Abstract
The present study was carried out to evaluate the effect of dietary taurine (Tau) on performance, digestive enzymes, antioxidant activity, and resistance of common carp, Cyprinus carpio L., fry to salinity stress. Fish (0.97 ± 0.033 g) were fed on different taurine levels of 0.0 (control), 5, 10, 15, or 20 g/kg diet up to satiation twice daily for 8 weeks. At the end of the feeding trial, fish were stressed by exposure to 10 ppt salinity for 3 days during which fish mortality was observed. Fish performance was significantly (P < 0.05) improved by dietary taurine up to 15 g Tau/kg diet after which fish growth and feed intake were almost the same. Also, taurine supplementation significantly (P < 0.05) elevated activities of intestinal amylase, lipase, and protease resulting in an improving in feed intake giving better performance. Furthermore, Tau-stimulated antioxidant activity of common carp was observed in a dose-related manner, where activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were significantly (P < 0.05) higher, but malondialdehyde (MDA) value was significantly (P < 0.05) lower in Tau-fed fish groups than those fed the control diet. In salinity stress experiment, highest survival rate was observed at fish fed Tau-supplemented diets without significant (P > 0.05) differences over fish fed the control diet. It appears that taurine could be used as a feed supplement to confer better growth and health of common carp fry with optimal level of 15 g/kg diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture represents one of the fastest growing food-producing sectors all over the world. The expansion in aquaculture is accompanied by growing need for protein sources for aqua-feeds production. The most important ingredient in fish diets is fish meal (FM), which is mainly obtained from a wild fish catch. As the catch from natural fisheries has stabilized, supply of FM is stable, but its demand increases, thereby causing higher price (Tacon and Metian 2009; Tacon et al. 2011). Therefore, intensive efforts have been given to the replacement of FM with less costly and more available plant protein sources in aqua-feeds. However, these plant protein meals have lower protein content as compared to FM and they contain anti-nutritional factors, which negatively affect feed intake, digestion, and/or absorption of nutrients (Kissil et al. 2000; Hardy 2010). Additionally, the plants-source proteins may lead to deficiency in one or several essential amino acids depending on the plant source and species to which they are fed; they also are almost devoid of taurine (Oliva-Teles et al. 2015).
Taurine (Tau) is an amino acid, which participates in many biological processes and it is recognized as a potent antioxidant, having an oxygen-free radical scavenger effect, leading to a reduction of lipid peroxidation, reduction of membrane permeability, and reduction of intracellular oxidation and so protecting tissue from oxidative injury (Hagar 2004; Parvez et al. 2008; Yu and Kim 2009; Zeng et al. 2010). Taurine is abundant in animal tissues; however, FM is particularly rich in taurine (5–7 mg/g DM; Yamamoto et al. 1998), but taurine is found in trace amounts in plant protein sources (Kataoka and Ohnishi 1986; Huxtable 1992; Spitze et al. 2003; Dragnes et al. 2009). It is known that FM composition may vary depending on fish species, processing method, and fillet content prior to processing. Besides that, important components of raw fish like taurine may be lost during the FM process. Hence, different types of FM such as jack mackerel meal, white FM, menhaden FM, and brown FM contain different amounts of taurine: 0.23, 0.34, 0.5, and 0.6%, respectively (Gaylord et al. 2006; Kim et al. 2005, 2008; Lim et al. 2013).
Fish could synthesize taurine from methionine and cysteine metabolism, but in some specific conditions, fish cannot synthesize enough taurine to meet body needs and therefore require additional taurine supply (Huxtable 1992; Roysommuti et al. 2003; Hu et al. 2008; Battin and Brumaghim 2009). In fact, the taurine requirement for certain species especially marine fish is higher than its content in FM sources. Therefore, taurine is considered as an important nutrient for fish where its deficiency retarded fish growth and feed efficiency (Takeuchi et al. 2001; Brotons Martinez et al. 2004; Chatzifotis et al. 2008; Takagi et al. 2008).
Common carp, Cyprinus carpio L., is one of the most widely distributed freshwater fish species across the globe representing 71.9% of freshwater production (Dawood and Koshio 2016), and its global production increased gradually from 2.41 million tons in 2000 to 4.08 million tons in 2013 (FAO 2014). With expansion of the farmed fish and the stability of FM production, aqua-feeds should contain a minimal amount of FM. In order to develop sustainable aqua-feeds, many studies have investigated taurine supplementation to compensate for FM reduction in diets (El-Sayed 2014; Salze and Davis 2015). Although Kim et al. (2008) stated that common carp does not need taurine supplementation for growth, it is hypothesized that taurine supplementation may be necessary to improve protective and antioxidative capacity and the health of the farmed fish when fed on low-FM diet. Therefore, this study was carried out to evaluate the effect of taurine supplemented to a soybean-based diet on the performance, digestive enzymes, and antioxidant activities of common carp fry. The tolerance of taurine-fed fish to salinity stress was also evaluated.
Materials and methods
Diet preparation and fish culture
Taurine was added to the ingredients of each diet to be 0.0 (control), 5.0, 10.0, 15.0, or 20.0 g/kg diet. However, taurine of each diet was suspended in 100 ml and blended with the other ingredients for 30 min to make a paste of each diet. The pastes were separately passed through a grinder and pelleted (1 mm diameter) in a paste extruder. The diets were oven-dried at 55 °C for 24 h and stored in plastic bags at − 2 °C for further use. The diet ingredients, proximate, and amino acid composition are given in Tables 1 and 2.
Common carp, C. carpio L., fry were obtained from the fish hatchery, Central Laboratory for Aquaculture Research, Abbassa, Abo-Hammad, Sharqia, Egypt. Fish were kept in an indoor fiberglass tank for 2 weeks for acclimation to the laboratory conditions. Fish (0.97 ± 0.033 g) were randomly distributed at a rate of 25 fish per 100-L aquarium in triplicate, and each aquarium was supplied with compressed air via air-stone using an aquarium air pump. The tested diets were offered to fish up to satiation at 9:00 and 14:00 h for 8 weeks. Settled fish waste along with a three-quarter of the aquarium’s water was siphoned daily, which was replaced by clean and aerated tap water from a storage tank. Fish mortality was recorded daily and dead fish were removed.
Analysis of water quality parameters
Water samples were collected weekly from each aquarium and water quality parameters were monitored. Water temperature and dissolved oxygen of each aquarium were measured in site with an oxygen-meter (970 portable DO meter, Jenway, London, UK). The unionized ammonia was measured using a Multiparameters Ion Analyzer (HANNA Instruments, Woonsocket, Rhodes Island, USA). The pH was measured by using a pH-meter (Digital Mini-pH Meter, model 55, Fisher Scientific, Denver, CO, USA). In all treatments, range of water temperature was 28.3–29.7 °C, dissolved oxygen concentration was 5.4–5.7 mg/L, unionized ammonia concentration was 0.14–0.27 mg/L, and pH was 7.6–7.8. All the previous parameters are within the acceptable range for fish growth (Boyd and Tucker 2012).
Growth and feed utilization parameters
At the end of the experiment, fish were collected from each aquarium, counted, and group-weighed. Parameters of growth performance and feed utilization were calculated as follows:
Weight gain % = 100 [final weight (g) − initial weight (g)] / initial weight (g);
Specific growth rate (SGR; %g/day) = 100 (Ln W2 − Ln W1) / T, where W1 and W2 are the initial and final weight, respectively, and T is the experimental period;
Feed intake = the summation of the diet offered throughout the experiment;
Feed conversion ratio (FCR) = feed intake (g) / weight gain (g).
Proximate chemical analysis
Samples of tested diets and fish from each treatment were analyzed according to the standard AOAC methods (Helrich 1990) for moisture, crude protein, total lipids, and total ash. Moisture content was estimated by heating samples in an oven at 85 °C until constant weight and calculating weight loss. Nitrogen content was measured using a microkjeldahl apparatus, and crude protein was estimated by multiplying the nitrogen content value by 6.25. Total lipid content was determined by ether extraction, and ash was determined by combusting samples in a muffle furnace at 550 °C for 6 h. Crude fiber was estimated according to Goering and Van Soest (1970) and gross energy was calculated according to NRC (1993). Amino acid composition in triplicate samples of fish diets was determined in acid hydrolysate (6 N HCl under reflux for 24 h at 110 °C) using an automatic amino acid analyzer (LKB 4151 plus, Biochrom Ltd., Cambridge, UK). Tryptophan was determined colorimetrically after hydrolyzing triplicate samples in 4.2 N NaOH (Basha and Roberts 1977). For taurine determination (in the diets and tissues), sample extraction was performed using 0.1 M HCl; derivatization was performed using dansyl chloride (McCarthy et al. 2000).
Intestinal digestive enzyme activity assay
At the end of the feeding trial, fish were fasted for 24 h, and five fish from each aquarium were sampled randomly for determining activities of intestinal digestive enzyme (amylase, lipase, and protease activities). Fish were anesthetized by using MS-222 (20 μg/L), dissected immediately, and the whole intestines were removed and blotted dry with filter paper. Then, the intestine samples were weighed and homogenized in ice-cold 0.85% NaCl solution, with volumes nine times the weight of the intestine, using a manual glass homogenizer on ice. Homogenates were then centrifuged (4500×g for 10 min at 4 °C), and supernatants were transferred into clean test tubes and the enzyme activities were analyzed within 12 h. Activities of digestive enzymes were measured using the diagnostic reagent kits according to the manufacturer’s instructions (Cusabio Biotech Co. Ltd., Wuhan, Hubei, China). Activities of amylase, lipase, and protease were assessed following the methods proposed by Bernfeld (1955), Shihabi and Bishop (1971), and Ross et al. (2000), respectively.
Hepatic antioxidant activity assays
At the end of the feeding trial, five fish from each aquarium were anesthetized using MS-222 (20 μg/L) and dissected. After that, liver tissue of these fish was homogenized. The antioxidant enzyme activities were measured using the diagnostic reagent kits according to the manufacturer’s instructions (MyBioSource Inc., San Diego, California, USA). Malondialdehyde (MDA) level was measured at 532 nm by thiobarbituric acid method described by Ohkawa et al. (1979). Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities were measured according to methods of McCord and Fridovich (1969), Aebi 1984, and Paglia and Valentine (1967), respectively.
Salinity stress tolerance
In this experiment, challenge aquaria were filled by tap water and salinized with 10 ppt by sodium chloride. After feeding trial, fish of each treatment were collected and directly transferred to the challenge aquaria at a rate of 10 fish per 50-L aquarium in duplicates. Each aquarium was supplied with compressed air via air-stone using an aquarium air pump. Each tested diet was offered to the corresponding fish up to satiation at 9:00 and 14:00 h for 3 days. Settled fish waste along with a half of the aquarium’s water was siphoned daily, which was replaced by clean, aerated, and salinized water. Fish mortality was recorded daily and dead fish were removed.
Statistical analysis
Prior to statistical analysis, data in percentages was first transformed into arcsine and later back-transformed by squaring the sine of the arcsine after re-testing for normality and homogeneity of variance. All data were tested for normality of distribution using the Kolmogorov–Smirnov test. The homogeneity of variances among different treatments was tested using Bartlett’s test. Then, they were subjected to one-way ANOVA to evaluate the effect of taurine supplementation. Differences between means were tested at the 5% probability level using Duncan test. The optimum taurine level for fish growth was determined using polynomial regression analysis. All the statistical analyses were done using the SPSS program version 20 (SPSS, Richmond, VA, USA) as described by Dytham (2011).
Results
Fish growth represented by final fish weight, weight gain, weight gain %, and SGR increased significantly (P < 0.05) with increasing taurine levels with no significant difference when fish fed 10–15 g/kg diet after which fish growth declined (Table 2). The lowest fish growth was obtained at the control group. Furthermore, taurine supplementation did not significantly affect fish survival and its range was 96.7–100% (P > 0.05; Table 3). The relationship between final fish weight and dietary taurine levels (Fig. 1) was best expressed by the second-order polynomial regression equation as follows:
The regression curve showed that the most suitable taurine level for optimum fish growth was 15 g/kg diet. Moreover, fish fed on diets containing 15 g Tau/kg consumed more diet (10.1 ± 0.21 g feed/fish) than the other treatments; meanwhile, no significant change in FCR values was observed and its range was 1.33 ± 0.051–1.40 ± 0.046.
The dietary taurine level significantly affected the whole-fish body composition except moisture content, which did not show significant difference (P < 0.05; Table 4). Taurine supplementation increased significantly contents of protein and total lipids; meanwhile, it decreased significantly total ash content.
Compared to the control group, taurine-fed fish showed higher intestinal amylase, lipase, and protease activities; however, their activities increased significantly with increasing taurine levels reaching the optimum values (82.7 ± 8.83, 144.0 ± 10.54, and 217.3 ± 38.75 U/g protein for amylase, lipase, and protease, respectively) when fish fed diets containing 10 g Tau/kg diet (P > 0.05; Table 5).
It is noticed that MDA decreased significantly; meanwhile, SOD, CAT, and GPx activities were significantly elevated due to increasing taurine levels in the tested diets (P < 0.05; Table 6). Their optimum activities (21.5 ± 2.11, 29.5 ± 5.13, 25.2 ± 5.91, and 26.2 ± 2.86 IU/g for MDA, SOD, CAT, and GPx, respectively) were observed when fish fed 10 g Tau/kg diet (P > 0.05; Table 6).
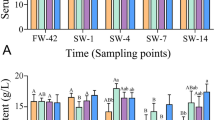
After the salinity challenge, the highest survival (100%) was observed at fish fed 10–20 g Tau/kg diet (Fig. 2). However, there was no significant difference in survival of fish fed taurine-enriched diets or the control diet.
Discussion
The results obtained revealed that dietary taurine enhanced growth performance and feed intake of common carp over the control diet with optimum level of 15 g/kg diet, which was sufficient for optimum performance and biological functions of common carp fry. These results may be due to the enhanced secretion of digestive enzymes resulting in improved feed digestion and utilization, and subsequently improved growth. In similar studies with carps, Liu et al. (2006) and Luo et al. (2006) found that taurine supplementation improved growth rates, feed digestibility, and feed efficiency. The results of the present study were similar to those reported in other fish, such as white seabass, Atractoscion nobilis (9.9 g/kg diet, Jirsa et al. 2014), Nile tilapia, Oreochromis niloticus (10 g/kg diet, Al-Feky et al. 2016), yellow catfish, Pelteobagrus fulvidraco (10.9 g/kg diet, Li et al. 2016), turbot, Psetta maxima (11.5 g/kg diet, Qi et al. 2012), and lower than the values for Japanese flounder, Paralichthys olivaceous (16.6 g/kg diet, Kim et al. 2005), while higher than the values reported for rainbow trout (8.5 g/kg diet, Gaylord et al. 2007), Florida pompano, Trachinotus carolinus (7.5 g/kg diet, Rossi and Davis 2012), cobia, Rachycentron canadum (5.0 g/kg diet, Lunger et al. 2007), common dentex, Dentex dentex (2.0 g/kg diet, Chatzifotis et al. 2008), and sea bass, Dicentrarchus labrax (2.0 g/kg diet, Brotons Martinez et al. 2004). The dietary taurine requirements of fish have a large range (from 2.0 to 16.6 g/kg diet), which is possibly affected by dietary protein sources and levels, assimilation rate, experimental conditions, and fish species and sizes (El-Sayed 2014; Salze and Davis 2015).
In contrast to the obtained results, Kim et al. (2008) reported that dietary taurine is not essential for growth of common carp juveniles. Salze and Davis (2015) reported that some fishes could biosynthesize taurine starting with methionine and cysteine. So, the availability of taurine precursors, i.e., methionine or cysteine in diets, could play a successful role in taurine biosynthetic in some (Yokoyama and Nakazoe 1992; Wang et al. 2014, 2016) but not all (Park et al. 2002; Kim et al. 2008) fish species. Diets of the present study were deficient in methionine and cysteine (Table 2) as compared with their requirement by common carp (Nose 1979). This suggests that common carp fed low-FM diets could not biosynthesize enough amount of taurine and needs external supply.
The proximate chemical analysis of whole-fish body including crude protein and total lipids improved significantly due to taurine supplementation, meanwhile ash content decreased significantly. These results suggest that taurine supplementation increased protein synthesis and deposition in fish body when it was supplemented at optimum levels (Li et al. 2009). The increased level of body protein content may be attributed to higher level of digestive enzyme activity and its beneficial effect on digestion and absorption of protein material in gut (Ye et al. 2011). Similar results were reported on juvenile turbot (Qi et al. 2012) and Nile tilapia (Al-Feky et al. 2016). Generally, the changes in body constituents such as protein and lipid contents could be linked with changes in their synthesis, deposition rate in muscle, and/or different growth rates (Fauconneau 1985; Abdel-Tawwab et al. 2006).
The inclusion of dietary taurine in fish diet herein enhanced the activities of intestinal amylase, lipase, and protease of common carp, which promoted the decomposition of dietary carbohydrate, lipids, and protein resulting in better feed utilization and better growth. In earlier studies, taurine has been reported to play a significant role in promoting nutrient digestion and absorption in aquatic animals (Salze et al. 2012; Nguyen et al. 2015; Richard et al. 2017). Enhanced digestive enzyme activities would enable better nutrient availability (Hoseinifar et al. 2017a); hence, partially explaining the improved growth observed in taurine-enriched fed fish. In similar studies, lipase activity measured in the intestinal digesta of yellowtail, Seriola quinqueradiata, fed several soybean meal-based formulations was increased when supplementing the diets with taurine (Nguyen et al. 2015).
Fish feeding plays an important role in their welfare by maintaining their oxidative balance, either by supplying nutrients that enhance the antioxidant system or avoiding those that would induce an increase of free radical production (Liew et al. 2015; Burgos-Aceves et al. 2016; Hoseinifar et al. 2017b). Further, SOD, CAT, and GPx enzyme system are principal components of antioxidant system. Thus, the higher values of hepatic SOD, CAT, and GPx activities in common carp fed taurine-enriched diets are indicative for the improved antioxidant capacity. Moreover, the hepatic MDA value in taurine-fed fish was significantly lower than those fed the control diet. Malondialdehyde (MDA) is a product of lipid peroxidation and directly reflects the level of lipid peroxidation, and a higher level of MDA leads to higher cell toxicity, accelerating the damage of cells and tissues (Buege and Aust 1978). In the present study, fish fed free-taurine diet showed highest MDA content, but that values decreased with a further increase in dietary taurine. The obtained results herein indicate that dietary taurine supplementation could enhance resistance of common carp to oxidative stress and would probably confer better fish health. In this regard, taurine is recognized as a potent antioxidant, having an oxygen-free radical scavenger effect, leading to a reduction of lipid peroxidation, reduction of membrane permeability, and reduction of intracellular oxidation and so protecting tissue from oxidative injury (Hagar 2004; Parvez et al. 2008; Yu and Kim 2009; Zeng et al. 2010; El-Sayed 2014; Salze and Davis 2015). In similar study, Rosemberg et al. (2010) observed that dietary taurine restored SOD and CAT activities and significantly reduced lipid peroxidation in zebrafish (Danio rerio). Li et al. (2016) found a taurine-enhancing SOD, CAT, and GPx activities in the liver of yellow catfish. Bañuelos-Vargas et al. (2014) reported that taurine supplementation led to a significant increase in CAT activity, as well as to a significant reduction of liver MDA in totoaba juveniles, Totoaba macdonaldi. They also suggested that taurine may play an important metabolic modulation action on fish fed soybean-based diets, contributing to the enhancement of the overall metabolism and to the reduction of liver oxidative damage.
Evaluation of stress response in fish is aimed to estimate the effect of feed supplements on improvement of fish health under stress conditions. Indeed, salinity stress challenge has been frequently used for determination of fry quality in many studies (Smith et al. 2004; Fazio et al. 2013; Imanpoor and Roohi 2016; Salze et al. 2008; Roohi et al. 2017). The results herein demonstrate that taurine supplementation had no effect on the survival of common carp stressed by 10 ppt salinity. These results agree with those previously obtained in Caspian roach (Rutilus rutilus) fed Sangrovit-supplemented diets (Imanpoor and Roohi 2016) and common carp fed diets enriched with fenugreek seeds (Roohi et al. 2017). On the other hand, Dimitroglou et al. (2010) reported that dietary mannanoligosaccharide significantly increased resistance of white sea bream larvae (Diplodus sargus L.) against salinity stress challenges. Soleimani et al. (2012) and Hoseinifar et al. (2013) revealed that dietary fructooligosaccharide (FOS) and galactooligosaccharide (GOS) significantly increased resistance of Caspian roach fry to salinity stress (15 ppt) where fish fed dietary FOS or GOS showed remarkable survival in dose-dependent manner, while no fish were survived in the control group. It could be hypothesized that anti-oxidative properties played a role in a defensive mechanism in intestinal environment, which could help animals to resist exo and endogenous oxidative stress (Burgos-Aceves et al. 2016; Lauriano et al. 2016; Aragona et al. 2017).
Conclusion
The present study shows that dietary taurine has a positive effect on the performance and health of common carp via elevating their intestinal digestive enzyme activities and improving their antioxidant capacity. However, taurine inclusion in common carp diets at a level of 15 g/kg diet could improve fish growth, feed utilization, and defense system of fish.
References
Abdel-Tawwab M, Khattab YAE, Ahmad MH, Shalaby AME (2006) Compensatory growth, feed utilization, whole-body composition, and hematological changes in starved juvenile Nile tilapia, Oreochromis niloticus (L.) J Appl Aquac 18(3):17–36. https://doi.org/10.1300/J028v18n03
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Al-Feky SSA, El-Sayed A-F, Ezzat AA (2016) Dietary taurine enhances growth and feed utilization in larval Nile tilapia (Oreochromis Niloticus) fed soybean meal-based diets. Aquac Nutr 22(2):457–464. https://doi.org/10.1111/anu.12266
Aragona M, Lauriano ER, Pergolizzi S, Faggio C (2017) Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat Prod Res 1–13
Bañuelos-Vargas I, López LM, Pérez-Jiménez A, Peres H (2014) Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on hepatic intermediary metabolism and antioxidant status of totoaba juveniles (Totoaba Macdonaldi). Comp Biochem Physiol B Biochem Mol Biol 170:18–25. https://doi.org/10.1016/j.cbpb.2014.01.003
Basha SMM, Roberts RM (1977) A simple colorimetric method for the determination of tryptophan. Anal Biochem 77(2):378–386. https://doi.org/10.1016/0003-2697(77)90251-2
Battin EE, Brumaghim JL (2009) Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys 55(1):1–23. https://doi.org/10.1007/s12013-009-9054-7
Bernfeld P (1955) Enzymes of carbohydrate metabolism. Methods Enzymol 1:149–158. https://doi.org/10.1016/0076-6879(55)01021-5
Boyd CE, Tucker CS (2012) Pond aquaculture water quality management. Springer Science & Business Media, Berlin
Brotons Martinez J, Chatzifotis S, Divanach P, Takeuchi T (2004) Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus Labrax fry fed with demand-feeders. Fish Sci 70(1):74–79. https://doi.org/10.1111/j.1444-2906.2003.00773.x
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
Burgos-Aceves MA, Cohen A, Smith Y, Faggio C (2016) Estrogen regulation of gene expression in the teleost fish immune system. Fish Shellfish Immunol 58:42–49. https://doi.org/10.1016/j.fsi.2016.09.006
Chatzifotis S, Polemitou I, Divanach P, Antonopoulou E (2008) Effect of dietary taurine supplementation on growth performance and bile salt activated lipase activity of common dentex, Dentex Dentex, fed a fish meal/soy protein concentrate-based diet. Aquaculture 275(1–4):201–208. https://doi.org/10.1016/j.aquaculture.2007.12.013
Dawood MAO, Koshio S (2016) Recent advances in the role of probiotics and prebiotics in carp aquaculture : a review. Aquaculture 454:243–251. https://doi.org/10.1016/j.aquaculture.2015.12.033
Dimitroglou A, Davies SJ, Sweetman J et al (2010) Dietary supplementation of mannan oligosaccharide on white sea bream (Diplodus sargus L.) larvae: effects on development, gut morphology and salinity tolerance. Aquac Res 41:245–251
Dragnes BT, Stormo SK, Larsen R, Ernstsen HH, Elvevoll EO (2009) Utilisation of fish industry residuals: screening the taurine concentration and angiotensin converting enzyme inhibition potential in cod and salmon. J Food Compos Anal 22(7–8):714–717. https://doi.org/10.1016/j.jfca.2009.01.020
Dytham C (2011) Choosing and using statistics: a biologist’s guide. John Wiley & Sons, Hoboken
El-Sayed A-FM (2014) Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev Aquac 6(4):241–255. https://doi.org/10.1111/raq.12042
FAO (2014) Food and Agriculture Organization of the United Nations. Global Aquaculture Production 1950–2012. FAO, Rome, Italy. (http://www.fao.org/fishery/statistics/global-aquacultureproduction/en)
Fauconneau B (1985) Protein synthesis and protein deposition in fish. Nutr Feed fish 17–45
Fazio F, Marafioti S, Arfuso F, Piccione G, Faggio C (2013) Influence of different salinity on haematological and biochemical parameters of the widely cultured mullet, Mugil Cephalus. Mar Freshw Behav Physiol 46(4):211–218. https://doi.org/10.1080/10236244.2013.817728
Gaylord TG, Barrows FT, Teague AM et al (2007) Supplementation of taurine and methionine to all-plant protein diets for rainbow trout (Oncorhynchus Mykiss). Aquaculture 269(1-4):514–524. https://doi.org/10.1016/j.aquaculture.2007.04.011
Gaylord TG, Teague AM, Barrows FT (2006) Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus Mykiss). J World Aquacult Soc 37(4):509–517. https://doi.org/10.1111/j.1749-7345.2006.00064.x
Goering HK, Van Soest PJ (1970) Forage fiber analysis. Agricultural handbook no. 379. US Dep Agric, Washington, DC, pp 1–20
Hagar HH (2004) The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol Lett 151(2):335–343. https://doi.org/10.1016/j.toxlet.2004.03.002
Hardy RW (2010) Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac Res 41(5):770–776. https://doi.org/10.1111/j.1365-2109.2009.02349.x
Helrich K (1990) Official methods of analysis 15th ed. Association of official analytical chemist Inc., Arlington
Hoseinifar SH, Ahmadi A, Khalili M, Raeisi M, van Doan H, Caipang CM (2017a) The study of antioxidant enzymes and immune-related genes expression in common carp (Cyprinus Carpio) fingerlings fed different prebiotics. Aquac Res 48(11):5447–5454. https://doi.org/10.1111/are.13359
Hoseinifar SH, Dadar M, Ringø E (2017b) Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: the functional feed additives scenario. Aquac Res 48(8):3987–4000. https://doi.org/10.1111/are.13368
Hoseinifar SH, Khalili M, Rostami HK, Esteban MÁ (2013) Dietary galactooligosaccharide affects intestinal microbiota, stress resistance, and performance of Caspian roach (Rutilus Rutilus) fry. Fish Shellfish Immunol 35(5):1416–1420. https://doi.org/10.1016/j.fsi.2013.08.007
Hu YH, Lin CL, Huang YW, Liu PE, Hwang DF (2008) Dietary amino acid taurine ameliorates liver injury in chronic hepatitis patients. Amino Acids 35(2):469–473. https://doi.org/10.1007/s00726-007-0565-5
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163. https://doi.org/10.1152/physrev.1992.72.1.101
Imanpoor MR, Roohi Z (2016) Effects of Sangrovit-supplemented diet on growth performance, blood biochemical parameters, survival and stress resistance to salinity in the Caspian roach (Rutilus Rutilus) fry. Aquac Res 47(9):2874–2880. https://doi.org/10.1111/are.12737
Jirsa D, Davis DA, Salze GP et al (2014) Taurine requirement for juvenile white seabass (Atractoscion Nobilis) fed soy-based diets. Aquaculture 422:36–41
Kataoka H, Ohnishi N (1986) Occurrence of taurine in plants. Agric Biol Chem 50:1887–1888
Kim S-K, Matsunari H, Takeuchi T, Yokoyama M, Furuita H, Murata Y, Goto T (2008) Comparison of taurine biosynthesis ability between juveniles of Japanese flounder and common carp. Amino Acids 35(1):161–168. https://doi.org/10.1007/s00726-007-0600-6
Kim S-K, Takeuchi T, Yokoyama M, Murata Y, Kaneniwa M, Sakakura Y (2005) Effect of dietary taurine levels on growth and feeding behavior of juvenile Japanese flounder Paralichthys Olivaceus. Aquaculture 250(3-4):765–774. https://doi.org/10.1016/j.aquaculture.2005.04.073
Kissil GW, Lupatsch I, Higgs DA, Hardy RW (2000) Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus Aurata L. Aquac Res 31(7):595–601. https://doi.org/10.1046/j.1365-2109.2000.00477.x
Lauriano ER, Pergolizzi S, Capillo G, Kuciel M, Alesci A, Faggio C (2016) Immunohistochemical characterization of toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius Auratus fed with a high-cholesterol diet. Fish Shellfish Immunol 59:250–255. https://doi.org/10.1016/j.fsi.2016.11.003
Li M, Lai H, Li Q, Gong S, Wang R (2016) Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus Fulvidraco fed all-plant protein diets. Aquaculture 450:349–355. https://doi.org/10.1016/j.aquaculture.2015.08.013
Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37(1):43–53. https://doi.org/10.1007/s00726-008-0171-1
Liew HJ, Fazio A, Faggio C, Blust R, de Boeck G (2015) Cortisol affects metabolic and ionoregulatory responses to a different extent depending on feeding ration in common carp, Cyprinus Carpio. Comp Biochem Physiol A Mol Integr Physiol 189:45–57. https://doi.org/10.1016/j.cbpa.2015.07.011
Lim S-J, Oh D-H, Khosravi S et al (2013) Taurine is an essential nutrient for juvenile parrot fish Oplegnathus Fasciatus. Aquaculture 414:274–279
Liu H, Li HW, Xu YJ et al (2006) Effects of taurine on growth and nutritional value of carps. Food Sci Technol 8:97
Lunger AN, McLean E, Gaylord TG, Kuhn D, Craig SR (2007) Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron Canadum). Aquaculture 271(1-4):401–410. https://doi.org/10.1016/j.aquaculture.2007.07.006
Luo L, Wen H, Wang L et al (2006) Effects of taurine on growth performance, quality, digestive and metabolic enzyme activity of grass carp (Ctenopharymgodon idellus). Chinese J Anim Nutr 18:166–171
McCarthy K, Hischenhuber C, Joyce N (2000) Determination of total taurine in pet foods by liquid chromatography of the dansyl derivative: collaborative study. J AOAC Int 83(4):784–788
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Metian AGJTM (2009) Fishing for feed or fishing for food: increasing global competition for small pelagic forage fish. AMBIO A J Hum Environ 38(6):294–302. https://doi.org/10.1579/08-A-574.1
Nguyen HP, Khaoian P, Fukada H, Suzuki N, Masumoto T (2015) Feeding fermented soybean meal diet supplemented with taurine to yellowtail Seriola Quinqueradiata affects growth performance and lipid digestion. Aquac Res 46(5):1101–1110. https://doi.org/10.1111/are.12267
NRC (1993) Nutrient requirements of fish. Committee on Animal Nutrition. Board on Agriculture. National Research Council. National Academy Press, Washington DC
Nose T (1979) Summary report on the requirements of essential amino acids for carp. In: Tiews K, Halfer JE (eds) Finfish nutrition and feed technology. Heenman, Berline, pp 145–156
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Oliva-Teles A, Enes P, Peres H (2015) Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In: Davis AD (ed) Feed Feed Pract Aquac. Elsevier Cambridge, UK, pp 203–233
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Park G-S, Takeuchi T, Yokoyama M, Seikai T (2002) Optimal dietary taurine level for growth of juvenile Japanese flounder Paralichthys Olivaceus. Fish Sci 68(4):824–829. https://doi.org/10.1046/j.1444-2906.2002.00498.x
Parvez S, Tabassum H, Banerjee BD, Raisuddin S (2008) Taurine prevents Tamoxifen-induced mitochondrial oxidative damage in mice. Basic Clin Pharmacol Toxicol 102(4):382–387. https://doi.org/10.1111/j.1742-7843.2008.00208.x
Qi G, Ai Q, Mai K et al (2012) Effects of dietary taurine supplementation to a casein-based diet on growth performance and taurine distribution in two sizes of juvenile turbot (Scophthalmus Maximus L.) Aquaculture 358:122–128
Richard N, Colen R, Aragão C (2017) Supplementing taurine to plant-based diets improves lipid digestive capacity and amino acid retention of Senegalese sole (Solea Senegalensis) juveniles. Aquaculture 468:94–101. https://doi.org/10.1016/j.aquaculture.2016.09.050
Roohi Z, Imanpoor MR, Jafari V, Taghizadeh V (2017) The use of fenugreek seed meal in fish diets: growth performance, haematological and biochemical parameters, survival and stress resistance of common carp (Cyprinus Carpio L.) Aquac Res 48(3):1209–1215. https://doi.org/10.1111/are.12962
Rosemberg DB, da Rocha RF, Rico EP, Zanotto-Filho A, Dias RD, Bogo MR, Bonan CD, Moreira JCF, Klamt F, Souza DO (2010) Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience 171(3):683–692. https://doi.org/10.1016/j.neuroscience.2010.09.030
Ross NW, Firth KJ, Wang A, Burka JF, Johnson SC (2000) Changes in hydrolytic enzyme activities of naive Atlantic salmon Salmo Salar skin mucus due to infection with the salmon louse Lepeophtheirus Salmonis and cortisol implantation. Dis Aquat Org 41(1):43–51. https://doi.org/10.3354/dao041043
Rossi W, Davis DA (2012) Replacement of fishmeal with poultry by-product meal in the diet of Florida pompano Trachinotus Carolinus L. Aquaculture 338:160–166
Roysommuti S, Azuma J, Takahashi K, Schaffer S (2003) Taurine cytoprotection: from cell to system. J Physiol Sci 16:17–27
Salze G, McLean E, Craig SR (2012) Dietary taurine enhances growth and digestive enzyme activities in larval cobia. Aquaculture 362:44–49
Salze G, McLean E, Schwarz MH, Craig SR (2008) Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 274(1):148–152. https://doi.org/10.1016/j.aquaculture.2007.11.008
Salze GP, Davis DA (2015) Taurine: a critical nutrient for future fish feeds. Aquaculture 437:215–229. https://doi.org/10.1016/j.aquaculture.2014.12.006
Shihabi ZK, Bishop C (1971) Simplified turbidimetric assay for lipase activity. Clin Chem 17(12):1150–1153
Smith ME, Kane AS, Popper AN (2004) Noise-induced stress response and hearing loss in goldfish (Carassius Auratus). J Exp Biol 207(3):427–435. https://doi.org/10.1242/jeb.00755
Soleimani N, Hoseinifar SH, Merrifield DL, Barati M, Abadi ZH (2012) Dietary supplementation of fructooligosaccharide (FOS) improves the innate immune response, stress resistance, digestive enzyme activities and growth performance of Caspian roach (Rutilus Rutilus) fry. Fish Shellfish Immunol 32(2):316–321. https://doi.org/10.1016/j.fsi.2011.11.023
Spitze AR, Wong DL, Rogers QR, Fascetti AJ (2003) Taurine concentrations in animal feed ingredients; cooking influences taurine content. J Anim Physiol Anim Nutr (Berl) 87(7-8):251–262. https://doi.org/10.1046/j.1439-0396.2003.00434.x
Tacon AGJ, Metian MR, Tacon MAGJ, et al (2011) Demand and supply of feed ingredients for farmed fish and crustaceans: trends and prospects
Takagi S, Murata H, Goto T, Endo M, Yamashita H, Ukawa M (2008) Taurine is an essential nutrient for yellowtail Seriola Quinqueradiata fed non-fish meal diets based on soy protein concentrate. Aquaculture 280(1-4):198–205. https://doi.org/10.1016/j.aquaculture.2008.05.012
Takeuchi T, Park G-S, Seikai T, Yokoyama M (2001) Taurine content in Japanese flounder Paralichthys Olivaceus T. & S. and red sea bream Pagrus Major T. & S. During the period of seed production. Aquac Res 32:244–248. https://doi.org/10.1046/j.1355-557x.2001.00021.x
Wang Q, He G, Wang X et al (2014) Dietary sulfur amino acid modulations of taurine biosynthesis in juvenile turbot (Psetta Maxima). Aquaculture 422:141–145
Wang X, He G, Mai K, Xu W, Zhou H (2016) Differential regulation of taurine biosynthesis in rainbow trout and Japanese flounder. Sci Rep 6(1):21231. https://doi.org/10.1038/srep21231
Yamamoto T, Akimoto A, Kishi S, Unuma T, Akiyama T (1998) Apparent and true availabilities of amino acids from several protein sources for fingerling rainbow trout, common carp, and red sea bream. Fish Sci 64(3):448–458. https://doi.org/10.2331/fishsci.64.448
Ye JD, Wang K, Li FD, Sun YZ (2011) Single or combined effects of fructo-and mannan oligosaccharide supplements and Bacillus Clausii on the growth, feed utilization, body composition, digestive enzyme activity, innate immune response and lipid metabolism of the Japanese flounder Paralichthys olivaceus. Aquac Nutr 17:902–911
Yokoyama M, Nakazoe J-I (1992) Accumulation and excretion of taurine in rainbow trout (Oncorhynchus Mykiss) fed diets supplemented with methionine, cystine and taurine. Comp Biochem Physiol A Physiol 102(3):565–568. https://doi.org/10.1016/0300-9629(92)90210-H
Yu J, Kim AK (2009) Effect of taurine on antioxidant enzyme system in B16F10 melanoma cells. Adv Exp Med Biol 7:491–499
Zeng K, Xu H, Chen K, Zhu J, Zhou Y, Zhang Q, Mantian M (2010) Effects of taurine on glutamate uptake and degradation in Müller cells under diabetic conditions via antioxidant mechanism. Mol Cell Neurosci 45(2):192–199. https://doi.org/10.1016/j.mcn.2010.06.010
Acknowledgements
This study was funded and supported by Central Laboratory for Aquaculture Research (CLAR), Abbassa, Abo-Hammad, Sharqia, Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Tawwab, M., Monier, M.N. Stimulatory effect of dietary taurine on growth performance, digestive enzymes activity, antioxidant capacity, and tolerance of common carp, Cyprinus carpio L., fry to salinity stress. Fish Physiol Biochem 44, 639–649 (2018). https://doi.org/10.1007/s10695-017-0459-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0459-8