Abstract
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is caused by autosomal dominant germline mutations in the fumarate hydratase (FH) gene and is characterized by cutaneous leiomyomas, uterine leiomyomas and aggressive renal malignancies. We conducted a retrospective chart review to characterize the patients referred to our Regional Genetics Program for assessment of HLRCC from 2004 to mid-2016. Forty-eight of 69 (69.5%) referred individuals were positive for a pathogenic or likely pathogenic variant in FH; they had an average age of 39.1 years. There were 11 different FH variants among them. As expected, the most sensitive indications for a positive genetic test were papillary renal cell carcinoma (RCC) at a young age (5/5; 100%) and multiple cutaneous leiomyomas (18/19; 95%). However, only twenty-two of 48 (46%) individuals with a positive molecular test had cutaneous leiomyomas, which is considerably lower than previously reported and supports the likelihood of ascertainment bias in previous reports. Notably, we have experience with 1 large family in which there were no cutaneous leiomyomas across a large age range. We confirm that multiple cutaneous leiomyomas and papillary RCCs at a young age have a high positive predictive value for a molecular diagnosis of HLRCC, but that cutaneous leiomyomas are less prevalent in HLRCC than previously understood, and therefore the condition is likely to be under-ascertained. Our understanding of the phenotypic spectrum of HLRCC is still evolving.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary leiomyomatosis and renal cell cancer (HLRCC, MIM 150800) is characterized by cutaneous leiomyomas, uterine leiomyomas and a predisposition to renal malignancies, typically an aggressive papillary type II renal cell carcinoma. HLRCC is caused by autosomal dominant germline mutations in the fumarate hydratase (FH) gene [1]. The enzyme fumarate hydratase catalyzes the conversion of fumarate to malate as part of the tricarboxylic acid cycle. Pathogenic variants in FH result in reduced enzyme activity by at least 50% [2], causing up-regulation of hypoxia-inducible factor (HIF) which may result in increased angiogenesis, glucose transport and growth stimulation. Fumarate may be an oncogenic metabolite as loss of fumarate hydratase has been shown to activate nuclear erythroid 2-like 2 transcription factor (NRF2), which may be involved in cancer development [3]. Fumarate also succinates cysteine to S-(2-succinyl) cysteine (2SC), and 2SC-modified proteins have been shown to be a reliable biomarker of FH pathogenic variants in uterine leiomyomas, RCCs [4] and cutaneous leiomyomas [5].
Germline pathogenic variants in FH are also implicated in other conditions: they predispose to malignant pheochromocytomas [6] and paragangliomas [6, 7] and they are also the cause of the autosomal recessive disorder fumaric aciduria (FA, MIM 606812) which is characterized by brain malformations, seizures, developmental delay and dysmorphic features.
Smit et al. proposed that the clinical diagnosis of HLRCC could rely on the single major criterion of multiple cutaneous leiomyomas, histopathologically confirmed, or the presence of at least 2 minor criteria which include severely symptomatic uterine leiomyomas requiring surgical treatment, type 2 papillary renal cell carcinoma before age 40 or a first degree family member with any of the above criteria [8]. Lehtonen et al. additionally suggested that collecting duct carcinomas be included in the criteria [9].
A definitive diagnosis of HLRCC is made with detection of a pathogenic FH mutation. The rate of positive genetic testing in individuals who meet the clinical criteria proposed by Smit et al. [8] is between 89 and 100% [8, 10]. The rate of positive testing when any of the major or minor clinical criteria is present has been shown to be 71% [2].
The prevalence of HLRCC has not been determined. Over 300 families with clinical findings of HLRCC and heterozygous mutations in the FH gene have been reported [2, 8, 11, 12]. The larger HLRCC characterization studies have relied on the presence of multiple cutaneous leiomyomas as a criterion for HLRCC testing [10, 13], thereby risking under-ascertainment of affected individuals.
Cutaneous leiomyomas have been reported in 76–100% of patients with HLRCC [8, 13]. The mean age of onset of cutaneous leiomyomas in the context of HLRCC is 25 years [10, 14], and 60–100% of FH germline mutation carriers over the age of 40 are reported to have cutaneous leiomyomas [8, 13]. The literature suggests that uterine leiomyomas are the most frequent finding in women with HLRCC, with greater than 80% having uterine leiomyomas by early adulthood [8] and up to 98% presenting with uterine leiomyomas at a mean age of 28–30 years [10, 15]. Renal tumors in HLRCC are typically of type 2 papillary histology and due to their aggressive nature are recognized as a distinct renal tumour subtype called HLRCC-associated RCC [16]. Collecting duct and clear cell cancers have also been reported in individuals with HLRCC [17]. Renal tumours in HLRCC are most often solitary and unilateral [18]. Estimates of a lifetime risk of renal cancer development in HLRCC vary widely and are approximated at 15% [19]. The mean age of RCC diagnosis in HLRCC has been reported as 41–43 years [2, 19, 20] however 4–7% of reported HLRCC cases have been in individuals below 20 years of age [19]. Genetic anticipation of RCC may occur in HLRCC [12]. Current recommendations are to consider genetic testing from the age of 8–10 years onward, and for individuals with positive genetic testing or a suspected clinical diagnosis to have an annual abdominal MRI [19]. There is no known genotype-phenotype association in HLRCC.
We believe that HLRCC is an under-reported cancer predisposition syndrome due to ascertainment bias for patients presenting with cutaneous leiomyomas. Here we describe our experience with patients referred to the Regional Genetics Program at the Children’s Hospital of Eastern Ontario for HLRCC from 2004 to mid-2016 and compare our experience with that described in the literature.
Methods
Seventy-one individuals were seen for assessment of HLRCC at the Regional Genetics Program in Ottawa, Ontario, Canada from 2004 to mid-2016. All were seen by a geneticist, genetic counsellor or both for a clinical assessment that included a review of medical and family history and examination of any skin lesions. Individuals were offered molecular genetic testing for HLRCC if they had more than one cutaneous leiomyoma, a positive family history of HLRCC or a personal history of renal cell carcinoma before the age of 40. Genetic testing, performed at clinical laboratories in Canada or the United States, consisted of sequence analysis of the FH gene (single gene testing), and if negative, duplication/deletion analysis. In some circumstances, individuals were offered genetic testing even if they did not meet the proposed HLRCC clinical guidelines; this included an individual seen for a presentation of papillary RCC at age 43, and 2 individuals seen for a single (biopsy proven) cutaneous leiomyoma.
We conducted a retrospective chart review for these 71 patients and extracted the following data: number of families (2 or more individuals that were related), age at first Genetics clinic appointment, sex, indication(s) for referral, specialty of referring provider, clinical history (biopsy proven cutaneous leiomyomas, self-reported uterine leiomyomas, self-reported hysterectomy, biopsy proven renal cell cancer), examination findings (suspected cutaneous leiomyomas), family history of renal cancer, whether genetic testing was performed and the results of genetic testing.
The 71 individuals included 2 healthy adult individuals referred with a family history of fumaric aciduria. Individuals referred to our cancer genetics program for an indication other than HLRCC, who were offered FH testing were excluded from this review. We included the following clinical laboratory terms as a positive molecular result: ‘positive for mutation’ (commonly seen in clinical laboratory reports prior to 2012), ‘variant, likely disease causing’, ‘likely pathogenic’ and ‘pathogenic’. We evaluated the pathogenic and likely pathogenic variants in FH for the in silico predicted effect of the pathogenic variant on the protein product using the software program Alamut.
Results
We saw 71 individuals for an assessment of HLRCC between 2004 and mid-2016. They ranged in age from 9 to 91 years (average 41.6 years). In the majority of cases, there was a single reason for referral; however in two instances the reason for referral was both biopsy-proven cutaneous leiomyomas and a self-reported history of uterine leiomyomas. The most common reason for referral was a family history of HLRCC or FA (41/71; 58%) and most of these referrals were from primary care providers. The second most common reason for referral was biopsy proven cutaneous leiomyoma(s) (22/71; 31%), with most of these referrals from dermatologists. Five individuals were referred to us for presentation of a renal cell carcinoma by an oncologist; 4 of these presentations were symptomatic, and the other was revealed on imaging indicated for vesicoureteral reflux.
Sixty-nine of the 71 individuals decided to pursue molecular FH testing following genetic counselling. Two individuals declined molecular FH testing, with both citing insurance discrimination concerns. Forty-eight of the 69 tested individuals (70%) had a heterozygous pathogenic or likely pathogenic variant in FH (29 female and 17 male). Of the individuals who had a positive molecular result for HLRCC, 22/48 (46%) had cutaneous leiomyomas (19 biopsy-proven and 3 suspected on clinical examination). Of the 29 females with a positive molecular test result, 18 (62%) self-reported uterine leiomyomas; they ranged in age from 24 to 63 years at the time of their visit to the Genetics clinic. Five of 48 individuals with a positive molecular test result had renal cell carcinomas (Table 1).
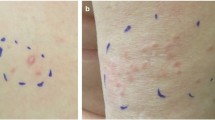
Twenty-one of the 22 individuals referred with biopsy proven cutaneous leiomyoma(s) decided to pursue FH molecular testing; of these, 18/21 (86%) had a positive pathogenic or likely pathogenic FH variant. Of the 3 individuals who did not have a positive result, 2 had only a single cutaneous leiomyoma. Therefore 18 of 19 individuals (95%) with multiple cutaneous leiomyomas had a positive molecular test. The other individual who did not have a positive molecular result was a 44 year old female with a history of multiple cutaneous leiomyomas, 1 of which was biopsy proven, as well as self-reported multiple uterine leiomyomas and a family history of RCC. Sequencing and deletion/duplication analysis of this individual’s FH gene did not reveal a pathogenic or likely pathogenic variant.
The five individuals referred with a presentation of RCC were positive for a pathogenic or likely pathogenic variant in FH (Table 2). The ages of these individuals at the time of RCC presentation ranged from 15 to 43 years. The RCC tumour histologies were papillary type II (3/5), tubulo-papillary and papillary with sarcomatoid features. None of these individuals had cutaneous leiomyomas at the time of their RCC presentation. Two of the 3 females that presented with RCC (at the ages of 24 and 43) self-reported a history of uterine leiomyomas. In 4 of these 5 individuals, the predicted effect of the FH pathogenic or likely pathogenic variant was a premature stop codon.
Twenty-five of the 29 females with a positive genetic test result were over the age of 20; the remaining 4 were ages 9, 10, 14 and 15. Of the 25 females over the age of 20 who tested positive, 18 (72%) self-reported a history of uterine leiomyomas. The remaining seven test positive females who did not have a known history of uterine leiomyomas ranged in age from 26 to 76 years (average 42.3 years).
Forty of 41 individuals referred because of a family history of HLRCC pursued FH testing, with 24/40 (60%) receiving a positive result of a pathogenic or likely pathogenic variant. One of the 24 individuals with a positive molecular test had a biopsy-proven cutaneous leiomyoma, and 3 of the 24 individuals had a skin lesion consistent with a cutaneous leiomyoma on examination in the Genetics clinic. The other 20 individuals with a positive FH molecular result did not have a history or clinical findings consistent with cutaneous leiomyomas. They ranged in age from 9 to 61 years (average 32.0 years).
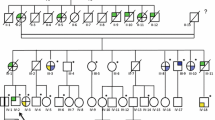
The 71 referred individuals included 12 families, defined as at least 2 individuals reported to be biologically related. Families were assigned a single pedigree number. One of these families consisted of 18 individuals of African-Haitian descent: a 24 year old female proband who presented with a papillary RCC and uterine leiomyomas, and 17 first, second and third degree relatives referred because of this family history. The family members reported at least 3 other relatives with RCCs who were diagnosed elsewhere and not seen at our centre. Eleven of these 18 individuals tested positive for the pathogenic variant: 4 female and 7 male. Three of the 4 positive females had a self-reported history of uterine leiomyoma(s) (ages 24, 50 and 58 years old); the remaining female did not self-report uterine leiomyomas at 26 years of age. It is notable that none of the 11 individuals in this family who tested positive had a history of, or clinical findings consistent with, cutaneous leiomyomas. They ranged in age from 9 to 58 years (average 32.6 years).
The 48 individuals who were positive for a pathogenic or likely pathogenic variant in FH had a total of 11 unique variants (Tables 2, 3). Two of these variants, c.1103T > C and c.1111A > T, are novel FH pathogenic variants and are not listed in ClinVar or the FH Leiden Open Variation Database, and the other 9 variants have been previously reported in ClinVar as of December 2017. There was an overlap of 3 variants found in individuals presenting with RCC and those referred for other indications; all 3 of these FH variants (c.797dup, c.1293delA and c.1430_1437dup) had a predicted effect of an early stop codon, likely truncating the protein. These 3 variants accounted for 37/48 (77%) of the individuals with a pathogenic or likely pathogenic variant: 16 individuals with 12 different pedigree numbers had the c.1293delA variant; 11 individuals with 1 pedigree number had the c.797dup variant; and 10 individuals with 6 different pedigree numbers who all reported French-Canadian ancestry had the c.1430_1437dup variant.
Discussion
Our lower rate of positive genetic testing for HLRCC is likely attributable to the subset of individuals referred because of a positive family history, often with no HLRCC clinical findings, whereas other study populations have all had cutaneous leiomyomas. Our rate of positive genetic testing in individuals referred because of RCC or multiple cutaneous leiomyomata (96%, 23/24 individuals) is consistent with previously described rates in this cohort. The lack of a positive molecular result when HLRCC is strongly clinically suspected may be due to technological limitations, mosaicism, or other undiscovered genes or mechanisms being implicated in HLRCC. In cases where we have a high clinical suspicion for HLRCC and molecular testing is negative, we counsel individuals to undergo RCC screening. It could be helpful to perform 2SC immunohistochemistry studies in cases where clinical criteria for HLRCC is met and no FH pathogenic variant is found. Our positive test population had lower than previously described rates of cutaneous leiomyomas and also uterine leiomyomas. It is possible that some individuals may develop cutaneous leiomyomas or present with symptomatic uterine leiomyomas over time. However, it also appears that cutaneous leiomyomas may not be present in all affected families. Notably, the 11 members of the Haitian family positive for a familial pathogenic FH variant did not have any cutaneous leiomyomas. It is unknown whether the presence of cutaneous leiomyomas in HLRCC is influenced by ethnicity.
Limitations of our study include the retrospective review approach that relied partially on self-reported information and also that individuals were not followed over time. Long term studies with large cohorts will be essential to further characterize HLRCC; these studies are necessary to provide better estimates of the risk of RCC in HLRCC and to determine whether screening for RCC reduces morbidity or mortality. As well, it would have been of interest to perform biochemical studies to assess for fumarate hydratase enzyme activity level.
Our data confirm that multiple cutaneous leiomyomas have a high positive predictive value (18/19 individuals, 95%) for a molecular diagnosis of HLRCC. However, our data also demonstrated that cutaneous leiomyomas in HLRCC are likely to occur less frequently than previously thought, and may not be expressed at all in some families with a pathogenic FH variant. While dermatologists thus remain critical players in the identification of individuals with HLRCC and an important target group for educational initiatives about HLRCC, cascade testing of family members (via a referral to a geneticist or genetic counsellor) will remain critical to identify at-risk individuals. HLRCC is likely more prevalent than currently recognized due to the ascertainment bias of multiple cutaneous leiomyomas being the predominant recruitment factor in previous HLRCC studies. Our data also confirm that papillary RCC at a young age has a high positive predictive value for a molecular diagnosis of HLRCC, indicating that oncologists, urologists and possibly pathologists are another important target group for educational initiatives about HLRCC. The proposed guidelines suggest that individuals with RCC under the age of 40 be tested; however, we found a pathogenic variant in a 43 year old male presenting with a papillary RCC and we would advocate for referral at or below the age of 45.
We also report 2 pathogenic FH variants that are not listed in ClinVAR as of December 2017: one of these, c.1111A > T, was found in an individual that presented with an RCC, while the other variant, c.1103T > C was not associated with RCCs. We note that the c.797dupT variant was found in 11 members of a Haitian family of African descent with several individuals who had RCCs and uterine leiomyomas, and no individuals with cutaneous leiomyomas. This particular variant may not be associated with cutaneous leiomyomas or possibly cutaneous leiomyomas are less prevalent in individuals with HLRCC of African descent. Although there is no clear association between cutaneous leiomyomas in HLRCC and ethnicity, a previous description of 2 African-American families with HLRCC found either a single suspected or no cutaneous leiomyomas in affected individuals [21]. Our report of 6 French-Canadian families with the same pathogenic variant c.1430_1437dup may be consistent with a founder effect mutation. This variant was not seen in previous reports of French cohorts of HLRCC patients [2, 22]. We plan to perform haplotype analysis studies to identify possible founder effects for the c.1293delA and c.1430_1437 variants.
It has been well established that multiple cutaneous leiomyomas and papillary renal cell carcinomas have a high positive predictive value in molecular testing for HLRCC. However, the absence of multiple cutaneous leiomyomas clearly does not rule out the presence of a pathogenic FH variant, and follow up cascade testing of family members is necessary to diagnose HLRCC and ensure appropriate RCC surveillance. Although not well estimated, HLRCC is likely more prevalent than previously understood. Educational initiatives about HLRCC should be targeted at oncologists and urologists in addition to dermatologists. Further long term studies of HLRCC are required to gain greater insights into the characteristics and clinical spectrum of HLRCC.
Abbreviations
- FA:
-
Fumaric aciduria
- FH:
-
Fumarase hydratase
- HIF:
-
Hypoxia inducible factor
- HLRCC:
-
Hereditary leiomyomatosis and renal cell cancer
- NRF2:
-
Nuclear erythroid 2-like 2 transcription factor
- RCC:
-
Renal cell carcinoma
- 2SC:
-
S-(2-succinyl) cysteine
References
Tomlinson IP, Alam NA, Rowan AJ et al (2002) Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30:406–410
Gardie B, Remenieras A, Kattygnarath D et al (2011) Novel FH mutations in families with hereditary leiomyomatosis and renal cell cancer (HLRCC) and patients with isolated type 2 papillary renal cell carcinoma. J Med Genet 48:226–234
Sandhu IV, Maksim NJ, Amouzougan EA et al (2015) Sustained NRF2 activation in hereditary leiomyomatosis and renal cell cancer (HLRCC) and in hereditary tyrosinemia type 1 (HT1). Biochem Soc Trans 43:650–656
Bardella C, El-Bahrawy M, Frizzell N et al (2011) Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol 225:4–11
Buelow B, Cohen J, Nagymanyoki Z et al (2016) Immunohistochemistry for 2-Succinocysteine (2SC) and fumarate hydratase (FH) in cutaneous leiomyomas may aid in identification of patients With HLRCC (hereditary leiomyomatosis and renal cell carcinoma syndrome. Am J Surg Pathol 40:982–988
Castro-Vega LJ, Buffet A, De Cubas AA et al (2014) Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Gen 23:2440–2446
Clark GR, Sciacovelli M, Gaude E et al (2014) Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab 99:E2046–E2050
Smit DL, Mensenkamp AR, Badeloe S et al (2011) Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet 79:49–59
Lehtonen H (2011) Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer 10:397–411
Toro JR, Nickerson ML, Wei M et al (2003) Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet 73:95–106
Bayley J, Launonen V, Tomlinson IP (2008) The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet 9:20
Wong MH, Tan CS, Lee SC et al (2014) Potential genetic anticipation in hereditary leiomyomatosis-renal cell cancer (HLRCC). Fam Cancer 13:281–289
Alam NA, Barclay E, Rowan AJ et al (2005) Clinical features of multiple cutaneous and uterine leiomyomatosis. Arch Dermatol 141:199–206
Schmidt LS, Linehan WM (2014) Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis 7:253–260
Stewart L, Glenn GM, Stratton P et al (2008) Association of germline mutations in the fumarate hydratase gene and uterine fibroids in women with hereditary leiomyomatosis and renal cell cancer. Arch Dermatol 144:1584–1592
Srigley JR, Delahunt B, Eble JN et al (2013) The International Society of Urological Pathology (ISUP) vancouver classification of renal neoplasia. Am J Surg Pathol 37:1469–1489
Haas NB, Nathanson KL (2014) Hereditary renal cancer syndromes. Adv Chronic Kidney Dis 21:81–90
Rosner I, Bratslavsky G, Pinto PA et al (2009) The clinical implications of the genetics of renal cell carcinoma. Urol Oncol 27:131–136
Menko FH, Maher ER, Schmidt LS et al (2014) Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer 13:637–644
Lehtonen HJ, Kiuru M, Ylisaukko-oja SK et al (2006) Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet 43:523–526
Wei M, Toure O, Glenn GM et al (2006) Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet 43:18–27
Muller M, Ferlicot S, Guillaud-Bataille M et al (2017) Reassessing the clinical spectrum assocation with hereditary leiomyomatosis and renal cell carcinoma syndrome in French FH mutations carriers. Clin Genet 92:606–615
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhola, P.T., Gilpin, C., Smith, A. et al. A retrospective review of 48 individuals, including 12 families, molecularly diagnosed with hereditary leiomyomatosis and renal cell cancer (HLRCC). Familial Cancer 17, 615–620 (2018). https://doi.org/10.1007/s10689-018-0076-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-018-0076-4