Abstract
In the present study, we tested the hypothesis that dietary histidine could improve antioxidant capacity of juvenile Jian carp (Cyprinus carpio var. Jian). A total of 1,200 juvenile Jian carp were fed graded levels of histidine at 2.3 (unsupplemented control), 4.4, 6.3, 8.6, 10.8 and 12.7 g/kg diet for 60 days. Results showed that the content of malondialdehyde (MDA) and protein carbonyl (PC) in serum and all tissues apparently decreased with increasing histidine levels up to an optimal level and increased thereafter. Anti-superoxide anion (ASA) capacity, glutathione peroxidase (GPX) activities and glutathione (GSH) content in serum and all tissues, anti-hydroxyl radical (a-HR) capacity, catalase (CAT) and glutathione-S-transferase (GST) activities in serum, muscle and intestine, superoxide dismutase (SOD) activities in serum and intestine, as well as glutathione reductase (GR) activity in serum, muscle and hepatopancreas were improved by dietary histidine. Fish fed diet with 8.6 g/kg histidine had lower serum glutamate-pyruvate transaminase (GPT) activity than that fed with control diet, whereas pattern of glutamate–oxaloacetate transaminase (GOT) activity was opposite. The present results suggested that histidine could improve antioxidant capacity and inhibit lipid peroxidation and protein oxidation of juvenile Jian carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Histidine is one of the essential amino acids for fish, for example, Pacific salmon (Oncorhynchus shawytscha) (Halver et al. 1957), Indian major carp (Cirrhinus mrigala) (Ahmed and Khan 2005) and Atlantic salmon (Salmo salar L.) (Breck et al. 2005) and many other species. Our previous study in juvenile Jian carp (Cyprinus carpio var. Jian) indicated that dietary histidine promoted growth, increased protein deposition and improved digestion and absorption ability, which may be partly related to its beneficial effects on intestine and hepatopancreas growth (Zhao et al. 2012). The growth and development of tissue and organs in fish rely on the structural integrity of cells (Hamlin et al. 2000). Chen et al. (2009) noted that the structural and functional integrity of intestinal epithelial cells in carp were associated with their antioxidant status. Our laboratory studies have demonstrated that the antioxidant status of Jian carp could be affected by glutamine (Lin and Zhou 2006) and methionine hydroxy analogue (Feng et al. 2011). However, only a little was known about the relationship between histidine and antioxidant defense of fish. It has been reported that histidine had an inhibition effect on lipid peroxidation in fish muscle sarcoplasmic reticulum suspension experimental system in vitro (Erickson and Hultin 1988, 1992). It suggested that dietary histidine may have beneficial effects on fish antioxidant status, which needs to be investigated.
Most components of cellular structure are likely to be the potential targets of reactive oxygen species (ROS), and the most susceptible substrates for oxidation are polyunsaturated fatty acids in the biomembrane (Mourente et al. 2007). On mammals, the lipid oxidation levels in kidney and liver of mice decreased with the administration of histidine (Lee et al. 2005). Histidine was also effective in inhibiting the oxidation of low-density lipoprotein in bovine serum (Decker et al. 2001). The effects of histidine on lipid peroxidation inhibition may be related to the interaction with toxic oxygen species (Wade and Tucker 1998). Hydroxyl radical (·OH), one kind of toxic oxygen species, can react with a wide variety of biomolecules and lead to oxidative damage of membrane lipids, proteins and nucleic acids (Mourente et al. 2007). Meanwhile, singlet oxygen (1O2) is a biologically nonradical toxic oxygen species that is highly reactive and potentially deleterious to biological systems (Wade and Tucker 1998). It has been demonstrated that histidine has preventive effects on ·OH generation in extracellular fluid of rat striatum (Obata et al. 2001). Besides, histidine has been recognized as a scavenger of the ·OH by interfering with the redox reactions (Nagy and Floyd 1984; Wade and Tucker 1998) and 1O2 by direct interactions with the imidazole ring (Foote and Clennan 1995; Wade and Tucker 1998). Moreover, toxic oxygen species or free radicals are considered to cause extensive oxidative damage to cells (Mourente et al. 2007). To prevent oxidative damage, fish have developed antioxidant defense system, which is mainly constituted of antioxidant enzymes and non-enzyme antioxidant (Martínez-Álvarez et al. 2005; Shiau and Hsu 2002). Antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and enzymes dependent on glutathione (glutathione peroxidase, GPX, glutathione reductase, GR and glutathione-S-Transferase, GST), serve as crucial part in antioxidant system (Martínez-Álvarez et al. 2005). Glutathione (GSH), the most abundant thiol-containing substance of low molecular weight in cells, is an effective non-enzyme antioxidant against free radicals and other oxidants (Mourente et al. 2007). To our knowledge, no research has been conducted to study the effect of histidine on the activities of antioxidant enzymes and GSH content in fish. Studies in mice have demonstrated that histidine supplement elevated CAT and GPX activities in kidney and liver (Lee et al. 2005; Liu et al. 2008). Post-intake of histidine increased GSH content in liver of mice (Liu et al. 2008). Taken together, dietary histidine improved the structure and function of fish tissues and organs may be partly related to the improvement of antioxidant defense, which warrants further investigation.
We conducted a series of studies to explore the effects of dietary histidine on juvenile Jian carp. Part 1 investigated the effects of histidine on growth performance, digestive and absorptive capacity of Jian carp (Zhao et al. 2012). This study was the second part, which shared the same growth trial with part 1, and the aim was to study the effects of dietary histidine on antioxidant capacity of Jian carp in serum, muscle, intestine and hepatopancreas. The present data can partly provide theoretical evidence for the effects of histidine on growth, protein deposition, digestive and absorptive ability of fish.
Materials and methods
Experimental diets
Formulation of the basal diet was the same as our previous study (Zhao et al. 2012) and was presented in Table 1. Fish meal (Pesquera Lota Protein Ltd., Villagram, Chile) and gelatin (Rousselot Gelatin Co., Ltd., Guangdong, China) were used as dietary protein sources. Crystalline amino acids (Jiangsu Nantong Eastern Amino Acid Co. Ltd., Nantong, China) were used to simulate the amino acid profile of diets with 34 g/kg whole chicken egg protein, except for histidine. The histidine concentrations in fish meal and gelatin were measured before the formulation by the method of Llames and Fontaine (1994). Experimental diets were supplemented with l-histidine hydrochloride monohydrate to provide histidine levels at 2.5 (unsupplemented control), 4.5, 6.5, 8.5, 10.5, 12.5 g/kg diet. All diets were made isonitrogenous with the addition of appropriate amounts of glycine. Zinc, ferrum, pyridoxine, pantothenic acid, inositol, thiamin and riboflavin were formulated to meet the nutrient requirements of Jian carp according to our laboratory’s studies (He et al. 2009; Wen et al. 2009; Jiang et al. 2009a, b; Li et al. 2010; Huang et al. 2011; Tan et al. 2011; Ling et al. 2010). The levels of other nutrients met the requirements for common carp according to the NRC (1993). Procedures for diet preparation and storage were the same as our previous study (Zhao et al. 2012). The histidine concentration in the experimental diets were measured to be 2.3 (unsupplemented control), 4.4, 6.3, 8.6, 10.8 and 12.7 g/kg diet as described by Llames and Fontaine (1994).
Feeding management
Juvenile Jian carp were obtained from Tong Wei Hatchery (Sichuan, China). Feeding management was the same as described in our previous study (Zhao et al. 2012). Fish were adapted to the experimental environment for 4 weeks. A total of 1,200 fish with an average initial weight of 8.76 ± 0.02 g were randomly assigned to 24 experimental aquaria (90 L × 30 W × 40 H cm), each of which was connected to a closed recirculating water system with continuous aeration. Feeding management was conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Animal Nutritional Institute, Sichuan Agricultural University. Water change rates in each aquarium were maintained at 1.2 L/min, and the water was drained through biofilters in order to decrease microorganism, reduce ammonia concentration and remove solid substances in the water. Dissolved oxygen was higher than 5 mg/L; temperature and pH of the water were maintained at 26 ± 1 and 7.0 ± 0.3 °C, respectively. The experimental units were maintained under natural light and dark cycle. For the feeding trial, each of six experimental diets was fed to quadruplicate of fish six times daily from 1 to 30 days and four times daily from 31 to 60 days. Fish were fed to satiation, and uneaten feed was removed by siphoning after each meal.
Sample collection and analysis
At the end of the feeding trial, fish were anaesthetized in a benzocaine bath (50 mg/L) 12 h after the last feeding according to the method described by Bohne et al. (2007). Blood of 15 fish collected from each aquarium was drawn from the caudal vein into heparinized syringes, stored at 4 °C overnight, and centrifuged at 3,000×g at 4 °C for 10 min, then stored at −70 °C until analyzed. The hepatopancreas, intestine and muscle of the same 15 fish were removed, weighed and frozen in liquid nitrogen, then stored at −70 °C until analyzed. Tissue samples of six fish from each aquarium were homogenized in 10 volumes (w/v) of ice-cold physiological saline and centrifuged at 6,000×g at 4 °C for 20 min respectively, and then, supernatants were collected for antioxidant parameters analysis. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Sichuan Agricultural University.
The protein concentration of tissue samples were determined by the method of Bradford (1976). Malondialdehyde (MDA) content was assayed as described by Livingstone et al. (1990) using the thiobarbituric acid reaction. Protein carbonyl (PC) content was determined according to the method described by Armenteros et al. (2009). The protein carbonyl content was calculated from the peak absorbance at 370 nm, using an absorption coefficient of 21,000/M cm. The anti-superoxide anion (ASA) capacity (O ·–2 -scavenging ability) and anti-hydroxyl radical (a-HR) capacity (·OH-scavenging ability) were determined by the method described by Zhang et al. (2005) and Jiang et al. (2010), respectively. Superoxide dismutase (SOD) and glutathione peroxidase (GPX) activities were assayed as described by Zhang et al. (2008). Catalase (CAT) activity was determined by the decomposition of hydrogen peroxide (Aebi 1984). Glutathione-S-transferase (GST) activity was measured by monitoring the formation of adduct between GSH and 1-chloro-2, 4-dinitrobenzene (CDNB) (Lushchak et al. 2001). Glutathione reductase (GR) activity was measured according to the method described by Lora et al. (2004). GSH content was determined as described by Vardi et al. (2008). GOT and GPT activities in serum were determined by the method of Bergmeyer and Bernt (1974a, b), respectively.
Calculations and statistical analysis
Results were presented as mean ± SD. All data were subjected to a one-way analysis of variance (ANOVA) followed by the Duncan’s multiple-range test to determine significant differences among treatment means at the level of P < 0.05 through SPSS 13.0 (SPSS Inc., Chicago, USA). The parameters with significant differences were subjected to a second-degree polynomial regression analysis.
Results
Serum antioxidant parameters
Effects of graded levels of dietary histidine on antioxidant parameters in serum of juvenile Jian carp are given in Table 2 and 3. MDA and PC content significantly decreased with increasing histidine levels up to 10.8 and 8.6 g/kg, respectively, and increased thereafter (P < 0.05). ASA capacity was the lowest in fish fed the diet with histidine concentration at 2.3 g/kg (unsupplemented control). a-HR capacity was improved with the increase in dietary histidine levels and was significantly higher in fish fed diets with 4.4, 6.3 and 8.6 g/kg histidine compared with other groups (P < 0.05). SOD activities in fish fed diets with 10.8 and 12.7 g/kg histidine were significantly higher than that in fish fed other diets (P < 0.05). The CAT, GPX, GST, GR activities and GSH content were significantly enhanced with the increase in dietary histidine levels up to 10.8, 6.3, 8.6, 8.6 and 8.6 g/kg, respectively, and decreased with further increase in dietary histidine concentration (P < 0.05). The relationship between serum a-HR capacity, CAT activity, GSH content and dietary histidine levels were described by the following quadratic equation: Y a-HR = −5.2929x 2 + 75.727x + 64.55, R 2 = 0.904, P < 0.05; Y CAT = −0.0585x 2 + 0.9881x + 1.3566, R 2 = 0.976, P < 0.01; Y GSH = −0.1685x 2 + 2.635x + 1.7548, R 2 = 0.970, P < 0.01.
Muscle antioxidant parameters
MDA, PC, and GSH content, ASA and a-HR capacities, SOD, CAT, GPX, GST, GR activities and in muscle are presented in Table 4 and 5. MDA content of the fish fed diet containing 2.3 g/kg (unsupplemented control) histidine was found to be significantly higher than those fed other dietary levels (P < 0.05), while no significant differences among other groups were evident (P > 0.05). PC content significantly decreased with dietary histidine levels up to 8.6 g/kg and significantly increased thereafter (P < 0.05). ASA capacity was significantly improved with increasing dietary histidine levels up to 8.6 g/kg diet (P < 0.05) and plateaued thereafter (P > 0.05). a-HR capacity followed a similar pattern to that observed in ASA capacity. There was no significant difference in SOD activities among the groups (P > 0.05). CAT, GPX and GR activities significantly increased with increasing dietary histidine levels up to 6.3 g/kg, after that significantly decreased (P < 0.05). GST activity was minimal in fish fed diet with 2.3 g/kg histidine and was maximum in fish fed diet with 8.6 g/kg histidine. Fish fed diets of histidine levels at 2.3 and 12.7 g/kg diet had lower GSH content than fish fed other diets. Regression analysis showed that muscle MDA, PC content, ASA capacity, GST activity and GSH content were quadratic response to graded levels of dietary histidine (Y MDA = 0.0146x 2 − 0.2699x + 3.7818, R 2 = 0.967, P < 0.01; Y PC = 0.0129x 2 − 0.1646x + 1.8524, R 2 = 0.861, P = 0.051; Y ASA = −0.011x 2 + 0.8036x + 30.935, R 2 = 0.903, P < 0.05; Y GST = −0.4353x 2 + 7.7412x + 161.9, R 2 = 0.875, P < 0.05; Y GSH = −0.1352x 2 + 1.9996x + 10.57, R 2 = 0.852, P = 0.060).
Intestine antioxidant parameters
As shown in Table 6, MDA and PC content, ASA and a-HR capacities in intestine were significantly affected by dietary histidine. MDA content was reduced with the increase in dietary histidine levels up to 8.6 g/kg diet and then significantly increased (P < 0.05). PC content was the lowest for fish fed diet containing 4.4, 6.3 and 8.6 g/kg histidine and was the highest for fish fed diet with 12.7 g/kg histidine. Both ASA and a-HR capacities were significantly improved with increasing dietary histidine levels up to 8.6 g/kg diet and declined thereafter (P < 0.05). Effects of graded levels of dietary histidine on SOD, CAT, GPX, GST, GR activities and GSH content in intestine are given in Table 7. SOD activities were the lowest in fish fed diet with 2.3 g/kg histidine and were the highest in fish fed with 8.6 and 10.8 g/kg dietary histidine. CAT and GPX activities were significantly enhanced with the increase in dietary histidine levels up to 8.6 and 6.3 g/kg diet, respectively, and decreased thereafter (P < 0.05). Fish fed diets with 6.3 and 8.6 g/kg histidine had significantly higher GST activities than those fed other diets (P < 0.05). No significant differences in GR activities among the groups were evident (P > 0.05). The GSH content was significantly lower in fish fed the diet with histidine concentration at 2.3 g/kg (unsupplemented control) than that in other groups (P < 0.05). The second-degree polynomial regression equations about the relationship between intestinal PC content, SOD, CAT, GST activity and dietary histidine levels were presented as following: Y PC = 0.0137x 2 − 0.1668x + 2.645, R 2 = 0.908, P < 0.05; Y SOD = −0.1685x 2 + 2.8623x + 32.701, R 2 = 0.868, P < 0.05; Y CAT = −0.751x 2 + 12.908x + 13.026, R 2 = 0.854, P = 0.056; Y GST = −3.2315x 2 + 46.649x + 39.586, R 2 = 0.920, P < 0.05.
Hepatopancreas antioxidant parameters
MDA and PC content, ASA and a-HR capacities in hepatopancreas of juvenile Jian carp fed diets containing graded levels of histidine are presented in Table 8. MDA content was the highest in fish fed diets with 2.3 and 4.4 g/kg histidine and the lowest in fish fed diet with 8.6 g/kg histidine. PC content significantly decreased with the increase in dietary histidine levels and was significantly lower in fish fed diets with 6.3 and 8.6 g/kg histidine compared with that in other groups (P < 0.05). ASA capacity was the highest in fish fed diet with 8.6 g/kg histidine, followed by those with 10.8 and 12.7 g/kg histidine, and was the lowest in fish fed diet of histidine level at 2.3 g/kg. No significant differences in a-HR capacities among the groups were found (P > 0.05). SOD, CAT, GPX, GST, GR activities and GSH content in hepatopancreas of juvenile Jian carp fed graded levels of dietary histidine are presented in Table 9. SOD activities were lowest in fish fed diets with 4.4, 6.3 and 8.6 g/kg histidine and were highest in fish fed diets containing 10.8 and 12.7 g/kg histidine. CAT activity was the highest in fish fed unsupplemented control diet and then significantly decreased (P < 0.05), while no significant differences were found among the other groups (P > 0.05). GST activities were gradually reduced with dietary histidine up to 6.3 g/kg diet and after that significantly increased (P < 0.05). GPX, GR activities and GSH content were significantly improved with the increase in dietary histidine levels up to 8.6 g/kg diet and decreased with levels further increasing (P < 0.05). Hepatopancreas PC content, ASA capacity, GPX and GR activity to dietary levels of histidine relationship were described by quadratic regression analysis: Y PC = 0.0255x 2 − 0.3713x + 2.3742, R 2 = 0.901, P < 0.05; Y ASA = −1.156x 2 + 27.308x + 278.21, R 2 = 0.907, P < 0.05; Y GPX = −2.7072x 2 + 41.234x + 17.337, R 2 = 0.911, P < 0.05, Y GR = = −1.4145x 2 + 18.868x + 52.031, R 2 = 0.923, P < 0.05.
Serum GOT and GPT activities
Effects of graded levels of dietary histidine on GOT and GPT activities in serum of juvenile Jian carp are shown in Table 10. GOT activities were lowest in fish fed diets with 2.3 and 4.4 g/kg histidine, and significantly increased with dietary histidine levels up to 10.8 g/kg diet and then decreased (P < 0.05). GPT activity was highest in fish fed the unsupplemented control diet and was lowest in those fed diet of histidine level at 8.6 g/kg. Regression analysis showed that serum GOT activity was quadratic response to graded levels of dietary histidine (Y GOT = 0.007x 2 + 1.5892x + 14.617, R 2 = 0.881, P < 0.05).
Histidine requirement
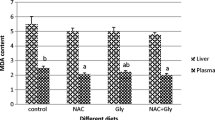
As shown in Fig. 1a, on subjecting the serum MDA content and dietary histidine levels to second-degree polynomial regression analysis, optimum histidine level was found at 9.2 g/kg diet or 2.80 g/100 g protein. The relationship was described by the following equation: Y MDA = 0.0743x 2 − 1.3717x + 14.122, R 2 = 0.933, P < 0.05. The PC content in serum to dietary levels of histidine relationship was described by quadratic regression analysis (Y PC = 0.9769x 2 − 16.89x + 116.49, R 2 = 0.932, P < 0.05) (Fig. 1b). Based on the above equation, the optimum histidine level was estimated to be 8.6 g/kg diet or 2.62 g/100 g protein.
Quadratic regression analysis of malondialdehyde (MDA) content (a) and protein carbonyl (PC) content (b) in serum for juvenile Jian carp (Cyprinus carpio var. Jian) fed diets containing graded levels of histidine for 60 days. Each point represents the mean of four replicates, with six fish per group. Optimal levels of dietary histidine for serum MDA and PC content were 9.2 and 8.6 g/kg diet, respectively
Discussion
Fish are a rich source of the n-3 polyunsaturated fatty acids (PUFA), which are vital constituents for cell membrane structure and function, but which are also highly susceptible to attack by oxygen and other organic radicals (Mourente et al. 2007). The highly unsaturated fatty acid composition of fish muscle makes it extremely susceptible to oxidation stress (Olsen and Henderson 1997). Intestine and pancreas are the main digestive organs for stomachless fish such as carp, and their antioxidant status play an important role for fish growth (Jiang et al. 2010). Study in vitro showed that histidine exerted an inhibition effect on lipid peroxidation in fish muscle sarcoplasmic reticulum suspension experimental system (Erickson and Hultin 1988, 1992). Therefore, the present study investigated the effects of dietary histidine on oxidative stress and antioxidant responses in serum, muscle, intestine and hepatopancreas of Jian carp.
Lipid peroxidation can be defined as the oxidative deterioration of PUFA and is an important consequence of oxidative stress, as indicated by the levels of malondialdehyde (MDA), which is a key metabolite production derived from lipid oxidation (Mourente et al. 2007). The current study showed that MDA content was reduced with histidine supplement in serum and tissues, which indicated that lipid peroxidation was suppressed by histidine. Similar results were documented with a fish muscle sarcoplasmic reticulum suspension experimental system in vitro (Erickson and Hultin 1988, 1992). Free radicals also catalyze the oxidation of amino acid residues in proteins, forming protein carbonyls (PC). PC content is the most widely used marker of oxidative modification of proteins (Uchida and Kawakishi 1993a). In our study, optimal level of dietary histidine decreased the PC content in serum and tissues, suggesting that protein oxidation were also inhibited by histidine. Decker et al. (2001) noted that histidine was effective in inhibiting formation of carbonyls on bovine serum albumin. It has been established that oxidative modification of proteins involves the conversion of amino acids to their oxidized forms and histidine residue is one of the major sites of damage during radical attack upon proteins, whereas supplement of histidine may donate itself to free radicals resulting in the stabilization of the protein (Uchida 2003). The findings of the present study suggest that histidine alleviated the oxidative damage of Jian carp in different tissues and organs and ensured the normal function of various tissues and organs as well as the whole body. Based on serum MDA and PC content data, the dietary histidine requirements of juvenile Jian carp were estimated to be 9.2 g/kg diet (2.80 g/100 g protein) and 8.6 g/kg diet (2.62 g/100 g protein), respectively, which were a little higher than that based on specific growth rate (7.8 g/kg diet or 2.38 g/100 g protein) (Zhao et al. 2012).
Increased ROS generation by monovalent reduction in cellular aerobic metabolism is responsible for increased oxidative injury to lipids and proteins (Livingstone 2003). Wade and Tucker (1998) implied that the effects of histidine to inhibit lipid peroxidation may be related to its interaction with toxic oxygen species. In this work, we determined the scavenging ability of histidine against superoxide radicals (O ·–2 ) and hydroxyl radical (·OH), two agents strongly involved in oxidative damage (Kohen and Nyska 2002). O ·–2 yielded from electron leakage in the mitochondrial respiratory transport chain imply a high toxicity to compound (Klotz and Sies 2009). In the present study, O ·–2 -scavenging ability (indicated by ASA capacity) was enhanced with histidine supplement in serum and tissues. Spectroscopic evidence has established the generation of singlet oxygen (1O2) in the water-induced dismutation of O ·–2 and in the electron transfer reaction or Haber–Weiss reaction of O ·–2 (Khan and Kasha 1994). Histidine has been recognized as an efficient quencher of 1O2 and may accelerate the consumption of O ·–2 through scavenging the product 1O2 (Foote and Clennan 1995). Thus, histidine may act as O ·–2 -scavenger indirectly through its interaction with 1O2. Furthermore, cellular O ·–2 is mainly reduced to form hydrogen peroxide (H2O2) and further reduced to generated ·OH (Klotz and Sies 2009). The present study showed that ·OH-scavenging ability (indicated by a-HR capacity) in serum, muscle and intestine were improved by dietary histidine. The effects of histidine to prevent ·OH generation or to eliminate ·OH had been established in vitro (Nagy and Floyd 1984; Obata et al. 2001). ·OH is generated from H2O2 via the Fenton reaction, which was mediated by divalent metal ion iron or copper (Klotz and Sies 2009). Histidine is an efficient chelator for copper and iron (Chevion 1988). Erickson and Hultin (1988, 1992) suggested that the effects of histidine to inhibit ·OH generation from its ability to coordinate with iron or copper, thus interfering with Fenton reaction that produce the ·OH. Histidine also could form a tight complex with H2O2 and thus lower the rate of ·OH formation (Chevion, 1988). Lipid peroxidation occurred when PUFA is attack directly by ·OH (Livingstone 2003). In this study, correlation analysis showed that MDA content was negative correlated to a-HR capacity in muscle (r = −0.760, P = 0.08) and in intestine (r = −0.671, P = 0.14). Therefore, the inhibition of lipid peroxidation by histidine may partly due to the promotion of ·OH-scavenging ability. In a word, histidine alleviated the oxidative damage in two main ways: decreasing the generation and/or increasing the elimination of ROS.
In the antioxidative defense of the host system, SOD catalyzes the conversion of O ·–2 to H2O2 and the dioxygen molecule (Khan and Kasha 1994). The present data showed that SOD activities were improved by dietary histidine in serum and intestine, whereas no difference in muscle. Tansini et al. (2004) reported SOD activity was not affected by histidine in brain of rats. Histidine residues serve as essential components in the active center of bovine erythrocyte Cu, Zn-SOD (Uchida and Kawakishi 1993b). When treated with its own reaction product H2O2, histidine residues at the active site were oxidized, which resulted in the inactivation of the enzyme (Uchida and Kawakishi 1993b). But whether dietary histidine supplementation can protect the histidine residues at the active site of Cu, Zn-SOD awaits investigation. The product of SOD dismutation, H2O2, can be removed by the CAT and GPX (Livingstone 2003). Our results demonstrated that CAT and GPX activities in serum, muscle and intestine as well as GPX activity in hepatopancreas were improved with dietary histidine up to an optimum level. Lee et al. (2005) and Liu et al. (2008) noted that histidine supplement elevated CAT and GPX activities in kidney and liver of mice. GST have a cytoprotective role involving elimination of reactive chemical species originating from the oxidative metabolism (Baez et al. 1997). In the present study, GST activity in serum, muscle and intestine were improved by histidine. Interestingly, our results showed that SOD, CAT and GST activities in hepatopancreas decreased with the increase in dietary histidine levels. a-HR capacity in hepatopancreas showed no difference among groups. A possible explanation could be that these antioxidant enzymes activities in hepatopancreas were inactivated by ROS. Growth rate was positive related to energy metabolism and amino acid metabolism, particularly in liver (Chessex et al. 1981). Our previous study has demonstrated that both specific growth rate and protein productive value exhibited positive quadratic relationship with dietary histidine levels (Zhao et al. 2012) O ·–2 is mainly generated from respiratory transport chain and 1O2 and ·OH were derived from O ·–2 (Khan and Kasha 1994). SOD and CAT could be inactivated by O ·–2 , 1O2 and ·OH (Pigeolet et al. 1990; Kim et al. 2001). Thus, fish with higher growth rate may associate with more ROS in liver, which thereby inhibited the activities of antioxidant enzymes. Serum glutamate–oxaloacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) activities are usually used as indicators of the function of vertebrate liver (Lin et al. 2010). The histology changes of liver are important indicators of the nutritional and physiological status of fish (Gatta et al. 2011). Chien et al. (2003) suggested GOT and GPT may be indirectly related to oxidant metabolites and also serve as indicators of oxidative status of liver. In the current study, Jian carp fed diet with 8.6 g/kg histidine had lower GPT activity than those fed control diet, while GOT activity in serum increased with the increase in dietary histidine levels. Liu et al. (2008) reported serum GOT and GPT activities were decreased with histidine supplementary in mice, whereas Easter and Baker (1977) reported that they were unaffected by histidine in gravid swine. The reason for these interesting results was not clear. Glutathione (GSH) is a tripeptide containing a thiol group and is an important protective non-enzyme antioxidant against free radicals and other oxidants (Rahman and MacNee 2000). The present study indicated that GSH content in serum and tissues was improved by histidine. Similar result was observed in liver of mice (Liu et al. 2008). GR catalyze the reduction of the oxidized of glutathione (GSSG) to GSH, at the expense of the NADPH (Reed 1990). The present work demonstrated that histidine was effective in promoting the activity of GR in serum, muscle and hepatopancreas. The elevation of GSH content may probably attribute to the promotion of GR activity. Our results suggested that histidine can promote antioxidant enzyme activity and non-enzyme antioxidant content, which contribute to the improvement of antioxidant capacity in Jian carp.
In summary, the present work showed that dietary histidine could elevate antioxidant enzymes activities and GSH content, enhance oxygen species-scavenging ability and thus inhibit lipid peroxidation and protein oxidation of Jian carp. The result of this study could partly provide theoretical evidence for the improvement of growth, protein deposition, digestion and absorption ability by histidine in our previous research. However, further studies should be carried out to reveal the underlying mechanisms of dietary histidine on antioxidant capacity of fish.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmed I, Khan MA (2005) Dietary histidine requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquacult Nutr 11:359–366
Armenteros M, Heinonen M, Ollilainen V, Toldrá F, Estévez M (2009) Analysis of protein carbonyls in meat products by using the DNPH-method, fluorescence spectroscopy and liquid chromatography-electrospray ionisation-mass spectrometry (LC-ESI-MS). Meat Sci 83:104–112
Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B (1997) Glutathione transferases catalyse the detoxication of oxidized metabolites (ο-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J 324:25–28
Bergmeyer HU, Bernt E (1974a) Glutamat-Oxalacetat-Transaminase. In: Bergmeyer HU (ed) Methoden der enzymatischen analyse, 3rd edn. Verlag Chemie, Weinheim, pp 769–775
Bergmeyer HU, Bernt E (1974b) Glutamat-Pyruvat-Transaminase. In: Bergmeyer HU (ed) Methoden der enzymatischen analyse, 3rd edn. Verlag Chemie, Weinheim, pp 785–791
Bohne VJB, Hamre K, Arukwe A (2007) Hepatic metabolism, phase I and II biotransformation enzymes in Atlantic salmon (Salmo salar, L.) during a 12 week feeding period with graded levels of the synthetic antioxidant, ethoxyquin. Food Chem Toxicol 45:733–746
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
Breck O, Bjerkås E, Campbell P, Rhodes JD, Sanderson J, Waagbø R (2005) Histidine nutrition and genotype affect cataract development in Atlantic salmon, Salmo salar L. J Fish Dis 28:357–371
Chen J, Zhou XQ, Feng L, Liu Y, Jiang J (2009) Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian). Aquaculture 288:285–289
Chessex P, Reichman BL, Verellen GJ, Putet G, Smith JM, Heim T, Swyer PR (1981) Influence of postnatal age, energy intake, and weight gain on energy metabolism in the very low-birth-weight infant. J Pediatr 99:761–766
Chevion M (1988) A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radical Bio Med 5:27–37
Chien YH, Pan CH, Hunter B (2003) The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture 216:177–191
Decker EA, Ivanov V, Zhu BZ, Frei B (2001) Inhibition of low-density lipoprotein oxidation by carnosine and histidine. J Agric Food Chem 49:511–516
Easter RA, Baker DH (1977) Nitrogen metabolism, tissue carnosine concentration and blood chemistry of gravid swine fed graded levels of histidine. J Nutr 107:120–125
Erickson MC, Hultin HO (1988) A unique role of histidine in Fe-catalyzed lipid oxidation by fish sarcoplasmic reticulum. Basic Life Sci 49:307–312
Erickson MC, Hultin HO (1992) Influence of histidine on lipid peroxidation in sarcoplasmic reticulum. Arch Biochem Biophys 292:427–432
Feng L, Xiao WW, Liu Y, Jiang J, Hu K, Jiang WD, Li SH, Zhou XQ (2011) Methionine hydroxy analogue prevents oxidative damage and improves antioxidant status of intestine and hepatopancreas for juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 17:595–604
Foote CS, Clennan EL (1995) Properties and reactions of singlet dioxygen. In: Foote CS, Valentine JS, Greenberg A, Liebman JF (eds) Active oxygen in chemistry. Chapman and Hall, London, pp 105–140
Gatta PP, Parma L, Guarniero I, Mandrioli L, Sirri R, Fontanillas R, Bonaldo A (2011) Growth, feed utilization and liver histology of juvenile common sole (Solea solea L.) fed isoenergetic diets with increasing protein levels. Aquac Res 42:313–321
Halver JE, Delong D, Mertz E (1957) Nutrition of salmonoid fishes. V. Classification of essential amino acids for Chinook salmon. J Nutr 63:95–105
Hamlin HJ, Herbing IHV, Kling LJ (2000) Histological and morphological evaluations of the digestive tract and associated organs of haddock throughout post-hatching ontogeny. J Fish Biol 57:716–732
He W, Feng L, Jiang J, Liu Y, Zhou XQ (2009) Dietary pyridoxine requirement of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 15:402–408
Huang HH, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Effects of dietary thiamin supplement on growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 17:233–240
Jiang J, Zheng T, Zhou XQ, Liu Y, Feng L (2009a) Influence of glutamine and vitamin E on growth and antioxidant capacity of fish enterocytes. Aquacult Nutr 15:409–414
Jiang WD, Feng L, Liu Y, Jiang J, Zhou XQ (2009b) Growth, digestive capacity and intestinal microflora of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary inositol. Aquac Res 40:955–962
Jiang WD, Feng L, Liu Y, Jiang J, Hu K, Li SH, Zhou XQ (2010) Lipid peroxidation, protein oxidant and antioxidant status of muscle, intestine and hepatopancreas for juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of myo-inositol. Food Chem 120:692–697
Khan AU, Kasha M (1994) Singlet molecular oxygen in the Haber-Weiss reaction. Proc Natl Acad Sci 91:12365–12367
Kim SY, Kwon OJ, Park JW (2001) Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie 83:437–444
Klotz LO, Sies H (2009) Cellular generation of oxidants: relation to oxidative stress. Red Sig Reg Biol Med 8:45–61
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions and methods for their quantification. Toxicol Pathol 6:620–650
Lee YT, Hsu CC, Lin MH, Liu KS, Yin MC (2005) Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol 513:145–150
Li W, Zhou XQ, Feng L, Jiang J, Liu Y (2010) Effect of dietary riboflavin on growth, feed utilization, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 16:137–143
Lin Y, Zhou XQ (2006) Dietary glutamine supplementation improves structure and function of intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 256:389–394
Lin JD, Lin PY, Chen LM, Fang WH, Lin LP, Loh CH (2010) Serum glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels in children and adolescents with intellectual disabilities. Res Dev Disabil 31:172–177
Ling J, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2010) Effect of dietary iron levels on growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 16:616–624
Liu WH, Liu TC, Yin MC (2008) Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food Chem Toxicol 46:1503–1509
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue Méd Vét 154:427–430
Livingstone DR, Garcia MP, Michel X, Narbonne JF, O’Hara S, Ribera D, Winston GW (1990) Oxyradical production as a pollution-mediated mechanism of toxicity in the common mussel, Mytilus edulis L. and other molluscs. Funct Ecol 4:415–424
Llames CR, Fontaine J (1994) Determination of amino acids in feeds: collaborative study. J AOAC Int 77:1362–1402
Lora J, Alonso FJ, Segura JA, Lobo C, Márquez J, Matés JM (2004) Antisense glutaminase inhibition decreases glutathione antioxidant capacity and increases apoptosis in Ehrlich ascetic tumour cells. Eur J Biochem 271:4298–4306
Lushchak VI, Lushchak LP, Mota AA, Hermes-Lima M (2001) Oxidative stress and antioxidant defenses in gold fish Carassius auratus during anoxia and reoxygenation. Am J Phys 280:100–107
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fisher 15:75–88
Mourente G, Bell JG, Tocher DR (2007) Does dietary tocopherol level affect fatty acid metabolism in fish? Fish Physiol Biochem 33:269–280
Nagy I, Floyd RA (1984) Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim Biophys Acta 790:238–250
NRC (1993) Nutrient requirements of fishes. In: Lovell RT, Cowey CB, Cho CY, Dabrowski K, Hughes S, Lall S, Murai T, Wilson RP (eds) National Research Council, Board of Agriculture. National Academy Press, Washington, DC
Obata T, Kubota S, Yamanaka Y (2001) Protective effect of histidine on para-nonylphenol-enhanced hydroxyl free radical generation induced by 1-methyl-4-phenylpyridinium ion (MPP+) in rat striatum. Biochim Biophys Acta 1568:171–175
Olsen RE, Henderson RJ (1997) Muscle fatty acid composition and oxidative stress indices of Arctic charr, Salvelinus alpinus (L.), in relation to dietary polyunsaturated fatty acid levels and temperature. Aquacult Nutr 3:227–238
Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, Zachary ME, Remacle J (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51:283–297
Rahman I, MacNee W (2000) Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 16:534–554
Reed DJ (1990) Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol 30:603–631
Shiau SY, Hsu CY (2002) Vitamin E sparing effect by dietary vitamin C in juvenile hybrid tilapia, Oreochromis niloticus × O. aureus. Aquaculture 210:335–342
Tan LN, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary zinc. Aquacult Nutr 17:338–345
Tansini CM, Durigon K, Testa CG, Belló-Klein A, Wajner M, Wannmacher CM, Wyse AT, Dutra-Filho CS (2004) Effects of histidine and imidazolelactic acid on various parameters of the oxidative stress in cerebral cortex of young rats. Int J Dev Neurosci 22:67–72
Uchida K (2003) Histidine and lysine as targets of oxidative modification. Amino Acids 25:249–257
Uchida K, Kawakishi S (1993a) 2-Oxo-histidine as a novel biological marker for oxidatively modified proteins. FEBS Lett 332:208–210
Uchida K, Kawakishi S (1993b) Identification of oxidized histidine generated at the active site of Cu, Zn-superoxide dismutase exposed to H2O2. J Biol Chem 269:2405–2410
Vardi N, Parlakpinar H, Ozturk F, Ates B, Gul M, Cetin A, Erdogan A, Otlu A (2008) Potent protective effect of apricot and β-carotene on methotrexate-induced intestinal oxidative damage in rats. Food Chem Toxicol 46:3015–3022
Wade AM, Tucker HN (1998) Antioxidant characteristics of l-histidine. J Nutr Biochem 9:308–315
Wen ZP, Zhou XQ, Feng L, Jiang J, Liu Y (2009) Effect of dietary pantothenic acid supplement on growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 15:470–476
Zhang Y, Zhan S, Cao P, Liu N, Chen X, Wang Y, Wang C (2005) The polypeptide in Chlamys farreri can protect human dermal fibroblasts from ultraviolet B damage. Chin J Oceanol Limnol 23:357–362
Zhang XD, Zhu YF, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280:136–139
Zhao B, Feng L, Liu Y, Kuang SY, Tang L, Jiang J, Hu K, Jiang WD, Li SH, Zhou XQ (2012) Effects of dietary histidine levels on growth performance, body composition and intestinal enzymes activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 18:220–232
Acknowledgments
This research was financially supported by National Department Public Benefit Research Foundation (Agriculture) of China (201003020) and Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-08-0905).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, L., Zhao, B., Chen, G. et al. Effects of dietary histidine on antioxidant capacity in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem 39, 559–571 (2013). https://doi.org/10.1007/s10695-012-9719-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9719-9