Abstract
As a flatfish, the turbot (Scophthalmus maximus) is one of the most important farmed fish species with great commercial value, which has a strong sexual dimorphism on growth rate and sexual maturity. In this study, using histology, the basic information on proliferation and migration of germ cells and early gonadal development during sex differentiation in turbot were described in detail. There were six to nine individual primordial germ cells (PGCs) with large nuclei until 15 days post-hatching (dph). The PGCs located under the mesonephric ducts undergo migration along the dorsal mesentery toward the region of the genital ridge from 0 to 15 dph. During migration, the number of PGCs was constant, and the expression of vasa had no significant changes. At 20 dph, the aggregation of somatic cells at genital ridge indicated the formation of primary gonad. Furthermore, the number of PGCs was increased to 60 and the expression of vasa was upregulated for the first time. The undifferentiated gonads with no morphological indications of sex differentiation grew larger with the increase in germ cells and somatic cells number/size from 20 to 35 dph. During 36–52 dph, cytological gonadal differentiation was observed. In presumptive testes of type I gonadal tissue (with a lance shape), the number of germ cells increased steadily and the germ cells had the same characteristics as before. Meanwhile, in presumptive ovaries of type II gonadal tissue (with a club-like shape), the germ cells proliferated and induced in two different populations of germ cells. One type had the morphological characteristics as undifferentiated germ cells, while the other type of germ cells underwent mitosis exhibiting smaller size and mottled nuclei. At 60 dph, ovarian cavity was present in the gonad of type II, which would develop into ovaries. However, spermatogonial cysts were not noticed in the gonad of type I until 90 dph, which indicated the formation of the testes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germ cells are highly specialized cells and play an important role in gonadal development and fertility. Proliferation of germ cells during embryogenesis and gonadogenesis is a critical biological issue. PGCs are precursors of germ cells before they arrive at the genital ridge and ultimately give rise to gamete-forming cells within the gonad, in other word, becoming oogonium in the ovary or spermatogonia in the testis(Devlin and Nagahama 2002; Nishimura and Tanaka 2014). In histology, PGCs have been identified by the characters of large size, large nucleus with clear nuclear borders and low nucleocytoplasmic ratio from somatic cells (Mandich et al. 2002; Nagai et al. 2001). Generally, PGCs are specified early in a position which is distinct from that of gonad. Through a long-range cell migration in development, PGCs finally reach to the somatic part of the gonad, where they further differentiate into gametes (Deniz Koç and Yüce 2012). The origin, migration or development at various stages of embryonic development of PGCs has been described in several teleost fishes (Deniz Koç and Yüce 2012; Hamaguchi 1982; Lin et al. 2012; Parmentier and Timmermans 1985; Raz 2003; Takashima et al. 1980). These studies indicate that, as in other vertebrates, once formed in the peripheral region of the endodermal layer, PGCs migration in fish occurs via the dorsal mesentery into the somatic mesoderm, and then, all the PGCs were accommodated in the presumptive genital ridge region before hatching.
Sex differentiation contains all occurrences from the primordial gonad to the differentiated testis and ovary, respectively. And it encompasses complex process all the events that take place in the primordial gonad, including the migration, proliferation and division of PGCs, the differentiation of germ cell, the development character of the male and female gonads and the formation of testis and ovary finally (Piferrer 2001; Yvan et al. 1983). The timing of sex differentiation varies among vertebrate species in fish. Simultaneously, many teleost are gonochorists, where individuals develop only as males or females, and remain the same sex throughout their life spans (Yamamoto 1969). Two major types have been summarized among gonochoristic species for different strategies of early gonadal development. In the first type of undifferentiated species, all individuals initially develop ovarian tissue, but later, in approximately half of the population, ovarian tissue subsequently degenerates and gonad masculinizes proceeding to generate an intersexual gonad, which will eventually develop into a normal testis, such as zebrafish. (Devlin and Nagahama 2002; Sandra and Norma 2009; Yamamoto 1969; Uchida et al. 2002; Dai et al. 2015; Orban et al. 2009). The other type names differentiated gonochorists, which manifest as early gonadal development proceeds from an undifferentiated gonad directly to an ovary or a testis, for example medaka and chub mackerel. In medaka, sex differentiation with the sexually dimorphic proliferation of germ cells occurs at stage 39 (Hamaguchi 1982; Satoh and Egami 1972). Gonadal sex differentiation takes place during the embryonic development between stages 5 and 6 in Chapalichthys encaustus (Guerrero and Moreno-Mendoza 2009). Ovarian differentiation occurred from 30 to 40 dph and testicular differentiation occurred at 30 dph, indicated by the formation of ovarian cavity and sperm duct primordium, respectively, in the chub mackerel (Scomber japonicus)(Kobayashi et al. 2011). The differentiated phase commences with the appearance of sex differentiation at 187 dph and undergoes further differentiation until the end at 215 dph in Salvelinus fontinalis (Fatima et al. 2011).
Some studies have indicated that one of the first clear signs of gonadal sex differentiation in teleost is an increase in PGC number before the undifferentiated gonad toward ovarian differentiation compared to those differentiate to testis (Lewis et al. 2008; Sandra and Norma 2009; Timmermans and Taverne 1989). Generally, ovarian differentiation occurs earlier than testicular differentiation in teleost (Arezo et al. 2007; Devlin and Nagahama 2002; Nakamura et al. 1998; Sacobie and Benfey 2005; Uguz et al. 2003). The PGCs are mitotically dormant during their migration toward the gonadal primordia; thereafter, they regain their proliferative activity (Hamaguchi 1982; Timmermans and Taverne 1989). The proliferation of germ cells is the first step in gametogenesis and becomes sexually dimorphic during gonadal sex differentiation. This difference provides the first morphological indication of sex differentiation.
When mentioned to gonadal sex differentiation, anatomical features are often used to describe the stages in gonadal differentiation. In some fishes, including marine species, the formation of ovarian cavity in histological evidence has been a common early sign of female sex differentiation (Luckenbach et al. 2003; Nagahama 1983; Nakamura et al. 1998; Nishimura and Tanaka 2014). In flatfishes, limited information is available on the timing of gonadal sex differentiation. Gonads have differentiated into testes and ovaries in fish ≥41 mm total length (TL) (98 %) in the winter flounder (Pseudopleuronectes americanus) (Fairchild et al. 2007). Gonadal sex differentiation occurs by 38.0 mm fork length (LF) in the Atlantic halibut (Hippoglossus hippoglossus) (Hendry et al. 2002). The Japanese flounder (Paralichthys olivaceus) differentiates between 15 and 30 mm TL (Yamamoto 1999).
Turbot belongs to marine flatfish species and owns the character of delicious meat and rapid growth. They have a great commercial value and are widely farmed in Europe (FEAP 2015) and Asia (Lei 2003). It shows sexual dimorphism in size as a gonochoristic species; thus, obtaining the all-female populations has become a hot topic in the farming. However, there is lack of information on early gonadogenesis of turbot under artificial farming conditions. This study aims to clarify the differentiation of germ cells, the development of gonad and the differences between the male and female gonad during the early stage of sex differentiation of turbot. To investigate the location and migration of PGCs, the aggregation of somatic cells which form primary gonad, as well as the size and position of gonad, the gonads tissue are sectioned in longitudinal and cross separately. In this paper, the development of the gonad and the timing of sex differentiation of turbot will be clarified, which would provide a better understanding of gonads development of marine fishes.
Materials and method
Fry production
A mature male and a few females in captivity maintained at Oriental Ocean Sci-Tech Co., Ltd (Shandong Province, China) were used to produce zygote. All zygotes were cultured under controlled temperature. Viable buoyant zygotes were collected following fertilization and transferred into an incubator with a gentle flow of seawater of 14 ± 0.5 °C. Before hatching, embryos were distributed into a cement tank (6 m× 4 m× 1.3 m). Incubation lasted for 5 days.
Rearing of fish
Larvae were reared in these tanks with 1 m depth of water. Rearing density was approximately 125 larvae l−1. Water temperature was raised to 18–19 °C and kept constant by using steam-heated iron pipelines installed at the bottom of the tank. During the experimental period, oxygen, salinity and pH were 7.0–8.5 mg l−1, 30–32 and 7.7–8.0, respectively. Water temperature, dissolved oxygen, salinity and pH were monitored daily. At the first 3 days of the rearing period, the water in the tank was static. From 4 to 10 dph, tank water was partially replaced (10 % daily). Water exchange rate was increased gradually with the age of the larvae, which was progressively adjusted to 50 % at 15 dph and 100 % at 25 dph. The photoperiod was natural and maintained at 10 h light and 14 h dark on an average.
Larvae were fed rotifers (Brachionus plicatilis) plus green water composed of Nannochloropsis sp., Chlorella sp. and Isochrysis sp. from mouth opening (4 dph) to 19 dph, Artemia nauplii from 12 to 34 dph and compound diets from 27 dph until the end of the experiment. The feeding scheme for larvae and juveniles is summarized in Fig. 1.
Sampling and preservation
The study described in this article was conducted following the EU Directive 2010/63/EU for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.html). About 30 larvae were sampled every phase after anesthesia with 0.05 % solution of ethyl 3-aminobenzoate methane sulfonate (Sigma-Aldrich, Shanghai, China).The whole larvae were collected every 5 days from 0 to 30 dph. The abdominal segments where the gonad located in were collected every 4 days from 30 to 60 dph. All the samples were fixed in Bouin’s solution for 24 h and stored in 70 % ethanol until histological processing. The whole larvae samples for RNA extraction were rapidly dipped into liquid nitrogen and stored at −80 °C.
Histology
Fixed samples were dehydrated in a series of ethanol (70, 80, 90, 95, 95, 100 and 100 %), clarified in xylene and embedded in paraffin. Finally 5-µm-thick sections were taken from the samples using a rotary microtome. All the tissue sections were stained with hematoxylin and eosin. Briefly, slides were deparaffinized in xylene, rehydrated in ethanol (100, 95, 80, 70 %), stained in hematoxylin and eosin solution, respectively, dehydrated through 70, 80, 90, 100 % ethanol series, clarified in xylene and sealed with neutral balsam. Images were captured by Nikon E50i microscope with Nikon DS-Fi1 imaging system.
Germ cell number on each section was counted to predict the total number of germ cell. To ensure that each germ cell was counted once, only germ cells with a prominent nuclear envelope and nucleolus were identified and counted. In this study, a few germ cells display a nuclear diameter greater than the thickness of the section, so the predicted germ cell number is likely a slight overestimate. Gonad dimensions (length × width diameter of ovoid or elongated structure) were taken at the place along the gonads where they were the largest in length and width in longitudinal section. Particularly, in longitudinal section, in this study, the length of the gonad was identified the dimension from the dorsum to abdomen, and the width was deemed as the total number of sections in which gonad tissue could be seen × the thickness of each section.
Real-time PCR
As a germ cell-specific marker, the vasa homolog has been used to trace the germline in several fish species including turbot (Lin et al. 2012). Expression of Scophthalmus maximus vasa (Smvas) during gonadal development was analyzed by relative real-time PCR using SYBR Green detection method. For each stage, three samples were used for RNA extraction. For 2, 6 and 9 dph turbot, each sample contained a pool of three whole larvae to get sufficient amount of total RNA; samples for 14–45 dph were from the gonad region of three larvae. Random primers were used to perform RT with 1 mg total RNA using PrimeScript RT reagent kit (Takara Bio Inc., Dalian, China). Samples were run in triplicates in a 25-µl reaction volume containing 2 µl of diluted cDNA (1:10), 0.4 µM of each primer, and 12.5 µl of 2× buffer. PCR was performed using 95 °C for 30 s, 40 cycles at 95 °C for 5 s and 68 °C for 30 s. A dissociation curve was added at the end of each program to check the amplification specificity. Real-time PCR was then performed using SYBR Premix Ex Taq Kit (Takara Bio Inc.) on a Mastercycler ep realplex (Eppendorf, Hamburg, Germany). The specific primers for Smvas (162 bp) and β-actin (187 bp) were as follows: VasaF: 5′-GTGGAGGTTACCGTGGAAAAGA-3′ and VasaR: 5′-AGTTGATGCCCTTCTCATAGTGG-3′; ActinF: 5′-GCTGTGCTGTCCCTGTATGCC-3′ and ActinR: 5′-AGGAGTAGCCACGCTCTGTCA-3′.
Comparative Ct method was used to analyze the results.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 software (www-01.ibm.com/software/analytics/spss). All the results were expressed as mean ± standard deviation. Data for all the groups were analyzed using two-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests.
Result
The migration of PGCs after hatching
The PGCs underwent a post-hatching migration in turbot. Originally, the PGCs were located under the mesonephric duct at 2–9 dph (Fig. 2a, b). Until 10 dph, lager individual PGCs migrated along the abdomen and still arranged under the mesonephric duct, and only got a little far away in the distance. The mesentery supporting the intestinal tract was found in the ventral body connected to the group of PGCs (Fig. 2c; Fig. 3a). After migration along the dorsal mesentery on the dorsal side of the abdominal cavity, the PGCs arrived at the gonadal primordium and hung at the mesentery at 15 dph (Fig. 2d; Fig. 3b). In gonadal primordium at 15 dph, the most obvious characteristic was the aggregation of stromal cell which could be observed on the surface of the peritoneal epithelium. So far, the migration of PGCs to the gonadal primordium finished at 15 dph.
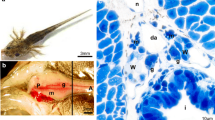
Photomicrographs of cross sections of undifferentiated gonads of turbot at 2–30 dph: a, b, c, d individual primordial germ cells at 2, 5, 10, 15 dph. e The germ cells arrive at the gonadal primordial under the migratory and somatic cells encompassed PGCs at 20 dph. f The enlargement of undifferentiated gonad for the increasing number of GC and somatic cells at 30 dph. A pair of primary gonad is formed. The higher magnification of GC located in the top left corner of the figure. M, Mesentery; MD, mesonephric duct; G, gut; GC, germ cell; PGC, primordial germ cells; S, spine; SC, somatic cell
Photomicrographs of longitudinal sections of undifferentiated gonads of turbot at 10–40 dph: a individual primordial germ cells at 10 dph. b The PGCs arrive at the gonadal primordial and hang at the mesentery at 15 dph. c Primary gonad formed at 20 dph. d, e, f The number of GCs and somatic cells increased and the primary gonad enlarged at 25, 30, 40 dph. The higher magnification of GC located in the top left corner of the figure. K, Kidney; M, mesentery; G, gut; GC, germ cell; PG, primary gonad; PGC, primordial germ cells; SC, somatic cell
The development of undifferentiated gonads
At 2 dph, PGCs were located at the posterior abdomen region and arranged under the mesonephric duct. There were about five single scattered and oval PGCs observed with relatively large diameter (6–8 µm) (Fig. 2a; Fig. 4a). At 5 dph, the PGCs were also easily recognized by their large size, conspicuous nucleus and low nucleocytoplasmic ratio. About six PGCs (6–8 µm diameter) (Fig. 4a, b) were observed with a single nucleolus of 2–3 µm in diameter. Morphologically, the PGCs were still slightly oval distinguished from somatic cells (Fig. 2b). At 10 dph, PGCs became 9 µm (Fig. 4a) in diameter and located below the mesonephric ducts. Each PGC contained one nucleus of 3–4 µm in diameter. The mesentery supporting the intestinal tract was found in the ventral body connected to the group of PGCs (Fig. 2c; Fig. 3a). At 15 dph, the most obvious characteristic was the appearance of stromal cells. The PGCs arrived at the genital ridge undergoing migration along the dorsal mesentery (Fig. 2d; Fig. 3b), which became larger about 10–13 µm (Fig. 4a) in diameter with one 3–4-µm-diameter nucleolus. The PGCs increased to nine in average (Fig. 4b). At 20 dph, the nucleoplasm and cytoplasm of PGCs were distinguished with clear nuclear borders by hematoxylin and eosin staining. And their number increased to 60 mitotically (Fig. 4b). Meanwhile, the aggregation of somatic cells encompassed PGCs herald the formation of primary gonads. A pair of primary gonads formed and located in the peritoneal cavity under the kidney. The primary gonad dimension among fish was 200 × 100 µm in average (Fig. 2e; Fig. 3c). At 25 dph, through mitosis, about 100 gonial cells could be seen (Fig. 4b). The size of primary gonads grew bigger to 230 µm in length (Fig. 3d). At 30 dph, the primary gonads elongated and reached to 300 µm in length and 130 µm in width (Fig. 2f; Fig. 3e). The diameter of gonial cells had increased to about 15 µm (Fig. 4a) with one nuclei (3–4 µm in diameter) containing condensed chromatin, but no meiotic activity was noted yet. Subsequently at 33 dph, the gonads of both sides were attached by a mesogonadium. The gonad of collected fish showed larger size with 500–750 µm in length, and a few blood cells began to appear.
Sex differentiation of turbot
Along with the increasing number of somatic cells and germ cells form 36 dph, two kinds of gonadal tissue could be observed. At 36 dph, nine of fifteen individuals showed relatively larger gonads (type I gonadal tissue with a lance shape) with gonad dimension ranging from 1100 × 70 to 1250 × 80 µm (Fig. 5a), while the rest had shorter gonads (type II with a club-like shape) ranging from 850 × 140 to 950 × 150 µm (Fig. 5f). The type I gonadal tissue showed fewer germ cells than that of type II. Specifically, the number of germ cells in the type I gonadal tissue was 105 in average, while that in type II gonadal tissue average was 447(Fig. 4b). And all the germ cells showed characteristics similar to those observed during the undifferentiated stage. At 40 dph, the germ cells also increased and present different trends of proliferation in two types of gonadal tissues (Fig. 5b, g). In gonadal tissue type I, the germ cells had the characteristics as expressed above. But in gonadal tissue type II, there were two different populations of germ cells. One population had the morphological characteristics of undifferentiated germ cells as the previous stage while the other population was undergoing mitosis and gradually multiplied in number. Moreover, the latter population of germ cells presented with different features and they exhibited smaller size and single prominent nuclei.
At 44–52 dph, the gonad dimensions of type I gonadal tissue (Fig. 5c, d, e) extended to 1450 × 100 µm in average, with the number of germ cells increased steadily. However, the stromal cells dramatically increased, aggregated and leaded to the extension of the gonad. One end of the primary gonads hung at the member of body cavity, while the other end dissociated in the abdominal cavity and attached to the abdominal wall. Meanwhile, the size of type II gonadal tissue (Fig. 5h, i, k) extended to about 1100 × 170 µm with a significantly increase of somatic cells and germ cells. Germ cells were mainly distributed in one side of the primary gonad, and the number was distinctly less than that of somatic cell. All these changes in germ cells and somatic cells indicated that the presumptive ovary began to be recognized. At 52 dph, the germ cells of type II gonadal tissue (Fig. 5k) underwent mitosis and formed clusters of PGCs, which caused a sudden decrease in the germ cells diameter (Fig. 4a). During these stages, the sex differentiation of the stromal cells could hardly be recognized. In addition, the other obvious difference of the two type gonadal tissues was the different existence morphology of blood vessel. In type I gonadal tissue, blood vessels became evident in the dorsal region of the primary gonads, while in type II gonadal tissue, the blood vessels were transversely sectioned and only scattered blood cells could be seen.
At 60 dph, the ovarian cavity was observed in a part of tested gonadal tissues. The presence of ovarian cavity indicated that the ovaries and testes could be distinguished clearly. Somatic cells near to the abdominal wall of ovarian cavity began proliferation in ovary. The individual germ cell was scattered into the somatic tissue on the sides facing the ovarian cavity. Germ cells, together with somatic cells, gradually multiplied in number by active mitosis. Thus, the germinal epithelium was clearly defined at this stage (Fig. 6c, d). A few blood cells could be found in the lateral region. In the presumptive testis, corresponding to the ovarian development, there was no sign of any histological differentiation at this stage (Fig. 6a, b). The germ cells proliferated slowly keeping the characteristic unchangeable as undifferentiated. In addition, an obvious blood vessel was seen at the side facing to body wall of the gonad.
Photomicrographs of longitudinal sections of ovary and testis: a presumptive testis at 60 dph. c Ovary with ovarian cavity at 60 dph. e Ovarian cavity increased in size. And the lamellae began to form at 75 dph. g Testis with spermatogonial cysts at 90 dph. b, d, f, h Higher magnification of a, c, e, g. BC, Blood cell; BV, blood vessel; GC, germ cell; O, ovary; OC, ovarian cavity; OG, oogonium; PT, presumptive testes; SG, spermatogonia; T, testes
At 75 dph, the ovarian cavity has increased in size, and the lamellae which would ultimately develop into ovarian follicles containing clusters of oogonium formed. In contrast, the presumptive testis was still undifferentiated at this time (Fig. 6e, f). At 90 dph (Fig. 6g, h) in presumptive testes, the number of germ cells increased obviously through mitosis. The stromal cells began to aggregation. This increasing of germ cells and stromal cells induced the enlargement of presumptive testes. The cluster of spermatogonia dividing from the single spermatogonia was smaller in diameter, and distributed in the edge of presumptive testes, which formed the spermatogonial cysts. The appearance of spermatogonial cysts around the edge of the gonad marked presumptive testes had turned into testes.
The expression of Smvas
The expression of Smvas during gonadal development from 2 to 45 dph was investigated by real-time PCR (Fig. 4c). The expression of Smvas was upregulated from 2 to 45 dph. Specifically, from 6 to 14 dph, the expression had no significant changes with the relative value lower than that of 2 dph. After 14 dph, there were two increasing points. One happened at 20 dph, the other came at 36 dph (P < 0.01). In addition, the expression of Smvas was consistent with the variations of germ cell number during gonadal development (2–45 dph), since the two increasing points of germ cell number also happened at 20 and 36 dph, respectively.
Discussion
In this study, we described the morphological characteristics of germ cell, the migration of PGCs, the formation of gonadal anlagen and the timing of sex differentiation of turbot in detail based on sex-specific histology. Initially, from 0 to 14 dph, PGCs underwent a post-hatching migration along the mesentery on the dorsal side of the abdominal cavity. About six to nine PGCs were observed (Fig. 4b), and there was no significant change in the vasa expression (Fig. 4c) which indicated that PGCs might be mitotically dormant during migration toward the gonadal primordium as reported in Barbus conchonius (Timmermans and Taverne 1989) and medaka (Hamaguchi 1982). At 15 dph, the PGCs arrived at the gonadal primordium and single somatic cells appeared around the PGCs. The presence of germ cells and somatic cells in gonadal primordium was necessary for the formation of gonadal anlage. At 20 dph, Vasa gene expression increased sharply (the first increasing point) (Fig. 4c) and the number proliferated to 60 (Fig. 4a, b). The bluegill sunfish (Lepomis macrochirus) (Gao et al. 2009), rainbow trout (Salmo gairdneri) (Lebrun et al. 1982), arctic charr (Salvelinus alpinus) (Chiasson and Benfey 2007) and coho salmon (Oncorhynchus kisutch) (Foyle 1993) also revealed that germ cells initiated continuous mitotic proliferation after their arrival in the gonadal primordium. It seemed that the mitotic proliferation of PGCs began at 20 dph in turbot. Meanwhile, PGCs were encompassed by the aggregation of somatic cells; thus, a pair of primary gonads formed and located in the peritoneal cavity under the kidney.
At 36 dph in turbot, we observed sex-related variations in gonadal differentiation (Fig. 5a, f) and two gonadal types were distinguished. One type appeared a club-like shape with gonad dimension ranging from 850 × 140 to 950 × 150 µm. Subsequently, exponential division of germ cell has been found in the club-like-shaped gonads from 40 to 52 dph. Therefore, a quick increase in germ cells number appeared. Another type with a lance shape showed relatively longer gonad with gonad dimension ranging from 1100 × 70 to 1250 × 80 µm. However, there were fewer germ cells in the lance-shaped gonad and no exponential division of germ cells observed. In light of the sex differentiation process, lance-shaped gonads and club-like gonads were also observed from 40 to 52 dph (Fig. 5). In addition, from 90 to 240 dph (8 months), in turbot, anatomical and histological analysis showed that the lance-shaped gonads had testicular structures, whereas the club-like gonads had ovarian structures (Fig. 7). Thus, we proposed that the gonads that showed greater proliferation of germ cells with a club-like shape were likely to develop into ovaries in adult, whereas the gonads with a lance-shaped and fewer germ cells were presumed to develop into testes in adult. In teleosts, such as Barbus conchonius (Timmermans and Taverne 1989), three-spined stickleback (Gasterosteus aculeatus) (Lewis et al. 2008), brook trout (S. fontinalis) (Sacobie and Benfey 2005), Austrolebias charrua (Arezo et al. 2007), bluegill sunfish (L. macrochirus) (Gao et al. 2009) and medaka (Saito et al. 2007), presumptive ovaries have more germ cells than presumptive testes during early gonadal differentiation and ovarian differentiation occurs earlier than testicular differentiation. It seemed that the increase in PGCs number might be a clear sign of gonadal sex differentiation. Therefore, we suggested that histology, especially the size and shape of gonads, combining with the cytological gonadal differentiation would be an effective way to distinguish female or male at the earliest stage of sex differentiation.
Ovary and testis at 90 dph and eight months post-hatching: a, b anatomical structure of ovary and testis at 90 dph. c Photomicrographs of ovary at 90 dph containing mainly PGCs. d Photomicrographs of testis at 90 dph. e, f Anatomical structure of ovary and testis at eight month. g Photomicrographs of Ovary at eight months containing mainly oogonium and some increased small oocytes. h Photomicrographs of testis at eight months with proliferated spermatogonia in testicular lobules. GC, Germ cell; I, increased small oocytes; OC, ovarian cavity; OG, oogonium; SG, spermatogonia
The knowledge that exogenous steroids could manipulate and effect sex ratio biases in fish has been comprehensively reviewed (Haffray et al. 2009; Luckenbach et al. 2003; Nakamura et al. 1998; Piferrer 2001). The most effective time of the onset of treatment to induce sex reversal with exogenous steroid hormones is the period when the gonads are still sexually undifferentiated (Nakamura et al. 1998). Our result that gonadal sex differentiation initiated at 36 dph was concert with previous research in turbot which showed that 100 % male or 100 % female can obtain when steroid treatments from 35 dph (Haffray et al. 2009). Therefore, we suggest that the most effective period for artificial sex reversal of turbot is before 36 dph.
In conclusion, our study demonstrates the process of development and differentiation of the gonad in turbot. Two gonadal types with club-like and lance shape were observed at 36 dph and cytological gonadal differentiation in female initiated at 40 dph. Overall, morphological changes associated with ovarian differentiation occurred at 36 dph (TL: 35.93 ± 1.10 mm) in presumptive ovaries. We propose that female and male gonads will be distinguished using histology combining with the cytological gonadal differentiation at early stage of gonadal differentiation. This research accumulates some basic data for sex differentiation in turbot and assists in understanding sex differentiation in other flatfish. The information will be useful to obtain all-female production in culture of turbot.
References
Arezo MJ, D’Alessandro S, Papa N, de Sá R, Berois N (2007) Sex differentiation pattern in the annual fish Austrolebias charrua (Cyprinodontiformes: Rivulidae). Tissue Cell 39:89–98
Chiasson M, Benfey TJ (2007) Gonadal differentiation and hormonal sex reversal in Arctic charr (Salvelinus alpinus). J Exp Zool Part A Ecol Genet Physiol 307:527–534
Dai XY, Jin X, Chen XW, He JY, Yin Z (2015) Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS ONE 10:15. doi:10.1371/journal.pone.0117824
Deniz Koç N, Yüce R (2012) A light- and electron microscopic study of primordial germ cells in the zebra fish (Danio rerio). Biol Res 45:331–336
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Fairchild EA, Rennels N, Howell WH, Wells RE (2007) Gonadal development and differentiation in cultured juvenile winter flounder, Pseudopleuronectes americanus. J World Aquac Soc 38:8
Fatima S, Adams M, Wilkinson R (2011) Histological study of gonadal development and sex differentiation in Salvelinus fontinalis under Tasmanian climate conditions. Aust J Zool 59:321. doi:10.1071/zo11092
FEAP (2015) European aquaculture production report 2005–2014. Federation of European Aquaculture Producers. http://www.feap.info/Default.asp?SHORTCUT=582. Accessed 20 Aug 2015
Foyle TP (1993) A histological description of gonadal development and sex differentiation in the coho salmon (Oncorhynchus kisutch) for both untreated and oestradiol immersed fry. J Fish Biol 42:699–712
Gao Z et al (2009) Gonadal sex differentiation in the bluegill sunfish Lepomis macrochirus and its relation to fish size and age. Aquaculture 294:138–146
Guerrero SM, Moreno-Mendoza N (2009) Gonadal sex differentiation in Chapalichthys encaustus (Teleostei: Goodeidae). Dev Biol 331:443
Haffray P, Lebègue E, Jeu S, Guennoc M, Guiguen Y, Baroiller JF, Fostier A (2009) Genetic determination and temperature effects on turbot Scophthalmus maximus sex differentiation: an investigation using steroid sex-inverted males and females. Aquaculture 294:30–36
Hamaguchi S (1982) A light- and electron-microscopic study on the migration of primordial germ cells in the teleost, Oryzias latipes. Cell Tissue Res 227:139–151. doi:10.1007/BF00206337
Hendry CI, Martin-Robichaud DJ, Benfey TJ (2002) Gonadal sex differentiation in Atlantic halibut. J Fish Biol 60:1431–1442
Kobayashi T, Ishibashi R, Yamamoto S, Otani S, Ueno K, Murata O (2011) Gonadal morphogenesis and sex differentiation in cultured chub mackerel, Scomber japonicus. Aquac Res 42:230–239
Lebrun C, Billard R, Jalabert B (1982) Changes in the number of germ cells in the gonads of the rainbow trout (Salmo gairdneri) during the first 10 post-hatching weeks. Reprod Nutr Dév 22:405–412
Lei J (2003) The development direction of turbot farming industry in China. Sci Fish Farm 7:3–4 (in Chinese)
Lewis ZR, McClellan MC, Postlethwait JH, Cresko WA, Kaplan RH (2008) Female-specific increase in primordial germ cells marks sex differentiation in threespine stickleback (Gasterosteus aculeatus). J Morphol 269:909–921
Lin F et al (2012) Germ line specific expression of a vasa homologue gene in turbot (Scophthalmus maximus): evidence for vasa localization at cleavage furrows in euteleostei. Mol Reprod Dev 79:803–813
Luckenbach JA, Godwin J, Daniels HV, Borski RJ (2003) Gonadal differentiation and effects of temperature on sex determination in southern flounder (Paralichthys lethostigma). Aquaculture 216:13
Mandich A, Massari A, Bottero S, Marino G (2002) Histological and histochemical study of female germ cell development in the dusky grouper Epinephelus marginatus (Lowe, 1834). Eur J Histochem 46:87–100
Nagahama Y (1983) The Functional-morphology of teleost gonads. Fish Physiol 9:223–275
Nagai T, Yamaha E, Arai K (2001) Histological differentiation of primordial germ cells in zebrafish. Zool Sci 18:215–223
Nakamura M, Kobayashi T, Chang X-T, Nagahama Y (1998) Gonadal sex differentiation in teleost fish. J Exp Zool 281:362–372
Nishimura T, Tanaka M (2014) Gonadal development in fish. Sex Dev 8:252–261
Orban L, Sreenivasan R, Olsson PE (2009) Long and winding roads: testis differentiation in zebrafish. Mol Cell Endocrinol 312:35–41
Parmentier HK, Timmermans LPM (1985) The differentiation of germ cells and gonads during development of carp (Cyprinus carpio L.). A study with anti-carp sperm monoclonal antibodies. J Embryol Exp Morphol 90:13–32
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281
Raz E (2003) Primordial germ-cell development: the zebrafish perspective. Nat Rev Genet 4:690–700
Sacobie CFD, Benfey TJ (2005) Sex differentiation and early gonadal development in brook trout. N Am J Aquac 67:181–186
Saito D et al (2007) Proliferation of germ cells during gonadal sex differentiation in medaka: insights from germ cell-depleted mutant zenzai. Dev Biol 310:280–290
Sandra G-E, Norma M-M (2009) Sexual determination and differentiation in teleost fish. Rev Fish Biol Fish 20:101–121
Satoh N, Egami N (1972) Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development. J Embryol Exp Morphol 28:385–395
Takashima F, Patino R, Nomura M (1980) Histological studies on the sex differentiation in rainbow. Bull Jpn Soc Sci Fish 46:1317–1322
Timmermans LPM, Taverne N (1989) Segregation of primordial germ cells: their numbers and fate during early development of Barbus conchonius (Cyprinidae, Teleostei) as indicated by 3H-thymidine incorporation. J Morphol 202:225–237
Uchida D, Yamashita M, Kitano T, Iguchi T (2002) Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol 205:711–718
Uguz C, Iscan M, Togan I (2003) Developmental genetics and physiology of sex differentiation in vertabrates. Environ Toxicol Pharmacol 14:9–16
Yamamoto T-O (1969) 3 Sex differentiation. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 3. Academic Press, New York, pp 117–175
Yamamoto E (1999) Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture 173:235–246. doi:10.1016/S0044-8486(98)00448-7
Yvan F et al (1983) La gonadogenèse des Poissons. Reprod Nutr Dev 23:453–491
Acknowledgments
This research was supported by National Natural Science Foundation of China (Nos. 31372514, 31472264, 31572602), Modern Agro-Industry Technology Research System (nycytx-50), China Postdoctoral Science Foundation (2014M551973), the Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology (Nos. 2015ASKJ02, 2015ASKJ02-03-03), Youth Innovation Promotion Association CAS and Chinese Academy of Science and Technology Service Network Planning (KFJ-EW-STS-060).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, C., Xu, S., Liu, Y. et al. Gonadogenesis analysis and sex differentiation in cultured turbot (Scophthalmus maximus). Fish Physiol Biochem 43, 265–278 (2017). https://doi.org/10.1007/s10695-016-0284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0284-5