Abstract
Many species differ genetically, physiologically, and morphologically between geographically distinct populations, typically in response to variation in ecological and climatic variables. Little is known, however, about geographical variation in sperm morphology. Sperm morphology is under strong sexual selection, has been shown to evolve rapidly, and often co-varies with other reproductive traits (e.g., testis size or mating system) that differ between populations in some species. The aim of this study was to establish whether sperm morphology varies between populations of the red-winged blackbird (Agelaius phoeniceus), a species with an enormous breeding range and marked inter-population variation in both body size and mating system. We found (1) highly significant variation in sperm morphology among study sites, (2) a gradual increase in sperm length from the southwest to the northeast of the breeding range, and (3) a strong negative association between sperm length and body size. However, the relationship with the mating system remains unclear. Several hypotheses to explain these patterns are proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many widely distributed species show genetic, physiological, and morphological variation across their geographical range, including growth rate or age and size at maturity (e.g., Bergmann 1847; James 1970; Gould and Johnston 1972; Atkinson and Sibly 1997; Blanckenhorn and Demont 2004). Variation in body size often also influences other traits, such as number and size of offspring (Roff 1992; Stearns 1992; Kiefer et al. 2008). Sexual traits may also vary between populations, including bird song (e.g., Podos and Warren 2007), sexual ornaments (e.g., Hill 1994; Møller 1995), testis size (Pitcher and Stutchbury 1998; Dziminski et al. 2010), and genital morphology (e.g., Holwell 2008).
Some of this spatial variation in reproductive traits is likely attributable to differences in habitat resources, which may determine the abundance and distribution of sexual partners, thereby resulting in variation of the mating system and the strength of sexual selection between populations (Emlen and Oring 1977). An additional factor likely to explain geographical variation is the duration of the breeding season, which is shorter at high than at low latitudes and may result in considerable variation in breeding synchrony, number of offspring per clutch, or the number of broods per year (e.g., Lack 1947; Murton and Westwood 1977; Weatherhead 1979; Stutchbury and Morton 1995). The generally high breeding synchrony at high latitudes, combined with the production of typically only a single brood per year, might increase male-male competition for paternity, resulting in more intense sperm competition than at low latitudes (Stutchbury and Morton 1995; Spottiswoode and Møller 2004; Macedo et al. 2008), although breeding synchrony may also reduce the opportunity for sperm competition by temporal segregation of male mate guarding and extra-pair copulation activity (e.g., Birkhead and Biggins 1987). Nonetheless, if there is latitudinal variation in the level of sperm competition, reproductive traits that are influenced by sperm competition are likely to exhibit latitudinal clines, as has been reported for testis size in some bird species (Pitcher and Stutchbury 1998).
Despite intense interest in evolutionary diversification of sperm morphology and the relationship between variation in sperm form and reproductive success, information on geographical variation in sperm morphology is very limited. Sperm evolve rapidly and exhibit a tremendous diversity in size and shape both between and within taxa (reviewed by Pitnick et al. 2009). Within species, sperm morphology is thought to be maintained by stabilizing selection (Parker 1993; Morrow and Gage 2001b; Calhim et al. 2007), but it can respond to directional selection, including selection pressure from the female reproductive tract (e.g., Woolley 1971; Morrow and Gage 2001a; Miller and Pitnick 2002). Hence, if spatially segregated populations vary in the strength or form of selection, sperm might be selected towards different optima across populations. Similarly, sperm length is positively associated with the rate of extra-pair paternity (Briskie et al. 1997) or relative testis size in various taxa (Gage 1994; Balshine et al. 2001; Byrne et al. 2003; Lüpold et al. 2009b) and, at least in passerine birds, also with the sperm-producing structures within the testes (Lüpold et al. 2009c) and the size of female sperm-storage tubules (Briskie and Montgomerie 1992). Therefore, latitudinal gradients in testis size and structures may show concomitant variation in sperm morphology. Despite these possible reasons for geographical variation in sperm morphology, the topic remains poorly studied.
The potential for (large-scale) geographical variation in sperm morphology is indicated by significant differences in sperm traits between geographically distinct populations in several taxa, including flies (Snook 2001; Pitnick et al. 2003; but see Hosken et al. 2003), sea urchins (Manier and Palumbi 2008), snails (Minoretti and Baur 2006), fish (Elgee et al. 2010), and frogs (Kuramoto 1996; Hettyey and Roberts 2006). However, most of these studies examined only two to four populations per species, sample sizes that render it difficult to investigate general geographical patterns across the species’ range. Probably the most detailed study to date is Snook’s (2001) on Drosophila subobscura, in which the inter-population variation in sperm morphology was considerable, but not gradual along the examined latitudinal transect, in contrast to the strong latitudinal clines in body size and chromosomal polymorphism in this species (Prevosti et al. 1990; Huey et al. 2000). In another study, yellow dung flies (Scathophaga stercoraria) were raised at different ambient temperatures to simulate latitudinal variation experimentally, and their sperm length increased with temperature and may thus also decrease with latitude (Blanckenhorn and Hellriegel 2002). This result contrasts somewhat with the lack of significant differences in sperm length in the same species among natural populations between Iceland, the United Kingdom, and Switzerland (Hosken et al. 2003). Hence, we still know little about the potential factors driving inter-population diversity in sperm morphology, and further detailed and large-scale studies are needed.
An ideal study species in which to investigate inter-population variation in sperm morphology is the red-winged blackbird (Agelaius phoeniceus), one of the most abundant and widespread bird species in North America, ranging from the east to the west coast and from Alaska to Costa Rica (Jaramillo and Burke 1999). The populations of A. phoeniceus have been classified into 23 subspecies based on morphological measurements and plumage coloration (Blake 1968; but see Van Rossem 1926; Power 1970), although the status of some of these subspecies is under debate (Garrido and Kirkconnell 1996; Jaramillo and Burke 1999). In contrast to the pronounced geographical variation in body morphology, there is relatively little genetic diversity among populations, based on mitochondrial DNA (Ball et al. 1988), allozymes (Gavin et al. 1991) or microsatellites (Williams et al. 2004). This may be due to relatively recent expansion from a common source, or because the dispersal of a few males not returning to their previous breeding site maintains sufficient gene flow between populations to limit genetic differentiation (Dolbeer 1978; Ball et al. 1988; Moore and Dolbeer 1989). The incongruity between genetics and morphology suggests that the morphological differences are probably driven by environmental variation, an idea supported by transplant experiments, in which eggs were transferred between distant populations and the size of body parts of nestlings consistently shifted towards that of the foster populations (James 1983).
Red-winged blackbirds also differ markedly in behavior across populations. For example, reported mean percentages of extra-pair paternity (EPP) across four distant populations differ significantly with a range of 24–40% (Gibbs et al. 1990; Westneat 1993a; Weatherhead and Boag 1995; Gray 1996; Westneat and Mays 2005). Among the same populations, breeding density also varies greatly, resulting in mean distances between territorial males of 12–75 m and explaining a large proportion of the variation in EPP (Westneat and Sherman 1997). As a result, populations are expected to differ in the level of sperm competition. Finally, inter-population differences also exist in the degree of polygyny, with typically 1–4 females per male among eastern populations (Westneat 1993a; Weatherhead 1995; Prather and Cruz 2006), but 4–6 and up to 20 females per male in some western populations (Beletsky 1996; Gray 1997b). Since males copulate frequently with each social partner (Westneat 1993b; Westneat et al. 1998), large harems can apparently result in temporary sperm depletion of territorial males, whereas this risk may be lower for small harems (Gray 1997a, b). Consequently, the combination of high polygyny and sperm competition is expected to exert stronger selection on male ejaculate production and composition compared to situations where both parameters are relatively low, thus potentially resulting in differential evolution of sperm morphology between populations. In fact, two studies indicate that sperm morphology varies across redwing populations (McFarlane 1963; Allen et al. 1967), but the small sample sizes of 1–4 males from each of four populations preclude conclusions on general geographical variation.
The aim of the present study was to examine sperm morphology across the distribution of the red-winged blackbird to test three predictions: (1) sperm morphology varies across populations, (2) sperm morphology exhibits a latitudinal or longitudinal gradient, and (3) this variation is associated with the geographical variation in male and female body size. Such an association with body size could be the result of (1) genetic covariation, (2) a dilution effect (i.e., size-dependent risk of dilution or loss of sperm in the female reproductive tract; Short 1981; Cummins and Woodall 1985), (3) effects of a size-dependent metabolic rate on the spermatogenic rate (Parapanov et al. 2008; Ramm and Stockley 2010), or (4) associations between pre- and post-copulatory sexual selection, such as size-dependent mate acquisition success (e.g., Webster 1992; Weatherhead and Boag 1995) and investments in sperm competitiveness (e.g., sperm velocity increasing with sperm length; Gomendio and Roldan 2008; Fitzpatrick et al. 2009; Lüpold et al. 2009a) or sperm numbers (associated with a trade-off between sperm size and number; Parker 1993).
Material and methods
Sample collection and sperm measurements
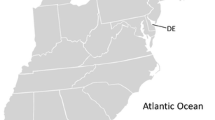
We analyzed the sperm of 459 male red-winged blackbirds from 17 locations throughout the continental United States (study locations in Fig. 1 and online Supplementary Table S1), collected by four different methods: (1) by cloacal massage (Burrows and Quinn 1937; Samour et al. 1986), (2) from natural ejaculations onto the false cloaca of model females (Pellatt and Birkhead 1994; Westneat et al. 1998), (3) from fecal samples (Immler and Birkhead 2005), or (4) by dissecting the distal end of the seminal glomera (i.e., sperm-storage organ at the end of the deferent duct) in birds that were culled for other research or management programs. Sperm collected through these different techniques do not differ in morphological measurements (Immler and Birkhead 2005; Lüpold, pers. obs.). All samples were fixed in 5% buffered formalin solution.
Map of the United States with the locations of sample collection. 1 Whidbey Island, WA; 2 Los Banos, CA; 3 Goleta Sanitary District, CA; 4 Alpaugh, CA; 5 Columbia NWR, WA; 6 Malheur NWR, OR; 7 Kern River Preserve, CA; 8 Fort Collins, CO; 9 Bismarck, ND; 10 Quivira NWR, KS; 11 Eagle Lake, TX; 12 Portageville, MO; 13 Beloit, WI; 14 Drakesboro, KY; 15 Hickory Corners, MI; 16 Fort Myers, FL; 17 Ithaca, NY. For further details on each location, see online Supplementary Table S1
For each sperm sample, we captured digital images of 5–10 morphologically normal and undamaged sperm cells at a magnification of ×250, using a Spot Insight QE camera (Diagnostic Instruments, Inc., Sterling Heights, MI) mounted onto a Leitz Laborlux S microscope (Vila Nova de Famalicão, Portugal). Five to ten sperm per sample appeared adequate for our inter-population comparisons because the intra-sample coefficients of variation were low for all sperm traits (<3.2%) except head length (<8.9%). Moreover, among 10 randomly selected males of a single population for which we measured 50 sperm each, 5 sperm explained >95% of the variation obtained from 50 sperm (>85% for head length). Using computer-assisted image analysis, we measured the following traits of each sperm cell to the nearest 0.1 μm: (1) head length, (2) straight midpiece length (see Birkhead et al. 2005), (3) flagellum length, and (4) total sperm length. All these measures are summarized in the online Supplementary Table S2.
Analyses of geographical variation
To analyze spatial variation in sperm morphology, we used longitude and latitude of all locations (to the nearest 0.1 degree) and constructed distance matrices for the 17 geographical locations and for mean length (per location) of each sperm trait. Accounting for the curvature of the earth (following Banerjee et al. 2004), the pairwise geographical distances among the 17 study sites ranged from 110 to 4,032 km (mean ± SE = 1,901 ± 85 km; N = 136 pairwise combinations), and each site was a minimum of 110 to 1,263 km (471 ± 79 km) away from any other. We performed Mantel tests (Mantel 1967) with 10,000 permutations to establish whether the difference in sperm morphology between locations increases with their geographical distance. For traits that were significantly correlated with geographical distance we calculated autocorrelation coefficients, Moran’s I values (Moran 1950), for each of five distance classes with proportional numbers of pairs (N = 26–28) to characterize the amount of spatial autocorrelation among sites. We calculated Moran’s I values using PASSaGE (Rosenberg 2001; www.passagesoftware.net), with an inverse distance weight matrix and testing for statistical significance against a random distribution. We used the changes of Moran’s I across distance classes (i.e. spatial correlogram) to infer spatial structures as described by Legendre and Fortin (1989) and assessed local significance of Moran’s I only if correlograms were globally significant after Bonferroni correction for multiple tests (Legendre and Fortin 1989). We also tested the presence of anisotropy with an angular correlation and determined the direction of the maximal gradient (Simon 1997).
Sperm morphology and mating system
We tested whether a link exists between sperm morphology and mating system. We had breeding data from six locations, including levels of polygyny (6 sites), nearest-neighbor distance (5 sites) and EPP (4 sites; online Supplementary Table S3). These sample sizes do not permit powerful statistical tests; yet, a consistent pattern among all three parameters could provide some information on potential links. For example, copulation frequency is predicted to increase with the rate of EPP and the level of polygyny resulting from increased numbers of copulations with each female and a greater number of females to inseminate, but also with the breeding density due to an increasing risk of EPP (see Introduction). Males exposed to high levels of EPP may additionally transfer more sperm per insemination to maximize their fertilizing success (e.g., Parker 1990). With constraints within ejaculates or testes likely to result in a trade-off between sperm size and number (e.g., Parker 1993; Lüpold et al. 2009c), we would expect a negative relationship between sperm length and polygyny or EPP, but a positive relationship with the nearest-neighbor distance. In contrast, longer sperm (with longer flagellum and midpiece) might be advantageous in situations of sperm competition because they are more competitive (Gomendio and Roldan 2008; Fitzpatrick et al. 2009; Lüpold et al. 2009a), in which case we would predict positive associations of sperm length at least with EPP (e.g., Briskie et al. 1997), and consequently a negative relationship with the nearest-neighbor distance.
Analyses of sperm length and body size
We examined whether sperm morphology (particularly sperm length) was associated with the geographical variation in male body size (e.g., Van Rossem 1926; Howell and van Rossem 1928; Power 1970). We measured wing, tarsus and tail length to the nearest 0.1 mm for all captured birds (using mist-nets or walk-in traps) before releasing them on site upon collection of a sperm sample. At some locations, we were unable to take measures on body size because males could not be captured due to unsuitable conditions for mist-nets and traps, or, as for the endangered A. p. aciculatus, due to stringent permitting restrictions, such that sperm samples could be collected only by natural ejaculation onto model females. In some other locations, body size measures were not taken by the researchers who provided the sperm samples. We obtained body size measurements for missing locations in the following ways (numbers refer to Fig. 1 and Table S2): for location 8 we used the data from Power (1970), who measured red-winged blackbirds in approximately the same site; for three other locations, the Field Museum of Natural History in Chicago kindly provided measurements from sites <100 km from ours, and the shortest distance of these sites to any other of our dataset was 300 km or more (Fig. 1). Finally, for three locations, from which no data were available, we used the mean values for the putative subspecies from the literature, with subspecies assignment based on the distribution maps in Gavin et al. (1991) (Table S1). This approach seemed justified because A. p. aciculatus and A. p. californicus are endemic to relatively small distinct areas in California (Kern River Valley and the northern part of the Central Valley, respectively), and for A. p. mearnsi, the vast majority of the birds measured by Howell and Van Rossem (1928) across Central Florida were from within about 100 km of our study site, such that the reported mean values were unlikely to deviate substantially from the presumed mean of our study site. Finally, the subspecies identity was unclear for location 4, because according to distribution maps (e.g., Van Rossem 1926; Gavin et al. 1991), this area would clearly be populated by A. p. californicus (the bicolored form with no yellow epaulet line), but all males from which we collected ejaculates using female models had a yellow line on the epaulet (Lüpold, pers. obs.). Agelaius p. neutralis appears to be the most likely candidate (Laymon and Garrett, pers. comm.), although morphological or genetic evidence is currently lacking. Nonetheless, all our results remained qualitatively the same when study site 4 was included or excluded. Due to the use of different sources for measurements of body size, we also verified our main results involving body size by re-analysis using only populations where body size was measured for this study directly (N = 9).
As a measure of body size we used the first principal component (PC1) from a principal components analysis (PCA) on the dimensions of wing, tail, and tarsus length, which explained 91.3% of the variance in size measures and was highly correlated with wing length (N = 17; wing: r = 0.98; tarsus: r = 0.81; tail: r = 0.92). We also performed a similar PCA on female body size, where PC1 explained 92.6% of the total variation and was again most strongly associated with wing length (r = 0.98; tarsus: r = 0.45; tail: r = 0.93).
We analyzed associations between sperm traits and body size in linear models to determine the sign of the relationships. However, in order to account for the spatial non-independence between data points, we confirmed the above results in partial Mantel tests (with 10,000 permutations), which are analogous to partial correlations but correlate the two morphological distance matrices of interest whilst controlling for the effects of geographical distances among the study locations (Legendre 2000). Note, however, that a significant Mantel statistic is much lower than would be expected from a conventional correlation analysis (Legendre 2000), such that relatively low r M values should not be interpreted as reflecting low biological significance.
In addition to relationships between sperm length and absolute male size (PC1), we also examined those with relative male size (i.e., corrected for female body size, PC1) as an index of sexual dimorphism. Across species of the New World blackbirds (Icteridae) male body size increases disproportionately with female size in response to increasing levels of polygyny (e.g., Björklund 1991; Webster 1992). Across the six sites with data on polygyny (online Supplementary Table S3), male body size, controlled for female size, also increased with polygyny (partial r = 0.82 (0.18 to 0.94), t = 3.25, P = 0.03; female size: partial r = –0.84 (–0.94 to –0.23), t = –3.46, P = 0.03).
Results
The three sperm components and total sperm length differed significantly among the 17 study locations (head: F 16,442 = 7.65, P < 0.0001; midpiece: F 16,442 = 4.34, P < 0.0001; flagellum: F 16,442 = 6.34, P < 0.0001; total length: F 16,442 = 6.80, P < 0.0001; also see Fig. 2). These results were qualitatively the same when grouping individuals by subspecies (N = 11) rather than collecting locations (all F 10,448 > 7.07, all P < 0.0001).
Mean sperm length and 95%CI for red-winged blackbirds from 17 different collecting locations. The location numbers refer to those in Fig. 1, arranged from west to east. The figures in italics indicate the number of males on each site, from which sperm samples were analyzed
In addition, in linear mixed-effects models with location as a random factor, total sperm length was highly correlated with all partial sperm measures (all r > 0.28, all P < 0.0001), but among the latter, only midpiece and flagellum length were strongly associated (r = 0.55, P < 0.0001; detailed results in Supplementary Table S4).
In all further analyses, we used mean values for each collecting location.
Geographical patterns in sperm morphology
In Mantel tests, the inter-site differences in midpiece, flagellum and total sperm length increased significantly with the distance between sites (r M = 0.17, P = 0.04, r M = 0.27, P = 0.01 and r M = 0.19, P = 0.03, respectively), whereas that of head length did not (r M = 0.05, P = 0.24). These results were also robust when controlling for body size in partial Mantel tests (i.e. holding the body size matrix constant; head: r M = 0.06, P = 0.24; midpiece: r M = 0.16, P = 0.05; flagellum: r M = 0.25, P = 0.02; total sperm length: r M = 0.17, P = 0.05).
All sperm traits with significant spatial correlation showed positive autocorrelation at short distances and negative autocorrelation for long distances (though not statistically significant for midpiece length; Table 1), which is the typical pattern of spatial gradients (Legendre and Fortin 1989). Angular correlograms showed that the degree of maximal gradients for midpiece, flagellum and total sperm length was 49.6° (P = 0.06), 50.5° (P = 0.007) and 56.6° (P = 0.02) from east (0°), respectively; that is, in a southwest to northeast direction. Along these gradients, the length of sperm traits increased to the northeast, as revealed by positive associations with both longitude and latitude in multiple regressions, corrected for body size (Table 2; Fig. 3).
Sperm morphology and mating system
Visual inspection of Fig. 4 did not reveal any systematic pattern of the three breeding parameters on sperm length (and all P > 0.47). For example, even in pairwise comparisons between the populations with the lowest and highest values of polygyny or nearest-neighbor distance, respectively, sperm length did not differ, even though it did for EPP (Fig. 4). These results do not permit any firm conclusions, but they suggest that sperm length might at best be weakly affected by the mating system (but see below).
Sperm morphology and body size
Given the variation in male body size across the geographical distribution of the red-winged blackbird (see Introduction), we tested whether the variation in sperm length was related to that of body size. Among the 17 locations, the differences in male body size increased with geographical distance (Mantel test: r M = 0.21, P = 0.03), but we detected no significant spatial autocorrelation across the five distance classes (global correlogram P = 0.17).
Sperm length was negatively correlated with male body size (r = –0.68 (–0.83 to –0.30), t = –3.57, P = 0.003), also after omitting the locations with body size measures taken from other sources than this study (N = 9; r = –0.71 (–0.88 to –0.08), t = –2.68, P = 0.03). This association was also robust to control for spatial effects in a partial Mantel test (partial r M = 0.37, P = 0.005; also see Table 2 and Fig. 5).
Relationship of total sperm length with male body size as PC1 (r = –0.85, P < 0.0001) across geographically distinct locations (N = 17), with both axes controlled for longitude and latitude. The black points (N = 9) indicate the data where body size was measured for this study, the gray points (N = 8) depict data with body size measures taken from the literature or museum collections (see Sect. “Material and methods”)
Male body size co-varied positively with female body size (r = 0.83 (0.58 to 0.91), t = 5.66, P < 0.0001; partial Mantel test corrected for spatial distances: r M = 0.70, P = 0.0004). Total sperm length was negatively correlated with male size corrected for female size and geographical coordinates (partial r = –0.65 (–0.82 to –0.25), t = –3.31, P = 0.006), and the significance of the relationship between sperm length and relative male body size was confirmed by a partial Mantel test holding both the female size and spatial distance matrices constant (partial r M = 0.27, P = 0.02). Furthermore, total sperm length was negatively associated with female body size in a simple regression (r = –0.59 (–0.79 to –0.16), t = –2.86, P = 0.01), but the partial Mantel test corrected for spatial effects was not significant (partial r M = 0.14, P = 0.11). Discarding the eight populations with body size measures taken from other sources yielded results that were consistent with all the above results with respect to the sign of associations, but they were not statistically significant (all N = 9; male size—female size: r = 0.08 (–0.55 to 0.64), t = 0.22, P = 0.83; sperm length—male size corrected for female size: partial r = –0.50 (–0.81 to 0.23), t = –1.53, P = 0.18; sperm length—female size: r = –0.41 (–0.77 to 0.32), t = –1.19, P = 0.27).
Finally, we investigated the relationship between sperm length and male body size within six populations with sperm morphology and body size measured for at least 15 individuals (N = 15–53). All these associations were not statistically significant (online Supplementary Table S5).
Discussion
The main results of this study were significant geographical variation in sperm morphology across the breeding range of the red-winged blackbird, with an increase in sperm traits from the southwest to the northeast, and a strong inverse association with body size across but not within populations.
Geographical variation in sperm morphology
Across taxa the variation in sperm morphology is considerable (reviewed in Pitnick et al. 2009). Although intra-specific variation in sperm morphology decreases with the intensity of sexual selection (e.g., Calhim et al. 2007) and red-winged blackbirds exhibit one of the lowest values of intra-specific variation in sperm length of any Icterid species studied so far (Lüpold et al. 2009b), we found marked differences among our 17 study sites. The absolute differences were small (approximately 6 μm or 4% of total sperm length between the longest and shortest location means), but statistically highly significant. Two previous studies (McFarlane 1963; Allen et al. 1967) have documented some level of sperm variation in this species, with measurements that are comparable to ours. Both of these previous studies, however, were based on small sample sizes and mostly restricted to populations along the east coast, making it difficult to investigate large-scale geographical variation.
More powerful across-population comparisons of sperm traits have been conducted in flies and revealed significant variation between different populations (e.g., Snook 2001; Pitnick et al. 2003). However, to our knowledge the only study to specifically examine gradual variation in sperm morphology found no such pattern in D. subobscura (Snook 2001). Gradual variation of traits is typically found along the latitudinal axis, particularly due to gradients in temperature, seasonality, and other climatic and ecological variables (Mayr 1963; Endler 1977). In the red-winged blackbird, however, sperm length (and in particular flagellum length) increased in a southwest to northwest direction across the United States.
One explanation for the observed geographical patterns of sperm morphology is that they may reflect historical processes associated with the expansion of the red-winged blackbird across the North American continent. Agelaius phoeniceus is thought to have diverged from the tricolored blackbird (A. tricolor) around 3.35 million years ago (Klicka and Zink 1997). Fossil records of A. phoeniceus are too scarce to provide information on evolutionary trends by region or expansion pathways across the continent (Emslie, pers. comm.), but the oldest fossil records of eastern populations (i.e. Florida) are about 2.5 million years old (Emslie 1998). Assuming that divergence times of fossil records are reasonably coherent with molecular data, A. phoeniceus may have colonized North America from the southwest (where extant populations of A. tricolor are located) and expanded towards the north and east, reaching the north only after the Wisconsin glacier started to retreat about 18,000 years ago (Ball et al. 1988). Sperm morphology might be under genetic drift and subtle changes might have occurred during species expansion [also note that A. tricolor sperm are shorter (124.4 ± 0.84 μm) than the shortest of our Californian populations of A. phoeniceus (140.3 ± 0.47 μm)]. This scenario would be consistent with our finding that differences in sperm morphology increase with the distance between populations. However, to reconstruct potential expansion pathways and for a more complete picture of whether genetic drift is important for the inter-population differences in sperm morphology, we clearly need highly resolved molecular inter-population studies.
Sperm morphology and mating system
Emlen and Oring (1977) showed how ecological factors can determine mating systems. In the red-winged blackbird, the duration of the breeding season differs between regions (e.g., Gray 1996; Prather and Cruz 2006), and the size and density of populations vary with habitat types across different climatic zones of the North American continent, resulting in variation of the mating system (Orians 1980; Searcy and Yasukawa 1995). Given the relatively high levels of sperm competition compared to other Icterid species (Lüpold et al. 2009b), red-winged blackbirds probably are under relatively strong sexual selection, such that sperm of this species could be expected to evolve rapidly in response to the variation in mating systems.
Our dataset was limited regarding breeding data, but the negative association between sperm length and relative male body size, which was linked with polygyny, suggests that it is too early to discard the possibility of sexual selection being one mechanism driving the variation in sperm length in the red-winged blackbird (also see below). Information on the mating system of additional populations is clearly needed before firm conclusions can be drawn.
Sperm length and body size
One of the most striking and unexpected relationships we found was a strong negative association between sperm length and body size across our study sites. It currently remains unclear whether there is a genetic (e.g., pleiotropic) or functional link between the two variables or whether the observed relationship is the result of two independent trends. However, in the following we discuss potential mechanisms based on male and female effects. The first two explanations are based on the idea that large males might be under stronger selection to transfer increased numbers of sperm than small males, but since sperm production is limited, sperm number is traded off against sperm length (e.g., Parker 1993; Parker et al. 2010). A third explanation will focus on direct selection on sperm length.
First, in a large female (with a large reproductive tract), sperm may experience a higher risk of being diluted or ‘getting lost’ than in a small female (‘dilution effect’; Short 1981; Cummins and Woodall 1985). If sperm number is traded off against sperm length, the inverse relationships of sperm length with both male and female body size across our sites would be consistent with this idea. Although one might also expect males to increase sperm length rather than sperm number in response to a dilution effect, recent sperm raffle models predict that in conditions with no constraint on the space for fertilization (as in birds where the female genital tract is substantially larger than sperm), sperm number has a much stronger effect on the outcome of sperm competition or dilution effect than sperm length (Parker et al. 2010).
Second, relatively larger males may, on average, attract more females and copulate more frequently, resulting in an increased risk of sperm depletion. Within populations of red-winged blackbirds, relatively large males indeed tend to defend larger territories and harems (e.g., Searcy and Yasukawa 1995; Weatherhead and Boag 1995; Westneat 2006). In addition, our data indicated a positive relationship between male size relative to female size and the level of polygyny as has previously been reported across Icterid species (e.g., Björklund 1991; Webster 1992). With a higher risk of sperm depletion, an increase in sperm number at the cost of sperm length (or quality) might again be more beneficial similar to the dilution effect. Detailed studies are now needed to establish whether polygyny or any other measure of the mating system results in variation in the risk of sperm depletion and whether this might result in a trade-off between sperm length and number. Addressing these links in wild bird populations, however, might be challenging. For example, although collecting natural ejaculates and quantifying sperm therein is relatively straightforward, these data are only useful with a male’s detailed mating history preceding sample collection as sperm counts are greatly influenced by previous ejaculations (e.g., Westneat et al. 1998). Similarly, relative testis size as an index of sperm competition or investment in sperm production changes considerably across the season (e.g., Wright and Wright 1944), and it is thus only reliable if collected during a relatively brief period around the peak of the breeding season for each population (Calhim and Birkhead 2007), resulting in logistic challenges in a large-scale study across populations like ours.
For both the dilution and sperm depletion effects, the underlying mechanism resulting in the trade-off between sperm length and number might be physiological or morphological constraints during sperm production. For example, at least across species, larger males exhibit a relatively slower metabolic rate and thus presumably a slower spermatogenic rate (Rezende et al. 2002; Parapanov et al. 2008). If such variation also exists in the red-winged blackbird and the production of long sperm entails a longer spermatogenic cycle (Ramm and Stockley 2010) or is less efficient (Lüpold et al. 2009c; Lüpold, Wistuba and Birkhead, manuscript), populations differing in average male size might also vary in average sperm length. This may be particularly the case if sperm number is more critical than sperm length (see above) and the production of shorter sperm might compensate for a relatively slower spermatogenic turnover. Additionally, males may differ in the size of their testes or sperm-producing structures, and testis morphology per se might be associated with sperm length (see Lüpold et al. 2009c).
A third possible explanation for the negative relationship between sperm length and body size could be direct selection on sperm length. For example, if relatively large males are better at acquiring mates (see above), smaller males may in turn invest more in postcopulatory competition by producing longer and thus potentially more competitive sperm. For example, in the domestic fowl (Gallus domesticus), subordinate males have limited access to females and hence trade up sperm velocity (Cornwallis and Birkhead 2007; Pizzari et al. 2007), which can increase their fertilization success in competitive situations (e.g., Birkhead et al. 1999). A similar situation may exist in red-winged blackbirds if small males gain mating opportunities predominantly through extra-pair copulations rather than defending a harem of females. If sperm velocity co-varies positively with sperm length, for which there is evidence both across and within passerine birds (Lüpold et al. 2009a; Mossman et al. 2009; but see Kleven et al. 2009; Lüpold et al. 2009b), smaller males might gain by producing longer sperm. Although Lüpold et al. (2009b) have not found a significant association between sperm velocity and sperm morphology within a population of red-winged blackbirds, we cannot eliminate the possibility that populations differ in the intensity of selection on both pre- and postcopulatory sexual traits and that these could be associated in one way or another.
Finally, it is also possible that increased body size is not primarily the result of variation in the mating system within a species, even if such an evolutionary trend exists across species. For example, the red-winged blackbird is thought to be one of many bird species in North America with increasing body size north- and westward in response to climatic gradients, probably for thermoregulatory reasons (e.g., Power 1969; James 1970). Similar effects are also indicated by James’s (1983) transplant experiment, in which environmental factors influenced nestling growth. Part of this differential growth or the variance in body size may be due to variation in habitat types across different climate zones. Consequently, body size and mating system might both be driven by a combination of ecological factors, potentially even independently in the same direction. Irrespective of the processes selecting for larger males, increased investment in sperm quantity by larger males may be one explanation for the inverse association between body size and sperm length. However, until further detailed information is available, independent selection on these two traits can be just as likely (e.g., thermoregulation in body size and genetic drift of sperm morphology).
Conclusions
We observed striking geographical variation in sperm morphology across different populations of red-winged blackbirds, but in contrast to the patterns found across species, our data are not conclusive about whether this variation is the result of different mating systems among populations. Strong relationships between sperm length and body size, however, suggest that sperm length might be driven primarily by body size, irrespective of whether this is mediated by variation in the mating system. Further research is needed to disentangle the factors driving these patterns. In addition, detailed and sensitive genetic studies might shed light on the expansion pathways to investigate the effect of historical processes. Finally, it would also be interesting to transplant males across populations or manipulate the breeding conditions to establish whether sperm morphology changes according to differences in the breeding environment in a similar way as body size has been shown to adjust to the foster population, but this may logistically be difficult with wild populations.
References
Allen JP, Hamon JH, McFarlane RW (1967) Some studies of the spermatozoa of certain species of the Icteridae (Blackbirds). Proc Indiana Acad Sci 77:434–441
Atkinson D, Sibly RM (1997) Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol Evol 12:235–239
Ball RM Jr, Freeman S, James FC, Bermingham E, Avise JC (1988) Phylogeographic population structure of red-winged blackbirds assessed by mitochondrial DNA. Proc Natl Acad Sci USA 85:1558–1562
Balshine S, Leach BJ, Neat F, Werner NY, Montgomerie R (2001) Sperm size of African cichlids in relation to sperm competition. Behav Ecol 12:726–731
Banerjee S, Carlin BP, Gelfand AE (2004) Hierarchical modeling and analysis for spatial data. Chapman & Hall/CRC, Boca Raton
Beletsky L (1996) The red-winged blackbird. The biology of a strongly polygynous songbird. Academic Press, London
Bergmann C (1847) Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien 1:595–708
Birkhead TR, Biggins JD (1987) Reproductive synchrony and extra-pair copulation in birds. Ethology 74:320–334
Birkhead TR, Martínez JG, Burke T, Froman DP (1999) Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc R Soc Lond B 266:1759–1764
Birkhead TR, Pellatt EJ, Brekke P, Yeates R, Castillo-Juarez H (2005) Genetic effects on sperm design in the zebra finch. Nature 434:383–387
Björklund M (1991) Evolution, phylogeny, sexual dimorphism and mating system in the grackles (Quiscalus spp.: Icterinae). Evolution 45:608–621
Blake (1968) Family Icteridae. In: Paynter RA Jr (ed) Check-list of birds of the world, vol XIV. Museum of Comparative Zoology, Harvard University, Cambridge, pp 138–202
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Blanckenhorn WU, Hellriegel B (2002) Against Bergmann’s rule: fly sperm size increases with temperature. Ecol Lett 5:7–10
Briskie JV, Montgomerie R (1992) Sperm size and sperm competition in birds. Proc R Soc Lond B 247:89–95
Briskie JV, Montgomerie R, Birkhead TR (1997) The evolution of sperm size in birds. Evolution 51:937–945
Burrows WH, Quinn JP (1937) The collection of spermatozoa from domestic fowl and turkey. Poult Sci 16:19–24
Byrne PG, Simmons LW, Roberts JD (2003) Sperm competition and the evolution of gamete morphology in frogs. Proc R Soc Lond B 270:2079–2086
Calhim S, Birkhead TR (2007) Testes size in birds: quality versus quantity—assumptions, errors, and estimates. Behav Ecol 18:271–275
Calhim S, Immler S, Birkhead TR (2007) Postcopulatory sexual selection is associated with reduced variation in sperm morphology. PLoS ONE 2:e413. doi:10.1371/journal.pone.0000413
Cornwallis CK, Birkhead TR (2007) Changes in sperm quality and numbers in response to experimental manipulation of male social status and female attractiveness. Am Nat 170:758–770
Cummins JM, Woodall PF (1985) On mammalian sperm dimensions. J Reprod Fertil 75:153–175
Dolbeer WA (1978) Movement and migration patterns of red-winged blackbirds: a continental overview. Bird Band 49:17–34
Dziminski MA, Roberts JD, Beveridge M, Simmons LW (2010) Among-population covariation between sperm competition and ejaculate expenditure in frogs. Behav Ecol 21:322–328
Elgee KE, Evans JP, Ramnarine IW, Rush SA, Pitcher TE (2010) Geographic variation in sperm traits reflects predation risk and natural rates of multiple paternity in the guppy. J Evol Biol 23:1331–1338
Emlen ST, Oring LW (1977) Ecology, sexual selection, and evolution of mating systems. Science 197:215–223
Emslie SD (1998) Avian community, climate, and sea-level changes in the Plio-Pleistocene of the Florida Peninsula. Ornithol Monogr 50:1–113
Endler JA (1977) Geographic variation, speciation, and clines. Princeton University Press, Princeton
Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S (2009) Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc Natl Acad Sci USA 106:1128–1132
Gage MJG (1994) Associations between body size, mating pattern, testis size and sperm lengths across butterflies. Proc R Soc Lond B 258:247–254
Garrido O, Kirkconnell A (1996) Taxonomic status of the Cuban form of the red-winged blackbird. Wilson Bull 108:372–374
Gavin TA, Howard RA, May B (1991) Allozyme variation among breeding populations of red-winged blackbirds: the California conundrum. Auk 108:602–611
Gibbs HL, Weatherhead PJ, Boag PT, White BN, Tabak LM, Hoysak DJ (1990) Realized reproductive success of polygynous red-winged blackbirds revealed by DNA markers. Science 250:1394–1397
Gomendio M, Roldan ERS (2008) Implications of diversity in sperm size and function for sperm competition and fertility. Int J Dev Biol 52:439–447
Gould SJ, Johnston RF (1972) Geographic variation. Annu Rev Ecol Syst 3:457–498
Gray EM (1996) Female control of offspring paternity in a western population of red-winged blackbirds (Agelaius phoeniceus). Behav Ecol Sociobiol 38:267–278
Gray EM (1997a) Do female red-winged blackbirds benefit genetically from seeking extra-pair copulations? Anim Behav 53:605–623
Gray EM (1997b) Intraspecific variation in extra-pair behavior of red-winged blackbirds (Agelaius phoeniceus). Ornithol Monogr 49:61–80
Hettyey A, Roberts JD (2006) Sperm traits of the quacking frog, Crinia georgiana: intra- and interpopulation variation in a species with a high risk of sperm competition. Behav Ecol Sociobiol 59:389–396
Hill GE (1994) Geographic variation in male ornamentation and female mate preference in the house finch: a comparative test of models of sexual selection. Behav Ecol 5:64–73
Holwell GI (2008) Geographic variation in genital morphology of Ciulfina praying mantids. J Zool 276:108–114
Hosken DJ, Garner TWJ, Blanckenhorn WU (2003) Asymmetry, testis and sperm size in yellow dung flies. Funct Ecol 17:231–236
Howell AH, van Rossem AJ (1928) A study of the red-winged blackbirds of souteasthern United States. Auk 45:155–163
Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–309
Immler S, Birkhead TR (2005) A non-invasive method for obtaining spermatozoa from birds. Ibis 147:827–830
James FC (1970) Geographic variation in birds and its relationship to climate. Ecology 51:3
James FC (1983) Environmental component of morphological differentiation in birds. Science 221:184–186
Jaramillo A, Burke P (1999) New World blackbirds: the icterids. Helm, London
Kiefer MC, Van Sluys M, Rocha CFD (2008) Clutch and egg size of the tropical lizard Tropidurus torquatus (Tropiduridae) along its geographic range in coastal eastern Brazil. Can J Zool 86:1376–1388
Kleven O, Fossøy F, Laskemoen T, Robertson RJ, Rudolfsen G, Lifjeld JT (2009) Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution 63:2466–2473
Klicka J, Zink RM (1997) The importance of recent ice ages in speciation: a failed paradigm. Science 277:1666–1668
Kuramoto M (1996) Generic differentiation of sperm morphology in treefrogs from Japan and Taiwan. J Herpetol 30:437–443
Lack D (1947) Significance of clutch-size, parts I and II. Ibis 89:302–552
Legendre P (2000) Comparison of permutation methods for the partial correlation and partial Mantel tests. J Stat Comput Simul 67:37–73
Legendre P, Fortin M-J (1989) Spatial pattern and ecological analysis. Vegetatio 80:107–138
Lüpold S, Calhim S, Immler S, Birkhead TR (2009a) Sperm morphology and sperm velocity in passerine birds. Proc R Soc Lond B 276:1175–1181
Lüpold S, Linz GM, Birkhead TR (2009b) Sperm design and variation in the New World blackbirds (Icteridae). Behav Ecol Sociobiol 63:899–909
Lüpold S, Linz GM, Rivers JW, Westneat DF, Birkhead TR (2009c) Sperm competition selects beyond relative testes size in birds. Evolution 63:391–402
Macedo RH, Karubian J, Webster MS (2008) Extrapair paternity and sexual selection in socially monogamous birds: are tropical birds different? Auk 125:769–777
Manier MK, Palumbi SR (2008) Intraspecific divergence in sperm morphology of the green sea urchin, Strongylocentrotus droebachiensis: implications for selection in broadcast spawners. BMC Evol Biol 8:283. doi:10.1186/1471-2148-8-283
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mayr E (1963) Animal species and evolution. Harvard University Press, Cambridge
McFarlane RW (1963) The taxonomic significance of avian sperm. Proc XIII Int Ornithol Congr 91:91–102
Miller GT, Pitnick S (2002) Sperm-female coevolution in Drosophila. Science 298:1230–1233
Minoretti N, Baur B (2006) Among- and within-population variation in sperm quality in the simultaneously hermaphroditic land snail Arianta arbustorum. Behav Ecol Sociobiol 60:270–280
Møller AP (1995) Sexual selection in the barn swallow (Hirundo rustica). V. Geographic variation in ornament size. J Evol Biol 8:3–19
Moore WS, Dolbeer WA (1989) The use of banding recovery data to estimate dispersal rates and gene flow in avian species: case studies in the red-winged blackbird and common grackle. Condor 91:242–253
Moran PAP (1950) Notes on continuous stochastic phenomena. Biometrika 37:17–23
Morrow EH, Gage MJG (2001a) Artificial selection and heritability of sperm length in Gryllus bimaculatus. Heredity 87:356–362
Morrow EH, Gage MJG (2001b) Consistent significant variation between individual males in spermatozoal morphometry. J Zool 254:147–153
Mossman J, Slate J, Humphries S, Birkhead TR (2009) Sperm morphology and velocity are genetically co-determined in the zebra finch. Evolution 63:2730–2737
Murton RK, Westwood NJ (1977) Avian breeding cycles. Clarendon Press, Oxford
Orians GH (1980) Some adaptations of marsh-nesting blackbirds. Princeton University Press, Princeton
Parapanov R, Nusslé S, Hausser J, Vogel P (2008) Relationships of basal metabolic rate, relative testis size and cycle length of spermatogenesis in shrews (Mammalia, Soricidae). Reprod Fertil Dev 20:431–439
Parker GA (1990) Sperm competition games: sneaks and extra-pair copulations. Proc R Soc Lond B 242:127–133
Parker GA (1993) Sperm competition games: sperm size and sperm number under adult control. Proc R Soc Lond B 253:245–254
Parker GA, Immler S, Pitnick S, Birkhead TR (2010) Sperm competition games: sperm size (mass) and number under raffle and displacement, and the evolution of P2. J Theor Biol 264:1003–1023
Pellatt EJ, Birkhead TR (1994) Ejaculate size in zebra finches Taeniopygia guttata and a method for obtaining ejaculates from passerine birds. Ibis 136:97–106
Pitcher TE, Stutchbury BJM (1998) Latitudinal variation in testis size in six species of North American songbirds. Can J Zool 76:618–622
Pitnick S, Miller GT, Schneider K, Markow TA (2003) Ejaculate-female coevolution in Drosophila mojavensis. Proc R Soc Lond B 270:1507–1512
Pitnick S, Hosken DJ, Birkhead TR (2009) Sperm morphological diversity. In: Birkhead TR, Hosken DJ, Pitnick S (eds) Sperm biology: an evolutionary perspective. Elsevier, London, pp 69–149
Pizzari T, Cornwallis CK, Froman D (2007) Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc R Soc Lond B 274:853–860
Podos J, Warren PS (2007) The evolution of geographic variation in birdsong. Adv Study Behav 37:403–458
Power DM (1969) Evolutionary implications of wing and size variation in the red-winged blackbird in relation to geographic and climatic factors: a multiple regression analysis. Syst Zool 18:363–373
Power DM (1970) Geographic variation of red-winged blackbirds in Central North America. Univ Kansas Publ Mus Nat Hist 19:1–83
Prather JW, Cruz A (2006) Breeding biology of red-winged blackbirds in South Florida. Southeast Nat 5:547–554
Prevosti A, Serra L, Segarra E, Aguadé M, Ribó G, Monclús M (1990) Clines of chromosomal arrangements of Drosophila subobscura in South America evolve closer to old world patterns. Evolution 44:218–221
Ramm SA, Stockley P (2010) Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biol Lett 6:219–221
Rezende EL, Swanson DL, Fernando Novoa F, Bozinovic F (2002) Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. J Exp Biol 205:101–107
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman & Hall, New York
Rosenberg MS (2001) PASSaGE. Pattern analysis, spatial statistics, and geographic exegesis. www.passagesoftware.net, Tempe, Arizona
Samour JH, Smith CA, Moore HD, Markham JA (1986) Semen collection and spermatozoa characteristics in budgerigars (Melopsittacus undulatus). Vet Rec 118:397–399
Searcy WA, Yasukawa K (1995) Polygyny and sexual selection in red-winged blackbirds. Princeton University Press, Princeton
Short RV (1981) Sexual selection in man and the great apes. In: Graham CE (ed) Reproductive biology of the great apes. Academic Press, New York, pp 319–341
Simon G (1997) An angular version of spatial correlations, with exact significance tests. Geogr Anal 29:267–278
Snook RR (2001) Absence of latitudinal clines in sperm characters in North American populations of Drosophila subobscura (Diptera: Drosophilidae). Pan-Pac Entomol 77:261–271
Spottiswoode C, Møller AP (2004) Extrapair paternity, migration, and breeding synchrony in birds. Behav Ecol 15:41–57
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stutchbury BJ, Morton ES (1995) The effect of breeding synchrony on extra-pair mating systems in songbirds. Behaviour 132:675–690
Van Rossem AJ (1926) The California forms of Agelaius phoeniceus (Linnaeus). Condor 28:215–230
Weatherhead PJ (1979) Ecological correlates of monogamy in tundra-breeding savannah sparrows. Auk 96:391–401
Weatherhead PJ (1995) Effects on female reproductive success of familiarity and experience among male red-winged blackbirds. Anim Behav 49:967–976
Weatherhead PJ, Boag PT (1995) Pair and extra-pair mating success relative to male quality in red-winged blackbirds. Behav Ecol Sociobiol 37:81–91
Webster MS (1992) Sexual dimorphism, mating system and body size in New World blackbirds (Icterinae). Evolution 46:1621–1641
Westneat DF (1993a) Polygyny and extrapair fertilizations in eastern red-winged blackbirds (Agelaius phoeniceus). Behav Ecol 4:49–60
Westneat DF (1993b) Temporal patterns of within-pair copulations, male mate guarding, and extra-pair events in eastern red-winged blackbirds (Agelaius phoeniceus). Behaviour 124:267–290
Westneat DF (2006) No evidence of current sexual selection on sexually dimorphic traits in a bird with high variance in mating success. Am Nat 167:E171–E189
Westneat DF, Mays HL (2005) Tests of spatial and temporal factors influencing extra-pair paternity in red-winged blackbirds. Mol Ecol 14:2155–2167
Westneat DF, Sherman PW (1997) Density and extra-pair fertilizations in birds: a comparative analysis. Behav Ecol Sociobiol 41:205–215
Westneat DF, McGraw LA, Fraterrigo JM, Birkhead TR, Fletcher F (1998) Patterns of courtship behavior and ejaculate characteristics in male red-winged blackbirds. Behav Ecol Sociobiol 43:161–171
Williams CL, Homan HJ, Johnston JJ, Linz GM (2004) Microsatellite variation in red-winged blackbirds (Agelaius phoeniceus). Biochem Genet 42:35–41
Woolley DM (1971) Selection for the length of the spermatozoan midpiece in the mouse. Genet Res 16:261–275
Wright PL, Wright MH (1944) Reproductive cycle of the male red-winged blackbird. Condor 46:46–59
Acknowledgments
We thank J. Homan, G. Linz, L. Reinhardt, A. Trutsch, and K. Yasukawa for their help in the field, R. Byrd, J. Cummins, D. Elwonger, L. Merrill, T. Muir, J. Rivers, V. Rohwer, S. Tupper, S. Werner, and M. Whitfield for providing additional sperm samples, the SPU meeting at Syracuse University for insightful discussion, and R. Montgomerie, R. Snook and three anonymous reviewers for valuable comments on the manuscript. S.L. was supported by the Janggen-Poehn Foundation, Swiss National Science Foundation, a Sheffield University ORS Award, a Lauff Research Award, a KBS Visiting Graduate Student Fellowship, and an NSF LTER Graduate Research Award; D.F.W. by the University of Kentucky; and T.R.B. by the Leverhulme Trust.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lüpold, S., Westneat, D.F. & Birkhead, T.R. Geographical variation in sperm morphology in the red-winged blackbird (Agelaius phoeniceus). Evol Ecol 25, 373–390 (2011). https://doi.org/10.1007/s10682-010-9410-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-010-9410-5