Abstract
In the present study, the testis histology, gonadosomatic index (GSI), germ cell proliferation and apoptosis, and the plasma 11-ketotestosterone (11-KT) and testosterone (T) levels of male Chalcalburnus tarichi were analyzed. According to the histological examinations of the specimens that were caught between February 2009 and January 2010, three testicular stages were determined. Those stages were as follows: (1) recrudescence or prespawning (July–April), (2) spawning (May–June), and (3) postspawning (July). It was observed that the GSI increased gradually, starting from the recrudescence stage, and it reached peak values at the spawning stage, while the lowest values were in the postspawning. Germ cell proliferation in the testis was detected using a proliferating cell nuclear antigen (PCNA), and germ cell apoptosis was detected by transferase dUTP nick end labeling staining. The germ cell PCNA and apoptosis index values were calculated. It was indicated that germ cell proliferation was observed in all of the testicular stages. The highest germ cell PCNA index (PI) levels were detected in July, August, and September, which then dropped in October and stabilized between February and April. The lowest PI values were detected in the spawning stage (May–June). Germ cell apoptosis was observed in all of the months, and the highest apoptotic index values were detected in August, September, October, May, and June. Plasma 11-KT and T levels were at their highest levels in May and June, and it was detected as stabile in the other months. There was a correlation between GSI, PI, and plasma androgen levels. In conclusion, the present data illustrate testicular development stages for C. tarichi and show changes in the level of GSI and sex steroid biosynthesis through spermatogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spermatogenesis in fish is the well-organized and coordinated process in which diploid spermatogonia proliferate and differentiate to form the mature spermatozoa. During testicular development, 11-ketotestosterone (11-KT) and testosterone (T) are the most important androgens synthesized in the fish testis (Borg 1994). 11-KT regulates the spermatogenesis by stimulating the Sertoli cells and spermatogonial proliferation (Schulz and Miura 2002). It was reported that 11-KT levels increase during spermatogenesis and it reaches its peak value in the spermiation (Billard et al. 1982; Billard 1986). In the male rainbow trout, in the annual reproduction season, when the gonadosomatic index (GSI) reaches its maximum value, the plasma T and 11-KT values were also observed at their highest levels (Hou and Han 2001). In the male European sea bass, Dicentrarchus labrax, T was shown to have a role in sex differentiation and the 11-KT has a role in spermatogenesis and maturation (Papadaki et al. 2005). Moreover, in the African catfish, Clarias gariepinus, spermatogenesis, which is stimulated by the 11-KT, is inhibited by T, and the balanced synthesis of both of these hormones plays a critical role in the testis development (Cavaco et al. 2001).
The proliferation cell nuclear antigen (PCNA), which is also known as cyclin, is a 36-kDa and multifunctional protein, and it is highly expressed in the G1 and S phases of the dividing cells. PCNA is a required component for the DNA replication mechanism and is considered as a marker for the actively replicated cells (Kelman 1997). Analysis of PCNA provided valuable information to evaluate the proliferating cells in the paraffin-embedded sections (Wolf and Dittrich 1992; Casasco et al. 1993).
Apoptosis is the cellular death mechanism that has roles in the fundamental biological steps, such as embryonic morphogenesis, metamorphosis, and remodeling of the hormone-stimulated tissues. Studies conducted with mammals showed that apoptosis takes place during spermatogenesis, and it was shown to have a critical role in keeping the germ cell-to-Sertoli cells ratio constant (Bartke 1995; Rodriguez et al. 1997). Apoptosis also prevents the maturation of the aberrant germ cells and the continuum of the germ cell integrity (Yin et al. 1998). It was shown that germ cell proliferation and apoptosis take place in the same stages as spermatogenesis, and the apoptosis in the seminiferous epithelium is the checkpoint in the germ cell cycle in the rabbit and cat (Blanco-Rodrίguez 2002a, b), and it continues during testis regression in the Brown Hare, Lepus europaeus (Štrbenc et al. 2003). Moreover, it was detected that the changes in the internal and external environment, such as the decrease in the levels of gonadotropin and testosterone (T), short photoperiod, and food restriction, lead to an increase in the testicular apoptosis of seasonally reproducing mammals (Young and Nelson 2001). Similar findings were also reported in the amphibians that apoptosis plays a role in the testicular regression in the newt, Cynops pyrrhogaster, and it has a role in the control of the different spermatogenic stages and differentiation of the germ cysts with the seasonal changes in the frog, Rana catesbeiana (Sasso-Cerri et al. 2006).
Despite a number of studies about the roles of apoptosis and germ cell proliferation in spermatogenesis in mammalian and amphibian species, a few studies were conducted to investigate the role of germ cell apoptosis and proliferation in spermatogenesis in fish. Apoptosis was observed during spermatogenesis where it plays a role in the testis homeostasis in the spotted ray, Torpedo marmorata (Prisco et al. 2003). In the hermaphrodite gilthead seabream, Sparus aurata, the proliferation of spermatogonial stem cells, Sertoli cells, and the primary spermatogonia takes place during the spermatogenesis, in parallel with the GSI, and apoptosis takes place in the spermatogonia during postspawning and germ cell proliferation starts again after this stage (Chavez-Pozo et al. 2005). In the swordfish, Xiphias gladius, 11-KT and T reach their highest levels in the spermatogenic phase in which the germ cell proliferation and apoptosis take place (Corriero et al. 2007).
The objectives of the present study were to investigate apoptosis and germ cell proliferation throughout the annual reproductive cycle in a cyprinid species Chalcalburnus tarichi, with emphasize on their relationships with 11-KT and T. The testicular development was described to characterize the spermatogenic cycle.
The C. tarichi is an endemic cyprinid species inhabiting the Lake Van basin. Its reproduction starts in the middle of April and continues into the middle of the July. This fish migrates into the rivers that are flowing into the lake for spawning (Danulat and Selcuk 1992). The fish become mature at 3 years (Elp and Çetinkaya 2000). Ünal et al. (1999) have already illustrated testicular development, but information about sex steroid levels through spermatogenesis is unknown.
Materials and methods
Fish sampling and tissue collection
Each month, from February 2009 to January 2010, seven male C. tarichi were killed and sampled from Lake Van (43°03′36″E, 38°23′53″N), with the exception of the May and June samples, which were caught in the Karasu river (43°14′22″E, 38°36′39″N). The monthly sampled fish were transported to the laboratory in containers with oxygen support. All of the procedures were approved by the Yüzüncü Yıl University Animal Experiments Ethics Committee for the ethical concerns of the study (decision number: 2012/04/16). The fish taken to the laboratory were anesthetized with MS222 (3-aminobenzoic acid ethyl ester, 200 mg/L), and their fork lengths and total weights were measured. After those measurements, the blood samples were collected from the caudal venous of the fish using heparinized injectors. Testis samples were isolated from each side, and samples were taken from the middle part of those tissues. Those samples were fixed in 10 % neutral buffered formalin solution for 24 h for histological studies. The collected blood samples were centrifuged at 1,500×g for 10 min, and the plasma samples were pipetted into new Eppendorf tubes. The plasma samples were kept at −72 °C until the hormone measurements were taken.
Gonadosomatic index and age determination
The GSI for each fish was calculated as: [(gonad mass (g)/body mass (g))] × 100. The ages of the fish were determined using the opercular bones according to the method described by Lagler et al. (1977).
The ages of the fish used in the study were determined as 4+/6+, the average fork length was 18.2 ± 1.2 cm, and the total weight was 65.2 ± 11.7 g.
Histological studies
Each of the testis samples that were fixed in the 10 % neutral buffered formalin was washed with phosphate-buffered saline, pH: 7.4, dehydrated using a gradual ethanol series, and embedded into paraffin. The 5-μm-thick sections from the paraffinized tissues were placed onto slides covered with egg albumin. Next, the samples were deparaffinized in xylene, and after rehydration with gradual ethanol concentrations, they were stained with hematoxylin–eosin for the examination of the developmental stages of the testis. The slides were examined under a Leica DMI 6000 B model microscope, and their photographs were taken.
Immunohistochemical PCNA labeling
For the immunohistochemical detection of the PCNA, the 5-μm-thick sections taken from the testis samples were transferred to polylysine-coated slides (Menzel Gläser, Germany). After the deparaffinization and rehydration processes of the sections, they were incubated in 3 % H2O2 solution and prepared with methanol for 5 min at room temperature for the inhibition of the tissue endogenous peroxidase activity. The samples were washed with Tris-buffered saline (TBS), pH: 7.4, and treated with target retrieval solution (Dako, Glostrup, Denmark) at 95–99 °C for 30 min for antigen retrieval, and then the samples were washed again with TBS. After washing with TBS three times for 5 min, the samples, which were incubated in horse serum for 20 min at room temperature to prevent nonspecific bindings, were then incubated overnight in a humidified environment with the primary antibody [monoclonal mouse antiproliferating cell nuclear clone PC10 (Dako, Ref. number: M0879)], which was diluted in a 1:300 ratio of TBS containing 1 % bovine serum albumin. After washing the sections with TBS, for the immunohistochemical visualization of the PCNA, a Dako SLAB + System-HRP kit (USA, Cat no: K0679) containing biotinylated antimouse secondary antibody was used. Briefly, the sections were incubated for 30 min at room temperature with the secondary antibody. After rinsing with TBS, the sections were incubated for 30 min with streptavidin-conjugated horseradish peroxidase complex mixture. For the visualization of peroxidase activity, the sections were incubated in a substrate–chromogen solution containing 3,3′-diaminobenzidine until a brown color appeared (for 1–3 min) and washed with double-distilled water. The counterstaining was done using Mayer’s hematoxylin stain. The slides were treated with gradual concentrations of ethanol (95 and 100 %) and covered with cover slips that were sealed using Entellan (Merck, Germany). In the preparation of the negative control slides, instead of primary antibody, TBS or horse serum was added. The slides were examined under a Leica DMI 6000 B model microscope, and their photographs were taken.
The histochemical labeling of the apoptotic cells
For labeling of the apoptotic cells in the sections taken from the testis tissues, the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) technique was used. TUNEL labeling was done with the use of commercial TdT-FragEL™ DNA Fragmentation Detection Kit (Cat. No: QIA33, Calbiochem, Merck). Briefly, after the deparaffinization and dehydration of the sections, which were 5 μm thick, fixed in 10 % neutral buffered formalin, and transferred to poly-lysine-coated slides, they were washed with TBS (20 mM Tris, pH: 7.6; 140 mM NaCl). Other steps of staining were conducted according to the manufacturer’s instructions as described by Kaptaner and Ünal (2011). After completion of the staining, the slides were covered with Entellan and examined under a Leica DMI 6000 B model microscope, and then their photographs were taken.
Plasma 11-ketotestosterone and testosterone measurement
For the determination of the 11-KT and T in the plasma samples, commercially available enzyme-linked immunosorbent assay (ELISA) kits were used (Cayman Chemical Inc., Germany). Hormone measurements and calculations were done according to the manufacturer’s instructions, using 100 μL plasma samples. Before the plasma-level measurement of the steroids was taken, the extraction procedure for 11-KT and T was done. 11-KT extraction was done using acetate/hexane (50:50), and T extraction was done using diethyl ether. After the evaporation of the organic extracts by cold airflow, the resulting pellet was dissolved in the EIA buffer supplied in the kit and they were kept at −72 °C until analyzed. The hormone measurements were taken in duplicate at 412 nm for both the standards and samples using an ELISA plate reader (DAS Plate Reader, Italy).
Cell counting and morphometry
For one section taken from the testis of each fish, 10 optical areas that were randomly chosen and did not intersect one another were visualized by magnification at 400 × , and the cells were counted as TUNEL/PCNA positive or negative. Cell counting was done using the cell counting module of the Image J software (National Institutes of Health, USA, http://rsbweb.nih.gov/ij/). Counting of the apoptotic and proliferating germ cells was done in the undifferentiated germ cells, primary and secondary spermatogonia, and spermatocytes. Sertoli cells and interstitial cells were not included in the counts. Next, for each fish, the apoptotic and proliferative cell index values were measured. The apoptotic index (AI) was calculated by the multiplication of the ratio of the TUNEL-positive cells counted in 10 different optical areas (each was 70,845 μm2) to the total cell number (positive + negative) with 100. The proliferative index (PI), on the other hand, was calculated as the multiplication of the ratio of the PCNA-positive cells counted in 10 different optical areas (each was 70,845 μm2) to the total cell number (positive + negative) with 100.
In the present study, the discrimination of the germ cells was done according to the properties such as the nucleus–cytoplasm diameter and nucleus morphology. The cytoplasm and nucleus diameters of the germ cells (the undifferentiated germ cells, primary spermatogonia, secondary spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids, and free spermatozoa) were measured manually using Image J software. For each germ cell type, at least 50 cells were used in the cytoplasmic and nuclear diameter measurements.
Statistical analyses
The data obtained from the AI, PI, GSI, and plasma steroid levels were analyzed using ANOVA in the SPSS 16.0 for Windows statistical program. Duncan’s multiple-range post hoc tests were used for the determination of the differences between months, and Pearson’s correlation coefficient was used for the evaluation of the correlation between the plasma steroid levels, GSI, AI, and PI. The data obtained from the analyses are represented as mean ± standard error. P < 0.05 was considered to be statistically significant.
Results
Testis morphology and histology
The testis of the C. tarichi was observed as a couple of structures confined into a thin connective tissue (tunica albuginea), placed longitudinally in the dorsal part. The testis tissue is basically composed of the seminiferous tubules, which are separated by a thin connective tissue. The germinal compartment of the testis is composed of Sertoli cells and germ cells. The germ cells in the seminiferous tubules grow in a synchrony of groups called germinal cysts. It was observed that spermatogonia were distributed in germinal compartments throughout the testis in an unrestricted-type organization in the testis of C. tarichi. The presence of the cell types, which are thought to be fibroblasts and Leydig cells, was observed, apart from the blood vessels, in the interstitial compartment of the testis.
In the C. tarichi, the undifferentiated spermatogonia are placed adjacent to the basal membrane of the germinal epithelium as single cells, having a high nucleocytoplasmic ratio and eccentric basophilic nucleolus, with a round nucleus and a light acidophilic cytoplasm. The primary spermatogonia are placed in groups, and the lower nucleus diameter and nucleocytoplasmic ratio are compared to the undifferentiated spermatogonia. The secondary spermatogonia were observed as placed in cysts, with rounded nucleus, and having more heterochromatin when compared to the primary spermatogonia. The nuclei of primary spermatocytes have a smaller diameter when compared to secondary spermatogonia, and the nuclei are rounded and having more dense heterochromatin, and the cells are placed in cysts. Secondary spermatocytes are observed in small numbers, and they have smaller nuclei and a denser heterochromatin structure when compared to primary spermatocytes. Spermatids are smaller than spermatocytes, and they are rather basophilic cells. Spermatozoa have flagella and a rounded head part that can be densely stained with hematoxylin and are observed as the cells that are free in the tubular lumen after maturation by the destruction of the cysts. The cytoplasmic/nuclear diameter of different germ cell types in the C. tarichi is shown in Table 1, and the illustrative figures are shown in Fig. 1.
The seasonal (monthly) changes in the testis were determined by considering the histological criteria and the ratio of the germ cells in the tubules. The differentiation of the stages of the testicular cycle was determined as follows (the illustrative figures of the different stages are shown in Fig. 2).
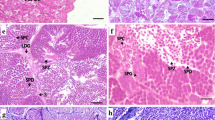
Illustration of the different testicular stages in Chalcalburnus tarichi: A June, when the recrudescence starts; mostly undifferentiated spermatogonia and few spermatogonia are seen. B September, the first primary spermatocytes are observed. C April, last month of recrudescence; presence of mostly primary spermatocytes in the seminiferous epithelium is shown. D May and E June, spawning months; the lumen seminiferous tubules are full of free spermatozoa. F July, postspawning stage; the seminiferous epithelium contains less primary and secondary spermatogonia and more undifferentiated spermatogonia and residual spermatozoa in the seminiferous epithelium (black arrow the undifferentiated spermatogonia, white arrow primary spermatogonia, black arrowhead secondary spermatogonia, white arrowhead primary spermatocyte, sSc secondary spermatocytes, Sd spermatid, Sz spermatozoa, and rSz residual spermatozoa)
-
a.
Recrudescence stage:
This stage lasts for a long time, starting in July and ending in April. The diameter of the seminiferous tubules increases with the progress of the spermatogenesis in the testis. In July and August, mostly undifferentiated spermatogonia and less spermatogonia cysts were observed in the seminiferous tubules (Fig. 2a). In September, high proportion of primary and secondary spermatogonia cysts were determined and primary spermatocytes are formed in the seminiferous tubules (Fig. 2b). In the progress of this stage, it was shown that the quantity of primary spermatocytes in the seminiferous tubules increases and they become dominant. In April, when the last samples of this stage were collected, this observation was more obvious and the dominance of the primary spermatocytes in the seminiferous tubules was seen, and there was no observation of the secondary spermatocyte and spermatid cysts (Fig. 2c).
-
b.
Spawning stage:
In this stage, covering May and June, it was shown that almost all of the seminiferous tubule lumens are full of free spermatozoa. It was observed that there are too few numbers of the undifferentiated spermatogonia and spermatogonia and few numbers of the spermatocytes and spermatid cysts (Fig. 2d, e). The fish in this stage were observed as ready for spawning, and when pressure was applied to the abdominal part, milt of all of the male fish might be stripped.
-
c.
Postspawning stage:
In this stage, which was observed in July, although not in all samples, a large number of the spermatozoa have been released; the tubular diameter is reduced, and only residual spermatozoa are observed in the lumen of seminiferous tubules (Fig. 2f). In addition to these observations, in some individuals, the presence of mostly undifferentiated spermatogonia and a few numbers of spermatogonia cysts was observed in the seminiferous epithelium in this month.
Gonadosomatic index
The changes in the GSI are shown in Fig. 3. In April, a statistically significant increase in the GSI was observed compared to February, and this increase reached peak values in May and June. In July and August, there were sharp decreases observed in the GSI (P < 0.05). The GSI started to increase again in September and October (P < 0.05), and this increase continued in November (P < 0.05). The GSI did not change in December and January, and it was similar in February.
Germ cell proliferation
The PI for each month is represented in Fig. 4. It was observed that the undifferentiated spermatogonia, primary and secondary spermatogonia, and primary spermatocytes proliferate at different ratios in the different testicular cycle stages (Fig. 5). The highest germ cell PI was identified in July (P < 0.05). In this month, approximately all of the undifferentiated spermatogonia and primary spermatogonia were observed to be PCNA positive. Proliferative activity resumed its high values up until September, and it showed a significant decrease in October. The lowest proliferative activity values were observed during May and June. PCNA immunoreactivity was observed in the undifferentiated spermatogonia, spermatogonia, and spermatocytes, but it could not be observed in the spermatids and spermatozoa (Fig. 5e, f).
Illustration of the testis samples taken from male Chalcalburnus tarichi between February 2009 (A) and January 2010 (L), labeled with anti-PCNA. The anti-PCNA immunopositive germ cells are represented by the color brown (black arrow undifferentiated germ cell, white arrow primary spermatogonium, black arrow head secondary spermatogonium, white arrowhead primary spermatocyte, sSc secondary spermatocytes, Sd spermatid, Sz spermatozoan; B: March; C: April; D: May; E: June; F: July; G: August; H: September; I; October; J: November; and K: December)
Germ cell apoptosis
The AI for each month is represented in Fig. 6. It was observed that apoptosis affects the undifferentiated spermatogonia, primary and secondary spermatogonia, and primary spermatocytes, starting from the postspawning stage. The highest AI values were observed in May and June. In this stage, apoptosis is mostly seen in the primary spermatocytes, but the undifferentiated spermatogonia and spermatogonia also undergo apoptosis (Fig. 7d, e). However, apoptosis in the secondary spermatocytes and spermatids was detected as being random. In July, the AI decreased and it was also detected that apoptosis takes place both in the undifferentiated spermatogonia and in the primary spermatogonia (Fig. 7f). It was shown that the AI increased significantly in August when compared to July and this increase continued until October. The AI started to decrease in November, and by January, it had reached the same value as the previous February.
Illustration of the testis samples taken from male Chalcalburnus tarichi between February 2009 (A) and January 2010 (L), labeled with anti-TUNEL. The anti-TUNEL immunopositive germ cells are seen represented by the color brown (black arrow the undifferentiated germ cell; white arrow primary spermatogonium; black arrow head secondary spermatogonium; white arrow head primary spermatocyte; B: March; C: April; D: May; E: June; F: July; G: August; H: September; I: October; J: November; and K: December)
Plasma T and 11-KT levels
The changes in the plasma T and 11-KT levels are shown in Fig. 8. The plasma T and 11-KT levels increased significantly in the spawning stage in May and June.
Discussion
In the C. tarichi, the GSI increased at the beginning of September and continued to increase up until April. The highest GSI values were observed in May–June. A sharp decrease in the GSI was observed in July and August. Similar GSI values were found in a previous study with male C. tarichi (Elp and Çetinkaya 2000). The GSI values correlated with the PCNA index. Thus, the increase in the testicular weight during the recrudescence period (July–April) is attributable to the germ cell proliferation. At the end of the spermatogenesis, the seminiferous tubules possessed wide lumens that were full of free spermatozoa, reflecting the maximal GSI values (May–June). The lowest GSI values were observed in July, reflecting the release of the spermatozoa.
In the present study, the histological examination of the testis and the rate of the germ cell distribution in the seminiferous epithelial cells helped to discriminate the three stages of the testicular cycle. The testicular organization and spermatogenesis of the C. tarichi were fundamentally similar to those of the findings described by Ünal et al. (1999). Undifferentiated spermatogonia were determined in all the stages of the testicular cycle in the C. tarichi. These cells were observed to be distributed throughout the testis and were located on the walls of the branching tubules as adjacent to the basal membrane. This observation displays that the germinal compartment in the C. tarichi testis has an unrestricted tubular type of organization, as in many other fish species (Grier 1981; Loir et al. 1995; Schultz and Miura 2002). A monthly examination of the testes showed that the undifferentiated spermatogonia were densely present in July, where empty seminiferous tubules and residual sperms were observed in the testis. On the other hand, in some of the individuals, the seminiferous tubule lumens narrowed and spermatogonia cysts appeared, and residual sperms were not observed in the tubule lumens, suggesting that there are two groups of fish population present in the lake in July: fish having newly spent testis and fish having spent testis. At the same time, the undifferentiated spermatogonia showed the highest proliferation activity in all of the individuals in July. Taken together, the above observations indicate that a new spermatogenic cycle rapidly starts following the postspawning period in C. tarichi. When the testicular cyst samples of the September individuals were examined, newly formed primary spermatocyte cysts were determined in the seminiferous epithelia. This finding shows that meiosis starts in September in C. tarichi. As the spermatogenesis progressed, further growth occurred with the increase in the cell number of the developing primary spermatocyte cysts. In April, the mainly primary spermatocytes in seminiferous epithelium cells were observed, while there were no secondary spermatocytes, spermatid, or spermatozoa in the tubules yet. In the spawning stage (May and June), secondary spermatocyte and spermatid cysts were rarely observed. Billard (1986) and Schulz et al. (2010) reported that secondary spermatocytes appear as a result of meiosis I and were rarely observed in the teleost testis and that they also rapidly entered into meiosis II. Similarly, the stage of sperm development in the secondary spermatocytes may occur rapidly, and it may take a short time for spawning in C. tarichi. On the other hand, we did not observe the gonadal intermediate stages as having tubules, which contain secondary spermatocyte, spermatid cysts, and spermatozoa between April and May–June. A factor for the intermediate missing stages between April and May–June may arise from the temporal and spatial population samplings. The April samplings were conducted in the first part of the month. Germ cell types such as the secondary spermatocyte, spermatid, and spermatozoa, expected to be observed together in intermediate stages, may appear toward the end of April. Similarly, the intermediate germ cell types were rarely observed in May. The May samples were caught only from the river population in the spawning period. Fish in the lake population in May might have gonads containing all of the intermediate germ cells.
It was determined that germ cell proliferation was observed in all the phases of the testicular cycle. The highest germ cell PI values were observed in July, August, and September. The PI began to decrease in October, and it stayed stabile with some fluctuations during spermatogenesis. The lowest PI values were in May and June. Similar findings were also reported by other researchers, such as in the gilthead seabream, Sparus aurata, a protandrous hermaphrodite teleost, where spermatogonial stem cells and developing germ cells proliferated during spermatogenesis and proliferative activity was observed to be scarce in the spawning, started to increase in the postspawning, and increased in the resting phase (Chaves-Pozo et al. 2005). With some differences from our study, the proliferative activity was at the highest levels in the spermatogenic phase in the swordfish (Xiphias gladius); it then decreased in the spawning phase and remained stabile in the postspawning phase (Corriero et al. 2007). Thus, germ cell proliferation is essential for fish testicular formation and development. On the other hand, interspecies differences in the germ cell proliferation between the stages may differ depending on the reproductive strategies and gonad types of the fishes. The undifferentiated spermatogonia and spermatogonia showed PCNA immunoreactivity in all of the samples and testicular stages of the C. tarichi. Specifically, almost all of these cells had a PCNA-positive nucleus in July and August, in which a new reproductive cycle began. Similarly, in the gilthead seabream, Sparus aurata, both the spermatogonial stem cells and spermatogonia showed proliferative activity during all of the stages of the reproductive cycle (Chaves-Pozo et al. 2005). PCNA immunoreactivity in the undifferentiated spermatogonia and spermatogonia during the year was also found in the testis of the frog (Rana esculenta) (Chieffi et al. 2000). Our findings showed that the undifferentiated spermatogonia proliferate not only during spermatogenesis, but also during the spawning and postspawning stages; thus, the total depletion of these cells is prevented. Spermatogonial stem cells, well characterized in mammalians, can renew and differentiate into spermatogonial cells that form sperm (De Rooij 2001). Hence, a special balance between the renewal and differentiation processes is needed for a continuous balance during spermatogenesis (Nóbrega et al. 2009). Similar to the mechanism in mammals, undifferentiated spermatogonia provide a basis for the remodeling of the testis by showing high proliferative activity at the beginning of that reproductive cycle of cyprinids.
PCNA is a ring-like protein that provides the DNA polymerase processivity of DNA replication (Kelman 1997), and it also plays a role in DNA repair by interacting with the partner proteins (Maga and Hübscher 2003). The gene sequence and function of PCNA are remarkably conserved among eukaryotes (Chieffi et al. 2000; Miura et al. 2002). The immunodetection of PCNA is widely accepted as a proliferation marker in the normal and pathological tissues of the fishes (Manera and Biavati 1994; Ortego et al. 1994; Leung et al. 2005). A study conducted with small laboratory fishes demonstrated the successful application of mammalian-based PCNA immunodetection in different tissues, i.e., the testis, ovary, gill, and hematopoietic tissues of the medaka (Oryzias latipes), guppy (Poecilia reticulata), and western mosquito fish (Gambusia affinis) (Ortego et al. 1994). In a recent study, an increased expression of PCNA-positive cells was reported in the epithelial cells in the intestine of Salmo trutta trutta naturally infected with a parasite, using PC10 monoclonal mouse antibody (Dezfuli et al. 2012). In agreement, PCNA-positive cells were successfully detected in the testicular tissue of the C. tarichi. Surprisingly, a high level of proliferation in the germ cells was observed in June, August, and September. One possible explanation for this is that the biochemical studies showed that PCNA interacts with proteins functioning in DNA repair and cell cycle control. Especially in cell cycling, PCNA interacts with several eukaryotic cell cycle proteins such as CDK–cyclin complexes. The eukaryotic cell cycle is divided into four phases: G1, S, G2, and M, and each phase is under the general control of specific CDK–cyclin complexes. The CDK4,6–cyclin-D complex regulates progression through G1, CDK2-cyclin-E is involved in regulating the transition from G1 to the S phase, and CDK2–cyclin-A and CDK1–cyclin-A act throughout the S phase, whereas CDK1–cyclin-A regulates mitosis (Maga and Hübscher 2003). Thus, “S phase-PCNA” detection with a heterologous antibody can give rise to false-positive results as a consequence of the additional underlying cellular activity associated with PCNA, such as cell cycle control. The other explanation is DNA damage; the senescence or differentiation of cells through either p53-dependent or p53-independent pathways induces the p21 protein, which blocks the progression from G1 to the S phase and the PCNA essential mediator of the regulatory action of p21 (Waga et al. 1994). In relation to these reasons, the immunocytochemical staining patterns of PCNA expressions may permit the recognition of the G1, S, G2, and M phases, not only the S phase. On the other hand, the observation of the PCNA immunoreactivity in the primary spermatocytes in our study coincides with other studies performed on teleost (Corriero et al. 2007), the frog (Chieffi et al. 2000; Chaves-Pozo et al. 2005), and mammalian (Schlatt and Weinbauer 1994) testis and might involve the function of PCNA in DNA excision repair as a part of recombination process (Chapmann and Wolgemuth 1994; Kelman 1997).
The results of this study show that apoptosis takes place continuously in every testicular stage of the C. tarichi and it affects the germ cells from the spermatogonia to the spermatids. In the gilthead seabream, Sparus aurata, apoptosis mainly takes place in the postspawning stage and it plays a role in the elimination of any remaining spermatogonia (Chaves-Pozo et al. 2005). It was reported that apoptosis occurs during spermatogenesis only in the spermatocytes and spermatids in the spotted ray, Torpedo marmorata (Prisco et al. 2003). Similar to our findings, it was stated that germ cell apoptosis is seen in all of the spermatogenesis, spawning, and postspawning stages and is mainly observed in the spermatogonium B and spermatocytes in the swordfish (Xiphias gladius) (Corriero et al. 2007). Even the apoptotic germ cell death shows differences according to the species of the fish; it is observed in all the stages of spermatogenesis and in the different germ cell types in fish (Nóbrega et al. 2009). One of the roles of apoptosis is the elimination of the damaged or unwanted cells (Alenzi 2004). The high levels of the germ cell apoptosis in the spawning stage of the C. tarichi might be due to the elimination of aberrant germ cells. All of these taken together show that apoptosis in C. tarichi is a fundamental and active cellular death mechanism for the regulation of spermatogenesis and it provides testis homeostasis continuity by affecting the germ cells, from the undifferentiated spermatogonia to the spermatids.
In August, September, and October, while the PI showed its highest levels, the germ cell AI values were also at their peak. On the other hand, in May and June, while the PI was at its lowest value, the AI was shown to have high values. The data obtained from the Pearson’s analysis showed that there was not a significant correlation between the germ cell AI and PI in C. tarichi. Similar to our findings, there was no significant correlation between the germ cell apoptosis and proliferation in the swordfish, Xiphias gladius (Corriero et al. 2007), and in the gilthead seabream, Sparus aurata (Chavez-Pozo et al. 2005).
In C. tarichi, 11-KT and T were well correlated with the GSI, and they reached their highest levels in the spawning stage (May and June). As in the case of this study, the high levels of 11-KT and T in the spawning stage were also reported in other fish species, such as the brown bullhead (Ictalurus nebulosus) (Burke et al. 1984), sea bass (Dicentrarchus labrax) (Prat et al. 1990), and Atlantic halibut (Hippoglossus hippoglossus) (Weltzien et al. 2002). Furthermore, high androgen levels were detected in the cyprinid species chub, Leuciscus cephalus and Alburnus albidus (Guerriero et al. 1998, 2005). We observed 11-KT levels of two to three times more than the T levels in the spawning stage. Supportingly, the correlations between 11-KT and T were also observed in other teleosts, such as the lake whitefish (Coregonus clupeaformis) (Rinchard et al. 2001), Atlantic halibut (Hippoglossus hippoglossus) (Weltzien et al. 2002), and swordfish (Xiphias gladius) (Corriero et al. 2007). Our data indicate that 11-KT is the major androgen in C. tarichi.
T is a vital factor in mammalian testis germ cells (Young and Nelson 2001). It was reported that a lack of T stimulates apoptosis in the rat testis (Nandi et al. 1999), and there is an inverse correlation between plasma T levels and apoptosis in seasonally reproducing mammals (Blottner et al. 1995, 1999). In this study, there was no correlation between the plasma T and 11-KT levels and apoptosis according to the Pearson’s analysis. Similar to our results, Corriero et al. (2007) could not find such an effect of T and 11-KT on the germ cell apoptosis in the swordfish (Xiphias gladius). Our findings displayed that a separate mechanism occurs regarding the androgen effect on testicular germ cell apoptosis occurring in fish compared to mammals. Nevertheless, further detailed studies are needed for the determination of the differences in the apoptotic process during the reproductive cycle in the teleosts and the increased germ cell death mechanism in the testicular regression phase in mammals.
In conclusion, basic information about the reproduction biology of the C. tarichi was obtained. It was shown that the GSI reflects the gonadal development in different stages of the reproduction cycle and that germ cell proliferation is well correlated in all of the testicular stages. Apoptosis is a basic mechanism in the regulation of spermatogenesis in the testis of the C. tarichi. Furthermore, information about the annual changes in the levels of the plasma steroids (11-KT and T) was obtained, and those results provide reference levels for spermatogenesis in C. tarichi. A positive correlation was observed between the GSI, germ cell proliferation, and plasma steroid levels. Specifically, it was determined that 11-KT is an effective hormone in the spawning stage. This basic information would be helpful for the reproduction biology and protection of the fish.
References
Alenzi FQ (2004) Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci 61:99–102
Bartke A (1995) Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology 136:3–4
Billard R (1986) Spermatogenesis and spermatology of some teleost fish species. Reprod Nutr Develop 26:877–920
Billard R, Fostier A, Weil C, Breton B (1982) Endocrine control of spermatogenesis in teleost fish. Can J Fish Aquat Sci 39:65–79
Blanco-Rodríguez J (2002a) DNA replication and germ cell apoptosis during spermatogenesis in the cat. J Androl 23:182–187
Blanco-Rodríguez J (2002b) Deoxyribonucleic acid replication and germ cell apoptosis during spermatogenesis in the rabbit. J Androl 23:484–490
Blottner S, Hingst O, Meyer HHD (1995) Inverse relationship between testicular proliferation and apoptosis in mammalian seasonal breeders. Theriogenology 44:320–328
Blottner S, Roelants H, Wagner A, Wenzel UD (1999) Testicular mitosis, meiosis and apoptosis in mink (Mustela vison) during breeding and non-breeding seasons. Anim Reprod Sci 57:237–249
Borg B (1994) Androgens in teleost fishes. Comp Biochem Physiol Part C 109:219–245
Burke MG, Leatherland JF, Sumpter JP (1984) Seasonal changes in serum testosterone, 11-ketotestosterone, and 17β-estradiol levels in the brown bullhead, Ictalurus nebulosus Lesueur. Can J Zool 62:1195–1199
Casasco A, Giordano M, Danova M, Casasco M, Cornaglia AI, Calligaro A (1993) PC10 monoclonal antibody to proliferating cell nuclear antigen as probe for cycling cell detection in developing tissues: a combined immunocytochemical and flow cytometric study. Histochemistry 99:191–199
Cavaco JEB, Bogerd J, Goos H, Schulz RW (2001) Testosterone inhibits 11-ketotestosterone-induced spermatogenesis in African catfish (Clarias gariepinus). Biol Reprod 65:1807–1812
Chapman D, Wolgemuth D (1994) Regulation of M-phase promoting factor activity during development of mouse male germ cells. Dev Biol 165:500–506
Chaves-Pozo E, Mulero V, Meseguer J, Ayala AG (2005) An overview of cell renewal in the testis throughout the reproductive cycle of a seasonal breeding teleost, the gilthead seabream (Sparus aurata L.). Biol Reprod 72:593–601
Chieffi P, Franco R, Fulgione D, Staibano S (2000) PCNA in the testis of the frog, Rana esculenta: a molecular marker of the mitotic testicular epithelium proliferation. Gen Comp Endocrinol 119:11–16
Corriero A, Desantis S, Briddges CR, Kime DE, Megalofonou P, Santamaria N, Cirillo F, Ventriglia G, Di Summa A, Deflorio M, Campobasso F, De Metrio G (2007) Germ cell proliferation and apoptosis during different phases of swordfish (Xiphias gladius L.) spermatogenetic cycle. J Fish Biol 70:83–99
Danulat E, Selcuk B (1992) Life history and environmental conditions of the anadromous Chalcalburnus tarichi (Cyprinidae) in the highly alkaline Lake Van, Eastern Anatolia, Turkey. Arch Hydrobiol 126:105–125
De Rooij DG (2001) Proliferation and differentiation of spermatogonial stem cells. Reproduction 121:347–354
Dezfuli BS, Giari L, Lui A, Squerzanti S, Castaldelli G, Shinn AP, Manera M, Lorenzoni M (2012) Proliferating cell nuclear antigen (PCNA) expression in the intestine of Salmo trutta trutta naturally infected with an acanthocephalan. Parasit Vectors 5:198–205
Elp M, Çetinkaya O (2000) İnci kefali (Chalcalburnus tarichi Pallas, 1811)’nin üreme biyolojisi üzerine bir araştırma. IV Su Ürünleri Sempozyumu 28–30 Haziran 2000 Erzurum 51–66
Grier HJ (1981) Cellular organization of the testis and spermatogenesis in fishes. Am Zool 21:345–357
Guerriero G, Paolucci M, Bianco PG, Botte V, Giarcia G (1998) The reproductive cycle of the endangered cyprinid Alburnus albidus: morphological changes of the gonads and plasma sex steroid fluctuations. Ital J Zool 65:223–226
Guerriero G, Ferro R, Ciarcia G (2005) Correlations between plasma levels of sex steroids and spermatogenesis during the sexual cycle of the chub, Leuciscus cephalus L. (Pisces: Cyprinidae). Zool Stud 44:228–233
Hou Y, Han XD (2001) Annual changes in plasma levels of cortisol and sex steroid hormones in male rainbow trout, Oncorhyncus mykiss. Chinese J Oceanol Limnol 19:217–221
Kaptaner B, Ünal G (2011) Effects of 17α-ethynylestradiol and nonylphenol on liver and gonadal apoptosis and histopathology in Chalcalburnus tarichi. Environ Toxicol 26:610–622
Kelman ZW (1997) PCNA: structure, functions and interactions. Oncogene 14:629–640
Lagler KF, Bardach JE, Miller RR, Passion DRM (1977) Ichthyology, 2nd edn. Wiley, New York, p 506
Leung AYH, Leung JCK, Chan LYY, Ma ESK, Kwan TTF, Lai KN, Meng A, Liang R (2005) Proliferating cell nuclear antigen (PCNA) as a proliferative marker during embryonic and adult zebrafish hematopoiesis. Histochem Cell Biol 124:105–111
Loir M, Sourdaine P, Mendis Handagama SM, Jégou B (1995) Cell–cell interactions in the testis of teleosts and elasmobranchs. Microsc Res Tech 32:533–552
Maga G, Hübscher U (2003) Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 116:3051–3060
Manera M, Biavati S (1994) An immuno-histochemical technique used to demonstrate the transition form of a squamous cell carcinoma in a mirror carp, Cyprinus carpio. J Fish Dis 17:93–96
Miura C, Miura T, Yamashita M (2002) PCNA protein expression during spermatogenesis of the Japanese eel (Anguilla japonica). Zoolog Sci 19:87–91
Nandi S, Banerjee PP, Zirkin BR (1999) Germ cell apoptosis in the testes of Sprague Dawley rats following testosterone withdrawal by ethane 1,2-dimethanesulfonate administration: relationship to Fas? Biol Reprod 61:70–75
Nóbrega RH, Batlouni SR, França LR (2009) An overview of functional and stereological evaluation of spermatogenesis and germ cell transplantation in fish. Fish Physiol Biochem 35:197–206
Ortego LS, Hawkins WE, Walker WW, Krol RM, Benson WH (1994) Detection of proliferating cell nuclear antigen in tissues of three small fish species. Biotech Histochem 69:317–323
Papadaki M, Piferrer F, Zanuy S, Maingot E, Divanach P, Mylonas CC (2005) Growth, sex, differentiation and gonad and plasma levels of sex steroids in male- and female-dominant populations of Dicentrarchus labrax obtained through repeated size grading. J Fish Biol 66:938–956
Prat F, Zanuy S, Carrilo A, de Mones A, Fostier A (1990) Seasonal changes in plasma levels of gonadal steroids of sea bass, Dicentrarachus labrax L. Gen Comp Endocrinol 78:361–373
Prisco M, Liguoro A, Comitato R, Cardone A, D’onghia B, Ricchiari L, Angelini F, Andreuccetti P (2003) Apoptosis during spermatogenesis in the spotted ray Torpedo marmorata. Mol Reprod Dev 64:341–348
Rinchard J, Dabrowski K, Ottobre J (2001) Sex steroids in plasma of lake whitefish Coregonus clupeaformis during spawning in Lake Erie. Comp Biochem Physiol Part C 129:65–74
Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P (1997) An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 16:2262–2270
Sasso-Cerri E, Cerri PS, Freymüller E, Miraglia SM (2006) Apoptosis during the seasonal spermatogenic cycle of Rana catesbeiana. J Anat 209:21–29
Schlatt S, Weinbauer GF (1994) Immunohistochemical localization of proliferating cell nuclear antigen as a tool to study cell proliferation in rodent and primate testes. Int J Androl 17:214–222
Schulz RW, Miura T (2002) Spermatogenesis and its endocrine regulation. Fish Physiol Biochem 26:43–56
Schulz RW, de França LR, Lareyre JJ, LeGac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Štrbenc M, Fazarinc G, Bavdek SV, Pogačnik A (2003) Apoptosis and proliferation during seasonal testis regression in the brown hare (Lepus europaeus L.). Anat Histol Embryol 32:48–53
Ünal G, Çetinkaya O, Elp M (1999) Histological investigation of gonad development of Chalcalburnus tarichi (P., 1811). Tr J Zool 23:329–338
Waga S, Hannon GJ, Beach D, Stillman B (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369:574–578
Weltzien FA, Taranger GL, Karlsen Ø, Birgitta N (2002) Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Physiol Part A 132:567–575
Wolf HK, Dittrich KL (1992) Detection of proliferating cell nuclear antigen in diagnostic histopathology. J Histochem Cytochem 40:1269–1273
Yin Y, Stahl BC, DeWolf WC, Morgentaler A (1998) p53-mediated germ cell quality control in spermatogenesis. Dev Biol 204:165–171
Young KA, Nelson RJ (2001) Mediation of seasonal testicular regression by apoptosis. Reproduction 122:677–685
Acknowledgments
This work was supported by the Yüzüncü Yıl University, Directorate of Scientific Research Projects, under Project Number: 2009 FED B013. The authors wish to thank Prof. Dr. Güler Ünal for her help during the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaptaner, B., Kankaya, E. Analysis of germ cell proliferation, apoptosis, and androgenesis in the Lake Van fish (Chalcalburnus tarichi) during testicular development. Fish Physiol Biochem 39, 1665–1679 (2013). https://doi.org/10.1007/s10695-013-9818-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9818-2