacific Ocean. Bull Environ Contam Toxicol 84:488–491. doi:10.1007/s00128-010-9966-3
Escobar-Sánchez O, Galván-Magaña F, Rosíles-Martínez R (2011) Biomagnification of mercury and selenium in blue shark Prionace glauca from the Pacific Ocean off Mexico. Biol Trace Elem Res 144:550–559. doi:10.1007/s12011-011-9040-y
Gehringer DB, Finkelstein ME, Coale KH, Stephenson M, Geller JB (2013) Assessing mercury exposure and biomarkers in largemouth bass (Micropterus salmoides) from a contaminated river system in California. Arch Environ Contam Toxicol 64:484–493. doi:10.1007/s00244-012-9838-4
Gelsleichter JJ, Walker CJ (2010) Pollutant exposure and effects on sharks and their relatives. In: Carrier JC, Musick JA, Heithaus MR (eds) Sharks and their relatives II: Biodiversity, adaptive physiology, and conservation. CRC Press, Boca Raton, pp 491–537
Gonzalez P, Dominique Y, Massabuau JC, Boudou A, Bourdineaud JP (2005) Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio). Environ Sci Technol 39:3972–3980
Hidalgo J, Flos R (1986a) Dogfish metallothionein I. Purification and characterization and comparison with rat metallothionein. Comp Biochem Physiol C 83:99–103
Hidalgo J, Flos R (1986b) Dogfish metallothionein II. Electrophoretic studies and comparison with rat metallothionein. Comp Biochem Physiol C 83:105–109
Hidalgo J, Tort L, Flos R (1985) Cd-, Zn-, Cu-binding protein in the elasmobranch Scyliorhinus canicula. Comp Biochem Physiol C 81:159–165
Hidalgo J, Bernues J, Thomas DG, Garvey JS (1988) Effect of 2-mercaptoethanol on the electrophoretic behavior of rat and dogfish metallothionein and chromatographic evidence of a naturally occurring metallothionein polymerization. Comp Biochem Physiol C 89:191–196
Ho NY, Yang L, Legradi J, Armant O, Takamiya M, Rastegar S, Strähle U (2013) Gene responses in the central nervous system of zebrafish embryos exposed to the neurotoxicant methyl mercury. Environ Sci Technol 47:3316–3325. doi:10.1021/es3050967
Hurtado-Banda R, Gomez-Alvarez A, Márquez-Farías JF, Cordoba-Figueroa M, Navarro-García G, Medina-Juárez LA (2012) Total mercury in liver and muscle tissue of two coastal sharks from the northwest of Mexico. Bull Environ Contam Toxicol 88:971–975. doi:10.1007/s00128-012-0623-x
Hylland K, Haux C, Hogstrand C (1995) Immunological characterization of metallothionein in marine and freshwater fish. Mar Environ Res 39:111–115
Kim MK, Zoh KD (2012) Fate and transport of mercury in environmental media and human exposure. J Prev Med Public Health 45:335–343. doi:10.3961/jpmph.2012.45.6.335
Kramer KK, Liu J, Choudhuri S, Klaassen CD (1996a) Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicol Appl Pharmacol 136:94–100
Kramer KK, Zoelle JT, Klaassen CD (1996b) Induction of metallothionein mRNA and protein in primary murine neuron cultures. Toxicol Appl Pharmacol 141(1):1–7
Lombardi-Carlson LA, Cortes E, Parsons GR, Manire CA (2003) Latitudinal variation in life-history traits of bonnethead sharks, Sphyrna tiburo, (Carcharhiniformes: Sphyrnidae) from the eastern Gulf of Mexico. Mar Freshw Res 54:875–883
Maz-Courrau A, López-Vera C, Galván-Magaña F, Escobar-Sánchez O, Rosíles-Martínez R, Sanjuán-Muñoz A (2012) Bioaccumulation and biomagnification of total mercury in four exploited shark species in the Baja California Peninsula, Mexico. Bull Environ Contam Toxicol 88:129–134. doi:10.1007/s00128-011-0499-1
Miero CL, Bervoets L, Joosen S, Blust R, Duarte AC, Pereira ME, Pacheco M (2011) Metallothioneins failed to reflect mercury external levels of exposure and bioaccumulation in marine fish—considerations on tissue and species specific responses. Chemosphere 85:114–121. doi:10.1016/j.chemosphere.2011.05.034
Monserrat JM, Martínez PE, Geracitano LA, Amado LL, Martins CM, Pinho GL, Chaves IS, Ferreira-Cravo M, Ventura-Lima J, Bianchini A (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol C: Toxicol Pharmacol 146:221–234
Nam DH, Adams DH, Reyier EA, Basu N (2011) Mercury and selenium levels in lemon sharks (Negaprion brevirostris) in relation to a harmful red tide event. Environ Monit Assess 176:549–559. doi:10.1007/s10661-010-1603-4
Nordberg N, Nordberg GF (2009) Metallothioneins: historical development and overview. Met Ions Life Sci 5:1–29
Oliveira M, Ahmad I, Maria VL, Serafim A, Bebianno MJ, Pacheco M, Santos MA (2010) Hepatic metallothionein concentrations in the golden grey mullet (Liza aurata)—relationship with environmental metal concentrations in a metal-contaminated coastal system in Portugal. Mar Environ Res 69:227–233. doi:10.1016/j.marenvres.2009.10.012
Pethybridge H, Cossa D, Butler EC (2010) Mercury in 16 demersal sharks from southeast Australia: biotic and abiotic sources of variation and consumer health implications. Mar Environ Res 69:18–26. doi:10.1016/j.marenvres.2009.07.006
Quirós L, Piña B, Solé M, Blasco J, López MA, Riva MC, Barceló D, Raldúa D (2007) Environmental monitoring by gene expression biomarkers in Barbus graellsii: laboratory and field studies. Chemosphere 67:1144–1154
Rhea DT, Farag AM, Harper DD, McConnell E, Brumbaugh WG (2013) Mercury and selenium concentrations in biofilm, macroinvertebrates, and fish collected in the Yankee Fork of the Salmon River, Idaho, USA, and their potential effects on fish health. Arch Environ Contam Toxicol 64:130–139. doi:10.1007/s00244-012-9816-x
Ronco AM, Arguello G, Suazo M, Llanos MN (2005) Increased levels of metallothionein in placenta of smokers. Toxicology 208:133–139
Rotchell JM, Clarke KR, Newton LC, Bird DJ (2001) Hepatic metallothionein as a biomaker for metal contamination: age effects and seasonal variation in European flounders (Pleuronectes flesus) from the Severn Estuary and Bristol Channel. Mar Environ Res 52:151–171
Ryvolova M, Krizkova S, Vojtech A, Beklova M, Trnkova L, Hubalek J, Kizek R (2011) Analytical methods for metallothionein detection. Curr Anal Chem 7:243–261
Saijoh K, Kuno T, Shuntoh H, Tanaka C, Sumino K (1989) Molecular cloning of cDNA for rat brain metallothionein-II and regulation of its gene expression. Pharmacol Toxicol 64:464–468
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12–18
Sevcikova M, Modra H, Kruzikova K, Zitka O, Hynek D, Adam V, Celechovska O, Kizek R, Svobodova Z (2013) Effect of metals on metallothionein content in fish from Skalka and Želivka reservoirs. Int J Electrochem Sci 8:1650–1663
Shariati F, Shariati S (2011) Review on methods for determination of metallothioneins in aquatic organisms. Biol Trace Elem Res 141:340–366. doi:10.1007/s12011-010-8740-z
Sinaie M, Bastami KD, Ghorbanpour M, Najafzadeh H, Shekari M, Haghparast S (2010) Metallothionein biosynthesis as a detoxification mechanism in mercury exposure in fish, spotted scat (Scatophagus argus). Fish Physiol Biochem 36:1235–1242. doi:10.1007/s10695-010-9403-x
Stefansson ES, Heyes A, Rowe CL (2013) Accumulation of dietary methylmercury and effects on growth and survival in two estuarine forage fish: Cyprinodon variegatus and Menidia beryllina. Environ Toxicol Chem 32:848–856. doi:10.1002/etc.2130
Storelli MM, Giacominelli-Stuffler R, Marcotrigiano G (2002) Mercury accumulation and speciation in muscle tissue of different species of sharks from Mediterranean Sea, Italy. Bull Environ Contam Toxicol 68:201–210
Storelli MM, Ceci E, Storelli A, Marcotrigiano GO (2003) Polychlorinated biphenyl, heavy metal and methylmercury residues in hammerhead sharks: contaminant status and assessment. Mar Pollut Bull 46:1035–1039
Tremain DM, Adams DH (2012) Mercury in grouper and sea basses from the Gulf of Mexico: relationships with size, age, and feeding ecology. Trans Am Fish Soc 141:1274–1286
U.S. Environmental Protection Agency (2001) Water Quality Criterion for the Protection of Human Health: Methylmercury. EPA-823-R-01-001. Office of Science and Technology, Office of Water, U.S. Environmental Protection Agency, Washington, DC 20460
Vélez-Alavez M, Labrada-Martagón V, Méndez-Rodriguez LC, Galván-Magaña F, Zenteno-Savín T (2013) Oxidative stress indicators and trace element concentrations in tissues of mako shark (Isurus oxyrinchus). Comp Biochem Physiol A: Mol Integr Physiol 165:508–514. doi:10.1016/j.cbpa.2013.03.006
Yasutake A, Nakamura M (2011) Induction by mercury compounds of metallothioneins in mouse tissues: inorganic mercury accumulation is not a dominant factor for metallothionein induction in the liver. J Toxicol Sci 36:365–372
Yasutake A, Nakano A, Hirayama K (1998) Induction by mercury compounds of brain metallothionein in rats: Hg0 exposure induces long-lived brain metallothionein. Arch Toxicol 72:187–191
Acknowledgments
The authors acknowledge J. Tyminski and additional staff and student interns from Mote Marine Laboratory for their roles in sample collection, which was made possible by Environmental Protection Agency Grant No. R826128-01-0 to C.A. Manire. Although the research described in this article has been funded in part by the United States Environmental Protection Agency, it has not been subjected to the Agency’s required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred. W. Habich and D. Tremain from the Florida Fish and Wildlife Conservation Commission are acknowledged for their roles in mercury analysis. Additional portions of this research were supported by the University of North Florida.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, C.J., Gelsleichter, J., Adams, D.H. et al. Evaluation of the use of metallothionein as a biomarker for detecting physiological responses to mercury exposure in the bonnethead, Sphyrna tiburo . Fish Physiol Biochem 40, 1361–1371 (2014). https://doi.org/10.1007/s10695-014-9930-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9930-y