Abstract
Testicular development and plasma levels of sex steroid [11-ketotestosterone (11-KT), testosterone (T) and 17,20β-dihydoxy-4-pregnen-3-one (17,20β-P)] were studied for the first time in wild golden mahseer, Tor putitora. Testicular development was investigated by macroscopic observation and histology of the gonads, whereas steroids were measured by enzyme-linked immunosorbent assay. Based on macroscopic observation and germ cell types present in gonad histology, the testes of T. putitora were divided into five developmental stages: immature [stage I; spermatogonia (SPG)], early spermatogenesis [stage II; SPG and spermatocytes (SPC)], late spermatogenesis [stage III; SPG, SPC, spermatids (SPD) and spermatozoa (SPZ)], spermiation (stage IV; SPZ) and post-spawning (stage V; SPG, SPD and SPZ). During the stage I of the testes, the lowest levels of plasma sex steroid and gonadosomatic index (I G) were recorded. The highest plasma level of T was 0.89 ± 0.09 ng/mL and 11-KT was 4.23 ± 0.54 ng/mL, which was during the stage III and IV, respectively. The peak in 11-KT was coincident with the peak in I G (1.65 ± 0.12 %). The lowest T and 11-KT levels were 0.25 ± 0.02 ng/mL and 0.47 ± 0.09 ng/mL, respectively, which was at stage I. Plasma levels of 17,20β-P increased significantly at stage III (1.04 ± 0.06 ng/mL) and stage IV testes (1.28 ± 0.03 ng/mL) and then declined in post-spawned fish. This indicates that 17,20β-P could also be a possible maturation-inducing steroid in this fish. The condition factor (K) significantly decreased during the testicular development and was lowest at spermiation stage (0.53 ± 0.02 %). The proportion of running male peaked concomitantly with the appearance of stage IV testes. Presence of germ cells of different developmental stages indicates that T. putitora male is a multiple spawner, and the information generated here is important for developing a captive breeding, culture and conservation programs for this endangered coldwater Himalayan fish species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Golden mahseer, Tor putitora (Cypriniformes: Cyprinidae), is an endemic food and game fish of the Himalayan region of South and Southeastern Asia (Nautiyal 1984). Once, this fish was abundantly distributed in hill streams and lakes of India, Nepal, Pakistan, Afghanistan, Bangladesh, Bhutan, Sri Lanka, Myanmar, Thailand and Iran (Nautiyal 1984; Islam and Tanaka 2007; Jayaram 2010) and can grow up to 3 m in length in its natural habitat (Bhatt et al. 2000). However, due to overfishing and habitat destruction, its natural population is declining significantly over the past few decades (Oliver et al. 2007; Froese and Pauly 2011) and listed as endangered. Therefore, attempts to culture and revive T. putitora stocks have been initiated in trans-Himalayan regions (Ingram et al. 2005; Dinesh et al. 2010). The major bottleneck for its intensive culture is the inability of this Tor sp. to attain complete gonadal maturity in captivity (Ismail et al. 2011). Artificial propagation through inducing maturation and spawning by using sex hormones has been attempted (Pandey et al. 1998), with limited success. Therefore, seed production in captivity is still dependent on spawning brooders collected from wild, mainly taking place during months of May to August in accordance with the breeding season in nature.
In teleost, gonads have two major functions: firstly to produce the germ cells through the process of oogenesis (in females) and spermatogenesis (in males) and secondly to produce sex steroids for the initiation and regulation of reproduction. In fishes, the testes can be categorised into two major types based on the morphology of germinal compartment and distribution of germ cells within this compartment (Santana and Quagio-Grassiotto 2014). In higher teleost, the germinal compartment is organised into branching lobules, while those of lower fish are organised into anastomosing tubules (Weltzien et al. 2002). Both the testes types can be further categorised into restricted spermatogonial type, where spermatogonia (SPG) are confined to the periphery of the testis and unrestricted spermatogonial type, where SPG can be found all along the length of the tubule (Schulz and Miura 2002; Schulz 2003). In the testes of a fish, germ cells and Sertoli cells are organised in spermatocysts, and each spermatocyst contains germ cells clones, all at the same stage of development (Billard et al. 1982). During spermatogenesis, where spermatogonial stem cell (SSC) differentiates into mature spermatozoa (SPZ), three major events take place. Initially, the SSC undergoes mitotic division to produce new undifferentiated SPG (type A) and differentiated SPG (type B) (De Rooij 2006; Schulz et al. 2010). Then, the differentiated SPG undergo meiosis, which leads to the production of haploid spermatids (SPD). Finally, the haploid SPD differentiate into matured SPZ by the process of spermiogenesis (Schulz 2003; Schulz et al. 2010).
Like other vertebrates, the gonad maturation of fish is regulated by several sex hormones (Lee and Yang 2002; Estay et al. 2003; Ismail et al. 2011). In males, androgen and progestin such as testosterone (T), 11-ketotestosterone (11-KT) and 17,20β-dihydoxy-4-pregnen-3-one (17,20β-P), respectively, are known to regulate testicular development by their direct influence on germ cell and also by exerting feedback effects in brain–pituitary–gonad (BPG) axis (Weltzien et al. 2002). Increase in 11-KT stimulates spermatogenesis (Aramli et al. 2014), whereas T stimulates the hypothalamus and pituitary, leading to the development and maturation of the testes (Chaves-Pozo et al. 2008). The progestins such as 17,20β-P are known to promote testicular hydration and spermiation (Miura et al. 1992; Nagahama 1994). Although 11-KT is considered as main androgen in teleost fish (Borg 1994; Koya et al. 2002; García-López et al. 2006; Ismail et al. 2011), sporadically T is found to be the main sex steroid in certain fish (Matsuyama et al. 1991). The temporal variation in plasma sex steroid levels in relation to testis development has been studied in Senegalese sole, Solea senegalensis Kaup (García-López et al. 2006), Atlantic halibut, Hippoglossus hippoglossus L. (Weltzien et al. 2002), Pacific herring, Clupea pallasii (Koya et al. 2002), spotted halibut, Verasper variegates (Koya et al. 2003), African catfish, Clarias gariepinus (Cavaco et al. 1997a, b), Japanese eel, Anguilla japonica (Miura et al. 1991), winter flounder, Pleuronectes americanus (Harmin et al. 1995), English sole, Pleuronectes vetulus (Sol et al. 1998), rainbow trout, Salmo gairdnerii Richardson (Scott et al. 1980) and mahseer Tor tambroides (Ismail et al. 2011). However, to our knowledge, there are no detailed studies, which focus on spermatogenic cycle of T. putitora in terms of testicular development in relation to plasma 11-KT, T and 17,20β-P.
Therefore, the aim of this study was to describe the testicular development of T. putitora in relation to plasma level of circulating androgens (T and 11-KT) and progestins (17,20β-P). So far little is known about the reproductive physiology of species from the genus Tor, and none of them correlate the testicular development with sex steroid dynamics.
Materials and methods
Fish
On the first week of every month from February 2014 to September 2014, live T. putitora were procured from local fisherman and brought back to wet laboratory of the Directorate of Coldwater Fisheries Research, Bhimtal, India. The fish were captured by hook and line from Bhimtal (latitude 24°48′N and longitude 73°54′E) and Sattal Lakes (latitude 29°20′N and longitude 79°31′E) of mid-Himalayan region of India. The average weight and length of the sampled fish were 429.62 ± 84.91 g and 37.48 ± 8.73 cm (mean ± SEM), respectively. The age of collected fish was determined by scale counting method (Nikolsky 1963), using the scale reader (Sipcon profile projector SP-300, Ambala cantonment, India). The fish were acclimatised in 1000-L capacity square fibreglass tanks with 0.45 m of maximum water depth for 7 days. During the acclimatisation, the tanks were continuously supplied with groundwater, and the water flow rate was 2.5 L/min. Body length (total length, standard length and fork length) was measured using a 60-cm measuring scale, and body weight and gonad weight were measured using a digital scale balance with 0.01 sensitivity. The temperature, dissolved oxygen and pH of the tank water during the acclimatisation period varied between 20.51 to 24.18 °C, 6.57 to 6.85 mg/L and 6.60 to 7.40, respectively. Feeding to satiation was done twice daily with a supplementary pellet diet (prepared by DCFR, Bhimtal), at the rate of 4 % of the body weight (35 % crude protein, 3 % moisture, 5 % fibre and 3 % crude fat).
Blood sampling
From acclimatised fish, blood sampling and tissue sampling were carried out. Fish were anesthetised using tricaine methane sulphonate (MS 222; 60 mg/L of water). Approximately, 1.5–2.0 mL of blood was drawn from the caudal vein of each fish using cold heparinised syringe (3 mL capacity) fitted with 22-gauge needle. The blood was collected in 2-mL Eppendorf tubes and before plasma separation allowed to stand at room temperature for 1 h. The plasma was separated from the blood by centrifugation at 5000×g for 10 min at 4 °C. The blood plasma was aliquot to 150 µL in 0.5 mL Eppendorf tubes and stored at −80 °C till further analysis.
Gonad development and histology
After collecting the blood, the fish were euthanised by an overdose of MS 222 (150 mg/L of water). The ventral surface of the fish was cut open and liver and testes were excised, weighed to the accuracy of 0.01 g. For macroscopic determination of gonad maturity, features such as degree of opacity of the gonads, colour, size, volume, length and sperm visibility were recorded (Rosenblum et al. 1987). Transverse fragments of testis tissue (approximately 0.4 cm3) from midlobe were fixed in 4 % phosphate-buffered (0.1 M, pH 7.2) formalin for 48–96 h at room temperature. For histological examination, the tissues were rinsed in running tap water, dehydrated in an ascending series of ethanol and processed by standard histological methods (Allen 1993). Testis sections were cut at 4–5 µm thickness on a semi-automatic microtome (Microm HM340E, Thermo Scientific) and stained with haematoxylin and eosin (H&E). The stained sections were examined and photographed on a Leica DM500 microscope (Leica, Germany). The testicular development stages were determined according to germ cell types present and their relative abundance (Schulz et al. 2010).
Sex steroid immunoassay
Sex steroid quantification (11-KT, T and 17,20β-P) in collected plasma was conducted using commercially available enzyme-linked immunosorbent assay (ELISA) kits from Cayman Chemical Company, Michigan, USA. The sex steroids were quantified following the assay kit procedures and methods described by Cuisset et al. (1994) and Nash et al. (2000). Microtiter plates (MaxiSorp™) for the assay were purchased from Nunc (Roskilde, Denmark). All samples and standards were assayed in triplicates, and the absorbance was read at 415 nm by using microplate spectrophotometer (EON, Bio Tek). The absorbance values were analysed using SoftMax Pro 5.4 software (Molecular Devices, LLC).
Calculations and data analyses
The gonadosomatic index (I G; %), condition factor (K) and hepatosomatic index (I H; %) were calculated as 100 × W G × (W B)−1, 105 × W B × (L3)−1 and 100 × W L × (W B)−1, respectively. W G is gonad weight (g), W B is total body weight (g), and W L is total liver weight (g). Data were analysed for statistical differences within spermatogenetic stages and spermiation condition by one-way analysis of variance (ANOVA) using SPSS (version 19.0), followed by Student–Newman–Keuls (SNK) multiple comparison tests with a significance level of p < 0.05. The data compliance with homogeneity of variance and normality was tested by the Levene and Kolmogorov–Smirnov methods, respectively, and log transformation was carried out, when necessary. Data are presented as mean ± standard error of mean (SEM).
Results
Altogether, 52 male T. putitora of age 3.0–4.5 years were sampled during the study period (Table 1). The standard length and fork length of the collected fish were 32.52 ± 6.78 and 35.69 ± 3.48 cm, respectively.
Testicular development
Five testicular development stages of spermatogenesis were defined in T. putitora, based on the macroscopic appearance, histological changes and relative abundance of various germ cell types present (Table 2 and Fig. 1).
Spermiation
In February and March, running males (RM) were not observed. RM was found from May to August (Fig. 2). The fraction of RM was low (25 %) in April and then gradually increased from May (around 64 %) to August (83 %). The percentage of RM was highest in August, which abruptly declined to 50 % in September. The proportion of RM was low in fish with stage II and III testes (10 and 25 %, respectively) and peaked in fish with testes at stage IV (90 %) (Fig. 3). At stage V testes, RM proportion was tremendously low (around 29 %). Non-RM (100 %) was observed at stage I of testicular development.
Photomicrographs of cross section (4-5 µm) of golden mahseer, Tor putitora, testes at different maturity stages. a, b Immature spermatogonia (SPG) (stage I); c early spermatogenic spermatocytes (SPC I and SPC II) (stage II); d, e, f late spermatogenic SPC and spermatids (SPD), (stage III); g, h functional maturation, spermatozoa (SPZ) (stage IV); i, j recovery, SPG and SPD (stage V). TA, Tunica albuginea; S, sertoli cell; BC, blood cell; L, lumen; V, vacuoles; LDG, Leydig cell; SFT, seminiferous tubule; scale bars 100 µm (a, d, g); 50 µm (b, c, j); 30 µm (e, h, i); 15 µm (f). Sections were stained with haematoxylin and eosin (H&E)
Proportion of running and non-running males of golden mahseer at different stages of testicular development. Sample size is given in Table 1
Morphometric changes
For fish with stage I testes, the mean I G was the lowest at 0.43 ± 0.01 % (Fig. 4a). The I G increased to 0.65 ± 0.04, 1.18 ± 0.06 and 1.68 ± 0.12 % for fish with stage II, III and IV testes, respectively. Subsequently, the index decreased to 1.20 ± 0.41 % for stage V. Mean I G was higher in running male (1.68 ± 0.12 %) than in non-running male (0.73 ± 0.09 %). The I H (mean 1.03 ± 0.32 %) did not vary significantly in between testicular development stages (Fig. 4b). The K (mean 0.76 ± 0.06 %) declined as the development of testes progressed from stage I testes (mean 1.12 ± 0.09 %) to stage II (mean 0.72 ± 0.06 %), stage III (mean 0.67 ± 0.03 %) and stage IV testes (mean 0.59 ± 0.02 %) (Fig. 4c). At stage V testes, the K increased significantly (mean 0.93 ± 0.10 %).
Variation in (mean ± SEM) a gonadosomatic index (I G), b hepatosomatic index (I H), c condition factor (K) of male golden mahseer, Tor putitora, at five gonadal development stage. Different letters indicate statistical differences (p < 0.05). Sample size is given in Table 1
Plasma sex steroid
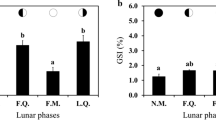
Plasma 11-KT, T and 17,20β-P in male T. putitora ranged from 0.47 to 4.23 ng/mL serum, 0.26 to 0.89 ng/mL serum, and 0.71 to 1.289 ng/mL serum, respectively, during the study period. 11-KT level significantly increased from the stage II testes (mean 0.56 ± 0.21 ng/mL) to stage III (mean 2.89 ± 2.81 ng/mL) and IV (mean 4.23 ± 0.51 ng/mL) (Fig. 5a). T level significantly increased from fish with the stage I testes (mean 0.25 ± 0.009 ng/mL) to stage III (mean 0.89 ± 0.02 ng/mL) (Fig. 5b). 17,20β-P in serum of fish increased from the stage II testes (0.71 ± 0.09 ng/mL) to stage III (1.04 ± 0.06 ng/mL) and IV (1.28 ± 0.03 ng/mL) (Fig. 5c). At stage V, the level of all the three sex steroids in fish was significantly lower (1.23 ± 1.01 ng/mL for 11-KT, 0.34 ± 0.001 ng/mL for T and 0.89 ± 0.04 ng/mL for 17,20β-P) than those of fish with stage IV testes. Average intra-assay and inter-assay coefficients of variations of 11-KT, T and 17,20β-P are shown in Table 3.
Plasma levels of sex steroids (mean ± SEM) in male golden mahseer, Tor putitora, at different testicular development stages. a 11-ketotestosterone, 11-KT; b testosterone, T; c 17,20β-dihydoxy-4-pregnen-3-one, 17,20β-P. Different letters above histograms indicate significant difference (p < 0.05). Sample size is given in Table 1
Discussion
In the present work, we describe for the first time the spermatogenic cycle of wild T. putitora in terms of testicular growth and development, in correspondence with androgen (11-KT and T) and progestin (17,20β-P) levels in blood plasma. This study was carried out from February to September 2014, during the breeding season reported for this species (Nautiyal 1984) in its natural habitat in Himalayan regions of northern India. Spermatogenesis was divided into five developmental stages based on macroscopic appearance and relative abundance of germ cell types present in testes of this fish. During the study period, distinct variations in I G, K and plasma level of sex steroids were observed, in association with different phases of testicular development.
In February and March, the testes of T. putitora were at stage I (Immature) and stage II (early spermatogenesis), respectively. It indicates that the spermatogenesis was initiated in few sampled fish during the start of summer (March). All sampled fish had testes at early and late spermatogenic stages (stage II and stage III) in April. In May, testes were at spermatogenic (stage II and stage III) and spermiation stage (stage IV). From June to July, mahseer had fully matured testes, with oozing of milt (spermiating stage, stage IV), which is in correspondence with the peak spawning seasons reported for this species in nature (Nautiyal 1984; Dinesh et al. 2010). Spawning is synchronised with heavy rainfall (June–August), a condition that ensures the influx of oxygen-rich water and dropping in water temperature, which stimulates the maturation of testes, and finally spermiation (Ingram et al. 2005; Islam and Tanaka 2007). In September, the sampled fish were at post-spawning (stage V) phase, which suggest the end of the breeding season for this fish. Therefore, this study indicates that spawning of T. putitora in the northern Himalayan region extends from May to August, with a peak in August, which is in agreement with the earlier observations (Bhatt et al. 2000; Dinesh et al. 2010).
Also, histologically five testicular development stages were distinguished in T. putitora, and spermatogenesis appears to be cystic type, similar to that reported in many other fishes, like winter flounder, Pseudopleuronectes americanus (Harmin et al. 1995), English sole, Parophrys vetulus (Sol et al. 1998), H. hippoglossus (Weltzien et al. 2002) and V. variegates (Koya et al. 2003). It occurs within individual cysts, which line the tubule walls (Santana and Quagio-Grassiotto 2014), and during and immediately preceding the spawning season (stage IV), the central lumen receives the SPZ released from cysts, which remain stationary along the lobule during spermatogenesis. Although T. putitora belongs to cyprinid group of fishes, its spermatogenetic pattern is different from all other cyprinids (mainly tropical cyprinids such as carps and few other Tor spp.) (Ingram et al. 2005). In other cyprinids, SPG and SPZ were present throughout the year in the testes, allowing continuous production of matured SPZ (Neetu Shahi, unpublished data), whereas in T. putitora matured spermatozoa is produced during certain month of the year (May–September). Histological examination of testes of this fish indicates that both SPD and SPZ were present at certain stage of testicular development. This may be the reason for the release of sperm during a certain month of year, mainly at the peak of gonad development and endocrinological activity. At late spermatogenesis, in the testes of T. putitora, meiotic activity leads to the accumulation of SPD and SPC in the cortical seminiferous lobules for a short period of time, since they get converted into SPZ heavily, a phenomenon which indicates synchronous spermatocyte development and complete maturation. Since spermiogenesis occurs simultaneously, at the final phase of gonad development, the testis accumulates large quantity of sperm (4–5 mL, personal observation), which can easily be extracted with a gentle pressure on the lower part of the abdomen. It has been recognised that in cystic spermatogenesis, synchronous germ cell development produces a large number of simultaneously mature SPZ, compared with semi-cystic spermatogenesis (García-López et al. 2006). Except for T. tambroides (Ismail et al. 2011), so far, there are no reports describing previously the testicular development of this species or any other Tor species. However, the study conducted by Ismail et al. (2011) in T. tambroides did not mention the type of spermatogenesis; rather, they described the annual sex hormone profile and germ cell development. Unlike T. putitora, which is a temperate water species and a seasonal spawner (Nautiyal 1984), the T. tambroides is a tropical species, which spawns throughout the year (non-seasonal spawner). Therefore, further study in detail is essential to clarify the relationship between cystic type spermatogenesis and the dynamics of the testicular development in T. putitora and any other Tor species as well.
Spermiating fish were present over a relatively long period of time from May to August, and almost all male fish examined during July to August had matured testes. The proportion of RM was low during the winter (February–March), increased in summer (April–May) and reaching its maximum at monsoon (July–August). Subsequently, as winter approaches the RM declined. This indicates that this fish breeds during rainy months, as most important spawning cues for breeding are flooding (Ingram et al. 2005). Within the five stages of testicular development, the highest and lowest proportion of RM was found in fish with testes at stage IV and II, respectively. Approximately 90 % of fish with testes at stage IV released sperm. However, RM was not found in fishes with stage I testes, which indicates that this species is a seasonal spawner and spawns only for few months during a year, which is in contrast to the observation of Ismail et al. (2011) in T. tambroides.
The I G in T. putitora increased from 0.43 % at the beginning of the gonadal development (stage I) to 1.68 % at maturity (stage IV). The same evolution in I G has been reported in C. pallasii (Koya et al. 2002), where I G increased from 0.38 % at the beginning of the testicular development to 17.20 % at maturity. In C. gariepinus (Cavaco et al. 1997a, b), the I G varied from approximately 0.1 % in immature male to 9 % in matured male. Similarly, Adebiyi et al. (2013) found higher I G (7.06 %) in mature river catfish, Hemibagrus nemurus. Such conspicuous variation in I G during spermatogenic cycle was also observed in H. hippoglossus L. (Weltzien et al. 2002), C. pallasii (Koya et al. 2002), V. variegates (Koya et al. 2003) and Japanese sardine Sardinops melanostictus (Matsuyama et al. 1991). In contrast, S. senegalensis (García-López et al. 2006) and giraffe nosed catfish, Auchenoglanis occidentalis (Shinkafi et al. 2011) showed slight changes in I G throughout the spermatogenetic cycle. In T. putitora, the I G increased a little from stage I to stage II, probably caused by cellular proliferation due to initiation of meiosis. From stage II to III, the I G showed a significant increase, probably in association with further accumulation of germ cells of various types. At stage IV, the I G increased considerably, which may be due to the excessive secretion of seminal fluid (observed mainly at anterior part of the gonad) in the testis of the fish after spermiogenesis. This was further supported by the observation that I G was significantly higher in RM in comparison with non-RM. At post-spawning phase, when the sperm was released, the I G gradually declined.
During spermatogenesis, the energy is mobilised from liver to gonad for development. In our study, the I H did not varied significantly throughout the spermatogenetic cycle. However, the values obtained for K suggest an inverse relationship between this factor and testis development, which confirms the influence of spermatogenesis on the physiological condition of the male fish. Similar results were obtained in Astyanax fasciatus (Gurgel 2004), which indicates a reduction in K during the reproductive cycle. This may be due to the use of energy reserves at the time of gonad development and reduction in feeding activity.
In teleosts, 11-KT, T and 17,20β-P are the main sex steroids known to control the initiation and progression of spermatogenesis (Koya et al. 2003). 11-KT stimulates the development of secondary sexual characteristics, spermatogonial proliferation and spermiation (Lintelmann et al. 2003), whereas T, a biosynthetic precursor of 11-KT, stimulates spermatogenesis (Fostier et al. 1983). 17,20β-P induces sperm motility (Miura et al. 1992). In the present study, plasma levels of 11-KT were being quantitatively higher than the T, as previously reported in few other fishes (Liu et al. 1991; Sol et al. 1998; Weltzien et al. 2002; García-López et al. 2006). During our study, increase in plasma titre of 11-KT was detected at late spermatogenesis (stage III), which indicates the onset of spermatozoa maturation and release of milt. Plasma 11-KT progressively increased and reached peak in association with the appearance of spermiating/running male fish (stage IV testes of T. putitora), which suggests the physiological role of 11-KT in spermiation rather than to spermatogenesis. Thereafter, the level of this androgen declined concomitantly with the appearance of post-spawning phase of spermatogenesis (stage V). The similar progression in 11-KT has also been observed in H. Hippoglossus (Weltzien et al. 2002) and in C. gariepinus (Cavaco et al. 1997a, b). The peak level of plasma 11-KT in this study (4.23 ng/mL) was quantitatively higher than the plasma level of 11-KT in river catfish Hemibagrus nemurus (0.017 ng/mL, Adebiyi et al. 2013) and T. tambroides (0.7 ng/mL, Ismail et al. 2011). However, the serum level of 11-KT in T. putitora was comparable with other fishes such as V. variegates (2.8 ng/mL, Koya et al. 2003), C. pallasii (6.58 ng/mL, Koya et al. 2002) and H. hippoglossus (4.0 ng/mL, Weltzien et al. 2002).
Plasma T levels gradually increased concomitantly in association with the progression of spermatogenesis (stage II and III) and peaked in late spermatogenic males. This situation differs from pleuronectiformes, in which peak levels of T were detected, when the testes accumulate a large quantity of SPZ (Harmin et al. 1995; Sol et al. 1998; Weltzien et al. 2002). Though such differences are not well understood, it may be related to the type of spermatogenesis and its sex steroid control. In T. putitora, the plasma T level gradually declined at the onset of spermiation and also in post-spawning phase. This suggests that in male golden mahseer, the physiological role of T may be related to spermatogenesis rather than to spermiation. This is in concurrence with earlier reports, where T is elevated during spermatogenesis, and falls on the spermiation stage (Fostier et al. 1983). The fall in T at spermiation may be due to the conversion of T into 17α,20β-P (Baynes and Scott 1985) or the synthesis of downstream metabolites of T (Matsuyama et al. 1991). Several previous information from other teleosts (Matsuyama et al. 1991) supports this concept that T possibly plays a role in the initiation and maintenance of spermatogenesis. Moreover, in golden mahseer, it remains to be investigated whether the androgens (T and 11-KT) are mainly produced by the testes, or also whether non-testicular tissues such as adrenal gland and inter-renal tissue can be a source of androgens (Mayer et al. 1990; Vermeulen et al. 1995; Cavaco et al. 1997a, b) and have the capacity of androgen production and conversion. In addition, the role of androgens in development of secondary sexual characteristic in maturing T. putitora also needs to be investigated.
Generally, 17,20β-P is considered as a maturation-inducing steroid in a number of fishes (Canario and Scott 1990; Canario 1991; Scott and Canario 1992) and involved in maturation of germ cells and spermiation (Nagahama 1994). Administration of this steroid induces spermiation and sperm mobility in salmonids (Miura et al. 1992) and black porgy, Acanthopagrus schlegelii (Yueh and Chang 1997). Peak in 17,20β-P during the periods of spermiation was also reported in sole (Canario 1991; García-López et al. 2006). The serum levels of 17,20β-P were considerably high (0.7–1.3 ng/mL) in our study and comparable to the observations in S. senegalensis (García-López et al. 2006). Its peak in concentration (1.3 ng/mL) was observed at spermiation stage, suggesting that this steroid may be the maturation-inducing steroid in T. putitora as suggested for S. senegalensis (García-López et al. 2006) and S. solea (Canario 1991). Moreover, in our study plasma levels of this steroid show significant variations and interestingly remained at relatively high concentrations throughout the testicular developmental stages. In contrast, study conducted by Degani et al. (1998) in male carp, Cyprinus carpio, reported that no significant changes was recorded for blood plasma of 17,20β-P during the breeding season. Although the exact cause of high 17,20β-P in the blood plasma of T. putitora could not be understand, it could possibly be related to the transformation of SPD into SPZ into successive batches (a pulsatile hormonal control may be acting) and also could be related to the short duration of 17,20β-P in the bloodstream of this fish (Rinchard et al. 1997), due to metabolisation (Scott and Canario 1992). Unfortunately, there are no reports describing previously the level and dynamics of this steroid in Tor species or other cyprinids to verify this hypothesis, and therefore, more precise and detailed studies must be carried out to determine the role and evolution of 17,20β-P in golden mahseer.
Conclusion
The current study describes testicular development stages of male T. putitora in terms of spermatogenetic cycle in relation to changes in plasma levels of 11-KT, T and 17,20β-P. Dynamics of plasma level of sex steroid reflected changes in I G and testicular histology, suggesting their significant role in regulation of spermatogenetic cycle of this fish. T. putitora males were also found to exhibit ‘associated reproductive pattern’ (Scott et al. 1980; Billard et al. 1982), which means that spawning occurs at the peak of gonadal activity. However, additional studies targeting on identifying the physiological effects and mechanism of sex steroid are urgently required, which will help to develop a captive breeding programme for this endangered species. To our knowledge, this is the first study relating the testicular development by gonad histology and sex hormone levels in T. putitora.
References
Adebiyi FA, Siraj SS, Harmin SA, Christianus A (2013) Plasma sex steroid hormonal profile and gonad histology during the annual reproductive cycle of river catfish Hemibagrus nemurus (Valenciennes, 1840) in captivity. Fish Physiol Biochem 39:547–557
Allen TC (1993) Haematoxylin and eosin. In: Phophet EB, Mills B, Arrington JB, Sobin MD (eds) Laboratory methods in histotechnology. American Registry of Pathology, Washington, pp 53–58
Aramli MS, Kalbassi MR, Nazari RM (2014) Sex steroid levels of Persian sturgeon, Acipenser persicus, Borodin, 1897, males in negative and positive responding to LH–RH-analogue. J Appl Ichthyol 30:18–19
Baynes SM, Scott AP (1985) Seasonal variations in parameters of milt production and in plasma concentration of sex steroids of male rainbow trout (Salmo gairdneri). Gen Comp Endocrinol 57:150–160
Bhatt JP, Nautiyal P, Singh HR (2000) Population structure of Himalayan mahseer, a large cyprinid fish in the regulated foothill section of the river Ganga. Fish Res 44:267–271
Billard R, Fostier A, Weil C, Breton B (1982) Endocrine control of spermatogenesis in Teleost fish. Can J Fish Aquat Sci 39:65–79
Borg B (1994) Androgens in teleost fishes. Comp Biochem Physiol 109C:219–245
Canario AVM (1991) Sex steroids in marine flatfish. In: Scott AP, Sumpter JP, Kime DE, Rolfe MS (eds) Proceedings of the fourth international symposium on the reproductive physiology of fish. FishSymp91, Sheffield pp 224–234
Canario AVM, Scott AP (1990) Effects of steroids and human chorionic gonadotropin on in vitro oocyte final maturation in two marine flatfish: the dab, Limanda limanda, and the plaice, Pleuronectes platessa. Gen Comp Endocrinol 77:161–176
Cavaco JEB, Vischer HF, Lambert JGD, Goos HJTh, Schulz RE (1997a) Mismatch between patterns of circulating and testicular androgens in African catfish, Clarias gariepinus. Fish Physiol Biochem 17:155–162
Cavaco JEB, Lambert JGD, Schulz RW, Goos HJTh (1997b) Pubertal development of male African catfish, Clarias gariepinus. In vitro steroidogenesis by testis and interregnal tissue and plasma levels of sexual steroids. Fish Physiol Biochem 16:129–138
Chaves-Pozo E, Arjona FJ, García-López A, García-Alcázar A, Meseguer J, García-Ayala A (2008) Sex steroids and metabolic parameter levels in a seasonal breeding fish (Sparus aurata L.). Gen Comp Endocrinol 156:531–536
Cuisset B, Pradelles P, Kime DE, Kuhn ER, Babin P, Le Menn F (1994) Enzyme immune assay for 11-ketotestosterone using acetylcholinesterase as label. Application to measurement of 11-ketotestosterone in plasma of Siberian sturgeon. Comp Biochem Phys C 108:229–241
De Rooij DG (2006) Regulation of spermatogonial stem cells behaviour in vivo and in vitro. Anim Reprod 3:130–134
Degani G, Boker R, Jackson K (1998) Growth hormone, sexual maturity and steroids in male carp (Cyprinus carpio). Comp Biochem Phys C 120:433–440
Dinesh K, Nandeesha MC, Nautiyal P, Aiyappa P (2010) Mahseers in India: a review with focus on conservation and management. Indian J Anim Sci 80:26–38
Estay F, Diaz A, Pendrazza R, Colihueque N (2003) Oogenesis and plasma levels of sex steroids in cultured females of brown trout (Salmo trutta, Linnaeus, 1758) in Chile. J Exp Zool 298A:60–66
Fostier A, Jalabert B, Billard R, Breton B, Zohar Y (1983) The gonadal steroids. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology IX A. Academic Press, New York, pp 277–345
Froese R, Pauly D (Ed) (2011) Fishbase. World Wide Web electronic publication. http://fishbase.org. Accessed 17 April 2014
García-López Ά, Fernández-Pasquier V, Couto E, Canario AVM, Sarasquete C, Martínez-Rodríguez G (2006) Testicular development and plasma sex steroid levels in cultured male Senegalese sol Solea senegalensis Kaup. Gen Comp Endocrinol 147:343–351
Gurgel HCB (2004) Population structure and breeding season of Astyanax fasciatus (Cuvier) (Characidae, Tetragonopterinae) from Ceara Mirim River, Poco Branco, Rio Grande do Norte, Brazil. Rev Bras Zool 21:131–135
Harmin SA, Crim LW, Wiegand MD (1995) Plasma sex steroid profiles and the seasonal reproductive cycle in male and female winter flounder, Pleuronectes americanus. Mar Biol 121:601–610
Ingram B, Sungan S, Gooley G, Sim SY, Tinggi D, De Silva S (2005) Induced spawning, larval rearing of two indigenous Malaysia Mahseer, Tor tromboides and Tor douronensis. Aquac Res 36:1001–1014
Islam SM, Tanaka M (2007) Threatened fishes of the world: Tor putitora Hamilton 1822 (Cypriniformes: Cyprinidae). Environ Biol Fish 78:219–220
Ismail MFS, Siraj SS, Daud SK, Harmin SA (2011) Association of annual hormonal profile with gonad maturity of mahseer (Tor tambroides) in captivity. Gen Comp Endocrinol 170:125–130
Jayaram KC (2010) The freshwater fishes of Indian region. Narendra Publishing House, New Delhi
Koya Y, Soyano K, Yamamoto K, Obana H, Matsubara T (2002) Testicular development and serum profiles of steroid hormone levels in captive male Pacific herring Clupea pallasii during their first maturational cycle. Fish Sci 68:1099–1105
Koya Y, Watanabe H, Soyano K, Ohta K, Aritaki M, Matsubara T (2003) Testicular development and serum steroid hormone levels in captive male spotted halibut Verasper variegates. Fish Sci 69:792–798
Lee WK, Yang SW (2002) Relationship between ovarian development and serum levels of gonadal steroid hormones and induction of oocyte maturation and ovulation in the cultured female Korean spotted sea bass Lateolabrax maculates (Jeom-nongoeo). Aquaculture 207:169–183
Lintelmann J, Katayama A, Kurihara N, Shore L, Wenzel A (2003) Endocrine disruptors in the environment. Pure Appl Chem 75:631–681
Liu HW, Stickney RR, Dickhoff WW (1991) Changes in plasma concentrations of sex steroids in adult Pacific halibut, Hippoglossus stenolepis. J World Aquac Soc 22:30–35
Matsuyama M, Adachi S, Nagahama Y, Kitajima C, Matsuura S (1991) Testicular development and serum levels of gonadal steroids during the annual reproductive cycle of captive Japanese sardine. Jpn J Ichthyol 37:381–390
Mayer I, Borg B, Schulz R (1990) Conversion of 11-ketoandrostenedione to 11-ketotestosterone by blood cells of six fish species. Gen Comp Endocrinol 77:70–74
Miura T, Yamauchi K, Takahashi H, Nagahama Y (1991) Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc Natl Acad Sci USA 88:5774–5778
Miura T, Yamauchi K, Takahashi T, Nagahama Y (1992) The role of hormones in the acquisition of sperm mobility in salmonid fish. J Exp Zool 261:359–363
Nagahama Y (1994) Endocrine regulation of gametogenesis in fish. Int J Dev Biol 38:217–229
Nash JP, Davail-Cuisset B, Bhattacharyya S, Suter H, Le Menn F, Kime DE (2000) An enzyme linked immunosorbent assay (ELISA) for testosterone, 17β-estradiol and 17α,20β-dihydroxy-4-pregnen-3-one using acetylcholinesterase as tracer: application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 22:355–363
Nautiyal P (1984) Natural history of the Garhwal Himalayan mahseer Tor putitora (Hamilton) breeding biology. Proc Anim Sci 93:97–106
Nikolsky GV (1963) Ecology of fishes. Academic Press, London, p 352
Oliver K, Sangma V, Basavaraja N (2007) Deccan Mahseer (Tor khudree) of Karnataka-on location of its wild brooders and fry and breakthrough in the hatchery production of its seed. Fish Chim 26:32–36
Pandey AK, Patiyal RS, Upadhyay JC, Tyagi M, Mahanta PC (1998) Induced spawning of the endangered golden mahseer, Tor putitora, with ovaprim at the state fish farm near Dehradun. Indian J Fish 45:457–459
Rinchard J, Kestemont P, Heine R (1997) Comparative study of reproductive biology in single and multiple spawner cyprinid fish. II. Sex steroid and plasma protein phosphorus concentrations. J Fish Biol 50:169–180
Rosenblum PM, Pudney J, Callard IP (1987) Gonadal morphology, enzyme histochemistry and plasma steroid levels during the annual reproductive cycle of male and female brown bullhead catfish, Ictalurus nebulosus Lesueur. J Fish Biol 31:325–341
Santana JCO, Quagio-Grassiotto I (2014) Extracellular matrix remodelling of the testes through the male reproductive cycle in Teleostei fish. Fish Physiol Biochem. doi:10.1007/s10695-014-9974-z
Schulz RW (2003) Endocrine regulation of spermatogenesis in teleost fish. ARBS Annu Rev Biomed Sci 5:57–68
Schulz RW, Miura T (2002) Spermatogenesis and its endocrine regulation. Fish Physiol Biochem 26:43–56
Schulz RW, de Franca LR, Lareyre JJ, LeGac F, Garcia HC, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Scott AP, Canario AV (1992) 17α,20β-Dihydroxi-4-pregnon-3-one 20 sulphate: a major new metabolite of the teleost oocyte maturation inducing steroid. Gen Comp Endocrinol 85:91–100
Scott AP, Bye VJ, Baynes SM, Springate JRC (1980) Seasonal variations in plasma concentrations of 11-ketotestosterone and testosterone in male rainbow trout, Salmo gairdnerii Richardson. J Fish Biol 17:495–505
Shinkafi BA, Ipinjolu JK, Hassan WA (2011) Gonad maturation stages of Auchenoglanis occidentalis (Valenciennes 1840) in River Rima, North-Western Nigeria. J Fish Aquat Sci 6:236–246
Sol SY, Olson OP, Lomax DP, Johnson LL (1998) Gonadal development and associated changes in plasma reproductive steroids in English sol, Pleuronectes vetulus, from Puget Sound, Washington. Fish B NOAA 96:859–870
Vermeulen GJ, Lambert JGD, Teitsma CA, Zandbergen MA, Goos HJTh (1995) Adrenal tissue in the male African catfish, Clarias gariepinus: localization and steroid hormone secretion. Cell Tissue Res 280:653–657
Weltzien FA, Taranger GL, Karlsen Ø, Norberg B (2002) Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Phys A 132:567–575
Yueh WS, Chang CF (1997) 17α,20β,21-Trihydroxy-4-pregnen-3-one and 17α,20β-dihydroxy-4-pregnen-3-one stimulated spermiation in protandrous black porgy, Acanthopagrus schlegeli. Fish Physiol Biochem 17:187–193
Acknowledgments
This research was supported by a project grant from Indian Council of Agricultural Research (ICAR), New Delhi, India, to first author (NS). The authors acknowledge Monalisa Sahoo of Indian Veterinary Research Institute (IVRI), Bareilly, for her assistance in histological preparations and data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahi, N., Mallik, S.K., Pande, J. et al. Spermatogenesis and related plasma androgen and progestin level in wild male golden mahseer, Tor putitora (Hamilton, 1822), during the spawning season. Fish Physiol Biochem 41, 909–920 (2015). https://doi.org/10.1007/s10695-015-0057-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0057-6