Abstract
Extra-pair paternity is common among socially monogamous bird species and considered an important driver of post-copulatory sexual selection on ejaculate traits including sperm traits. Patterns of extra-pair paternity and sperm size both show substantial variation among populations, yet we know little about the expression of these key reproductive traits at high latitudes. Here we report patterns of extra-pair paternity and describe variation in sperm dimensions in a Norwegian population of the socially monogamous Great Tit (Parus major) breeding beyond the polar circle at 69° northern latitude. Across six study years, we detected extra-pair paternity in 19.2% of 26 broods, and on average 4.7% of nestlings per brood were extra-pair offspring. As expected from results of previous intraspecific analyses of latitudinal variation in extra-pair paternity rates, the observed rate of extra-pair offspring was low in comparison to published estimates from more southern Great Tit populations (range: 2.9 − 20.4%). Our results therefore support a pattern of decreasing levels of extra-pair paternity with increasing latitude in this species also for extremely high latitudes. Overall mean sperm total length amounted to 97.5 ± 0.6 (SE) μm and 30.6% of the total phenotypic variation in sperm total length was explained by differences among sperm samples. The among-sample coefficient of variation in mean sperm total length per sample was 1.93%. Using previous comparative work as a yardstick, this value is substantially lower than expected for the observed frequency of 4.7% extra-pair offspring.
Zusammenfassung
Fremdvaterschaften und Variation in der Spermienlänge in einer hochnordischen Population der Kohlmeise (Parus major)
Vaterschaften außerhalb des Paarbundes kommen bei sozial monogamen Vogelarten häufig vor und gelten als wichtige Triebkraft postkopulatorischer sexueller Selektion auf Ejakulatmerkmale, inklusive Spermienmerkmale. Sowohl Fremdvaterschaftsraten als auch die Spermiengröße weisen erhebliche Variation zwischen Populationen auf. Wir wissen allerdings wenig über die Ausprägung dieser reproduktiven Schlüsselmerkmale in hohen Breiten. Wir beschreiben in diesem Beitrag Muster von Fremdvaterschaften sowie Variabilität in der Spermiengröße bei der sozial monogamen Kohlmeise (Parus major) in einer norwegischen Population, die jenseits des Polarkreises auf 69° nördlicher Breite brütet. Über sechs Studienjahre hinweg stellten wir bei 19,2% von 26 Bruten mindestens ein außerhalb des sozialen Paarbundes gezeugtes Jungtier fest. Im Mittel waren 4, 7% der Nestlinge pro Brut außerhalb des Paarbundes gezeugt. Wie aus Ergebnissen früherer intraspezifischer Analysen der Breitengradvariation von Fremdvaterschaften zu erwarten, war die beobachtete Fremdvaterschaftsrate niedrig im Vergleich zu Schätzungen für südlichere Kohlmeisenpopulationen (Spannbreite: 2,9%–20,4%). Unsere Ergebnisse stützen daher ein Bild abnehmender Fremdvaterschaftsraten mit höherem Breitengrad bei der Kohlmeise, das auch für extrem hohe Breitengrade zutrifft. Die mittlere Gesamtlänge der Spermien betrug 97,5 ± 0,6 (SE) μm und 30,6% der gesamten phänotypischen Variation in der Spermienlänge wurde durch Unterschiede zwischen Spermienproben erklärt. Der Variationskoeffizient der mittleren Spermienlänge pro Probe betrug 1,93% zwischen unterschiedlichen Proben. Legt man frühere komparative Studien als Maßstab zugrunde, ist dieser Wert für eine beobachtete Häufigkeit von 4,7% Fremdvaterschaften deutlich niedriger als erwartet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social monogamy in combination with biparental care is common in birds, yet extra-pair paternity is widespread particularly in passerines (reviewed by Brouwer and Griffith 2019). The frequency of extra-pair paternity varies considerably among individuals/pairs, populations and species in socially monogamous birds, ranging from zero to more than half of all offspring in a population resulting from extra-pair copulations (reviewed by Brouwer and Griffith 2019). Why extra-pair paternity varies so widely is not fully understood, but, e.g., breeding synchrony and life-history appear to be important factors associated with the variation in extra-pair paternity observed within and across species (e.g., Griffith et al. 2002; Brouwer and Griffith 2019; Lifjeld et al. 2019; Valcu et al. 2021).
The relationship between extra-pair paternity and latitude has recently been examined in two comprehensive comparative studies. Brouwer and Griffith (2019) found contrasting patterns in within-species approaches, with a negative association between latitude and the proportion of extra-pair paternity for some species (e.g., Great Tit Parus major) but a positive association for other species (e.g., Barn Swallow Hirundo rustica). Across species, Brouwer and Griffith (2019) found a weak negative association between latitude and the proportion of extra-pair paternity. Valcu et al. (2021), by applying an assemblage-level analysis controlling for, among others, shared environment and breeding range, also demonstrated a negative association between latitude and the frequency of extra-pair paternity across zoogeographical realms. Lower levels of extra-pair paternity at higher latitudes are consistent with several hypotheses for variation in the occurrence of extra-pair paternity. For instance, shorter breeding seasons at higher latitudes will often lead to more synchronous breeding and, according to the breeding synchrony hypothesis, males may then face a trade-off between investment in extra-pair mating and mate guarding (Westneat 1990).
Extra-pair copulations will result in sperm of different males competing for fertilizations (sperm competition) and/or an opportunity for cryptic female choice and such post-copulatory sexual selection may affect the evolution of ejaculate traits including sperm traits (reviewed in Birkhead et al. 2009; Birkhead and Montgomerie 2020). A recent meta-analysis across a variety of taxa revealed that the expression of most examined ejaculate traits, with the exception of sperm length, was positively associated with paternity success (Macartney et al. 2023). In birds, comparative studies have, for example, shown that spermatozoa are longer (Briskie and Montgomerie 1992; Kleven et al. 2009), swim faster (Kleven et al. 2009) and are less variable in length (Calhim et al. 2007; Kleven et al. 2008) in species with higher levels of extra-pair paternity.
The central aims of our study were to examine the frequency of extra-pair paternity and analyse sperm morphological variation in a population of Great Tits breeding north of the polar circle at 69° latitude. Little is known about the expression of such key reproductive traits of socially monogamous passerine birds breeding at high latitudes and it is unclear whether the scope of results from comparative studies also extends to extreme latitudes. Based on the results from recent analyses revealing a negative relationship between latitude and frequency of extra-pair paternity (Brouwer and Griffith 2019; Valcu et al. 2021), we expected relatively low levels of extra-pair paternity in our far northern study population. Furthermore, we also expected medium to high among-sample variation in mean sperm length, as the among-male coefficient of variation (CV) in mean sperm total length has been shown to decrease with the frequency of extra-pair paternity across passerine birds (Kleven et al. 2008; Lifjeld et al. 2010). Here, low levels of extra-pair paternity, and thus possibly less intense post-copulatory sexual selection, is assumed to result in weaker selection for an optimal sperm phenotype (Parker and Begon 1993). Relaxed selection may then lead to greater variation among males in mean sperm length compared to more promiscuous species. Recent modelling approaches indicate, however, that cryptic female choice may modify effects of sperm competition on sperm size (Cramer et al. 2023).
Materials and methods
Study species and study population
The Great Tit is a small (17–19 g), hole-nesting passerine bird (Cramp and Perrins 1993). Great Tits are socially monogamous (Cramp and Perrins 1993) but show frequent extra-pair mating behaviour (Lubjuhn et al. 2007; Brouwer and Griffith 2019). Females usually incubate the eggs alone while both parents feed the young (Cramp and Perrins 1993). We studied Great Tits in a nest box population in the Pasvik Valley (69°28’N, 29°50’W) in northeastern Norway. Our study area sustained 119 nest boxes placed on both sides along approximately 20 km of roads, with varying distances between the boxes. Over a 6-year study period, the number of Great Tit pairs occupying these nest boxes ranged from 6 to 19. A mixture of pine and birch forest characterizes the habitat; for more details about the study site see Kleven et al. (2020).
Field methods
The fieldwork was carried out during six breeding seasons between 2016 and 2022 (not in 2017). Permits to capture, handle and ring the birds were issued by the Norwegian Environment Agency to Oddmund Kleven (A-license 1082). No other animal ethics approval was required for this work under Norwegian law. Putative parents were trapped inside nest boxes while providing food to the nestlings. We trapped an adult male and female for nine broods and a male only for 17 broods. All were first broods. We considered the trapped adults to be the social parents of any focal brood. Adults were banded with a numbered aluminum ring provided by the Norwegian Bird Ringing Centre at Museum Stavanger. Adults were sexed in the field based on sexual plumage dimorphism (Cramp and Perrins 1993), according to the shape of their cloacal protuberance (far more extended in breeding males) and the presence (females) or absence (males) of a brood patch (Svensson 1992). In addition, sex of adult birds was verified molecularly by using the universal primers P2 and P8 (Griffiths et al. 1998). All adult individuals had been correctly sexed in the field.
Buccal swabbing was applied to sample DNA from adults and all nestlings present in the nest box at the time of sampling (Handel et al. 2006). Briefly, epithelial cells were sampled by gently rotating a buccal swab (4N6FLOQSwabs™, Copan, Italy) against the inside of the cheeks and on the tongue. The 4N6FLOQSwabs™ are manufactured for application in forensics and paternity testing in humans and have high recovery of DNA (Dadhania et al. 2013). Buccal swabs were stored at 4 °C in 2 mL microtubes (Sarstedt, Germany) containing 1 mL Queen’s lysis buffer (Seutin et al. 1991) until molecular genetic analysis.
We gently massaged the cloacal protuberances of a subset of breeding males during the nestling provisioning stage from 2019 to 2021 to obtain a sperm sample as described in detail by Laskemoen et al. (2013). The sample was first mixed with 10 µL standard phosphate-buffered saline (PBS) and immediately transferred into 250 µL of a 5% formaldehyde solution (equivalent to an approximately 12.5% formalin solution assuming a stock solution of 40% formaldehyde). Samples were stored at room temperature until sperm microphotography in autumn 2021 (differential storage duration in the used medium does not affect sperm length; Schmoll et al. 2016).
Parentage analysis
Genomic DNA was extracted from buccal swabs using the QIAamp 96 Blood Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. All samples were genotyped at six polymorphic autosomal microsatellite loci (Table 1). The universal primers P2 and P8 (Griffiths et al. 1998) were added for molecular sex determination. All primers were combined into a single multiplex polymerase chain reaction (PCR) run using fluorescently labeled forward primers and a multiplex PCR Kit (Qiagen). PCR products were separated on an ABI 3500xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) and allele sizes were assigned using GENEMAPPER v5.0 software (Applied Biosystems). The assignment was done without using information about family/sibship affiliation, but sample IDs were not blinded prior to assignment of allele sizes. All samples were successfully genotyped at all loci. Marker polymorphism and deviation from Hardy–Weinberg equilibrium were calculated using Arlequin v.3.5.2.2 (Excoffier and Lischer 2010), exclusion probabilities were calculated using GenAlEx v6.5 (Peakall and Smouse 2012), and frequencies of null-alleles (non-amplifying alleles) were estimated using MICRO-CHECKER (van Oosterhout et al. 2004); for results, see Table 1. None of the loci deviated significantly (α < 0.05) from Hardy–Weinberg equilibrium, and there was no indication of null alleles at any locus (see Table 1). The markers’ combined exclusion probability assuming the mother was known was 0.999 and the combined exclusion probability when the mother was unknown was 0.998, allowing reliable assignment of offspring paternity status as within-pair offspring (WPO) versus extra-pair offspring (EPO).
Nestlings were considered WPO if their allele sizes matched those of the putative parents at all loci or mismatched at a single locus (with either parent). Nestlings with two or more mismatches with the putative father’s allele sizes were considered EPO (mean number of allelic mismatches for EPO: 4.9; range: 4–6). There were two cases in different broods with a single allelic mismatch between a nestling and the putative father. In these two cases, the offspring allele differed in size by a single repeat unit compared to that of the closest-sized allele of the putative father. As microsatellite mutations typically occur by addition or deletion of a single repeat unit (e.g., Anmarkrud et al. 2008), we assumed these allelic mismatches to result from a mutation and considered the nestlings as being sired within-pair. All other nestlings in these two broods were WPO. There were no allelic mismatches between nestlings and putative mothers.
Sperm morphology analysis
Approximately 3 µL of solution from each sperm sample were transferred onto a standard microscope slide and air-dried over-night. The slide was then carefully rinsed with distilled water to remove dirt and salt crusts and air-dried again. Slides were subsequently examined by light microscopy at 400 times magnification under light-field conditions using an Olympus BX50 microscope and all pictures were taken by the same person (Sonja Schindler) using a Canon EOS 850 digital camera. A micrometer scale was pictured for each sperm sample immediately before slides were screened. Only spermatozoa that showed no obvious signs of damage were included. Pictures of 25 intact spermatozoa per sperm sample were used for further analysis, as measuring 25 spermatozoa has been shown to provide a sufficiently precise estimate of a sample’s mean sperm total length (Laskemoen et al. 2007). To ensure blind measurements with respect to sperm sample identity, all samples were anonymised before analysis by Tim Schmoll. Sperm head, midpiece and tail length were subsequently measured to a precision of 0.01 μm during a continuous measuring period by a single observer (Sonja Schindler) using ImageJ 1.52 a (Rasband 1997–2018). Sperm total length was calculated as the sum of these components. For each of twelve sperm samples that contained sperm, 25 spermatozoa were measured. All 300 spermatozoa were blindly measured twice to assess measurement error via repeatability analysis (see below). The mean of the two measurements was used for all subsequent analyses. For subsequent analysis we furthermore excluded the second sperm sample of one male sampled twice (in 2019 and 2021) resulting in a final sample size of 275 spermatozoa from eleven sperm samples of eleven different males.
Statistical analysis
To make explicit the uncertainty when estimating key metrics of extra-pair paternity, we fitted statistical models with the intercept as the only fixed effect, which provided us with estimates of grand means but also corresponding confidence intervals. More specifically, we used generalised linear models (GLMs) with logit link and i) binomial errors to model the probability for a brood to contain at least one EPO and ii) quasi-binomial errors for estimating the proportion of EPO per brood (the latter using the R function cbind to create the independent variable as a column-bind matrix of the number of EPO and the number of WPO of a given brood, respectively). Quasibinomial instead of binomial errors were assumed for modelling the proportion of EPO because inspection of dispersion parameters indicated moderate overdispersion here. We refrained from using a more complex mixed effects model to account for variation among sampling years as 2 out of 6 years were represented with a single brood only. We estimated the overall population-level probability that a brood contained at least one EPO and the population mean frequency of EPO per brood, including corresponding 95% Wald confidence intervals, by fitting the intercept/grand mean as the only fixed effect.
We used the R package rptR (Stoffel et al. 2017) to calculate the repeatability of sperm length measurements for repeated measurements of the same individual spermatozoa based on linear mixed effects models and including 95% confidence intervals (parametric bootstrapping, n = 10,000 replicates). We fitted the intercept/grand mean of the respective trait as the only fixed effect and sperm identity as the only random effect. Repeatabilities of measurements for sperm total length and sperm sections were generally high (see Table 2). To estimate variation in sperm total length among versus within sperm samples, we fitted the grand mean of sperm total length as the only fixed effect and sample identity as the only random effect. We refrained from using a more complex mixed effects model to account for variation among sampling years as one out of 3 years was represented with a single sperm sample only.
To quantify variation among sperm samples in mean sperm total length per sample we used the CV adjusted for small sample sizes as CVadj = (1 + 1/4n) × (SD × 100/mean) (Sokal and Rohlf 1995) with n = 11 (final number of sperm samples). Confidence intervals (95%) for the among-sample CVadj were obtained by non-parametric bootstrapping (n = 10,000 replicates).
We used R 4.2.2 (R Core Team 2023) for all computations.
Results
Patterns of extra-pair paternity
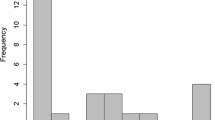
Across all six study years, we obtained parentage data for a total of 192 nestlings from 26 broods of 25 different pairs. The probability for a brood to contain at least one EPO was 5/26 = 19.2% (95% CI 8.2 − 38.7%). Overall, 9 out of 192 nestlings were not sired by the male providing parental care and thus on average 4.7% (95% CI 1.6 − 12.8%) nestlings per brood were EPO (Fig. 1).
Patterns of sperm morphological variation
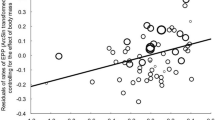
Across three study years, we obtained morphometric data for 275 spermatozoa from eleven sperm samples of eleven different males. The overall mean sperm total length was 97.5 ± 0.6 (SE) µm; patterns of variation in sperm length are described in Table 3. Differences among the sperm samples of the eleven different males explained 30.6% (95% CI 10.3−49.3%) of the total phenotypic variation in sperm total length (linear mixed effects model with sperm sample identity as random effect: χ2 = 65.7, df = 1, p < 0.001, Fig. 2). The adjusted among-sample CV in mean sperm total length per sperm sample was 1.93% (95% CI 0.99−2.53%).
Discussion
We detected extra-pair paternity in 19.2% of 26 broods, and on average 4.7% of nestlings per brood were EPO in our Great Tit study population. In line with results from recent intraspecific analyses of latitudinal variation in extra-pair paternity rates in Great Tits (Brouwer and Griffith 2019), the observed population-level rate of EPO (4.7%) was low in comparison to published estimates from more southern populations. Parameterising the regression equation for the latitudinal variation in extra-pair paternity rates in Great Tits from Brouwer and Griffith (2019; see model 6 in electronic supplementary material) for the latitude of our study site (69° north), predicts a population-level value of 4.0% EPO for our study population. This prediction lies very close to our actual point estimate and quite central within our 95% CI of 1.6−12.8%. Providing the northernmost estimate to date for the study species (indeed roughly 10° further north than the previous northernmost samples), our results hence support a pattern of decreasing levels of extra-pair paternity with increasing latitude (cf. Brouwer and Griffith 2019; Valcu et al. 2021) also for extremely high latitudes where our study species reaches the limit of its distribution range.
Several hypotheses have been proposed to explain the documented variation in extra-pair paternity in birds, including variation among populations within species (reviewed in Brouwer and Griffith 2019). A possible reason for low rates of EPP in our far northern study site include a low population density that decreases encounter rate (Westneat 1990) and makes extra-territorial forays of both sexes increasingly costly (Charmantier and Perret 2004).
Predation may also affect the rates of extra-pair paternity. A correlational study in the Japanese Tit (Parus minor) found higher levels of extra-pair paternity when nest predation was high (Yuta and Koizumi 2016). An experimental study in the Eurasian Blue Tit (Cyanistes caeruleus) documented higher rates of extra-pair paternity in broods exposed to predators during the fertile period (Santema et al. 2020), supporting the hypothesis that the perceived risk of predation affects the rate of extra-pair paternity (Abbey-Lee et al. 2018). Both the rate of nest predation and perceived risk of predation is unknown for our study population, but nest predation rates in passerines seem to be decreasing with increasing latitude (Matysioková and Remeš 2022).
The time period available for breeding also shrinks with increasing latitude and thus pairs in far northern populations can be expected to breed more synchronously than those of more southern populations. As a result, a trade-off between time investment in within-pair versus extra-pair mating effort may constrain the opportunity for extra-pair mating (discussed in Brouwer and Griffith 2019). Peripheral populations, as our study population, also display lower genetic variation (Eckert et al. 2008), thus likely reducing potential genetic benefits of female extra-pair mating. In addition, the harsh environmental conditions far north may increase the need for bi-parental care and limit the time available for extra-territorial forays and extra-pair copulatory behaviour.
While average sperm dimensions were quite similar to those of more southern Great Tit populations (cf. Losdat and Helfenstein 2018; Schmoll et al. 2018; Svobodová et al. 2018), with CVadj of just 1.93, our results revealed comparatively low variation in mean sperm total length among sperm samples of different males (cf. CVadj of 3.17 and 3.25 for two populations in Germany and Southern Norway, Schmoll et al. 2018). In a comparative study of passerine birds by Lifjeld et al. (2010), the CVadj value for 55 species ranged from 1.13 to 6.20 (median: 2.38). Parameterising the regression equation for the variation in extra-pair paternity rates across passerine birds from Lifjeld et al. (2010) for our CVadj value of 1.93, a population-level frequency of EPO of approximately 50% could have been expected, but we found 4.7% instead. While our measure for variation among samples in mean sperm total length is accounting for small sample sizes, our estimate is still based on eleven sperm samples only and accordingly needs to be treated with some caution. It is, however, also conceivable that the overall very strong predictive power of the relationships reported in Lifjeld et al. (2010) is undermined in populations experiencing extreme environmental or demographic conditions. For example, with limited dispersal options on a species distribution range, populations may be more strongly kin-structured/inbred and thus additive genetic variation as an important source for among-individual variation in quantitative traits may be less pronounced (presupposing heritable genetic variation in sperm length, e.g., Edme et al. 2019).
In conclusion, our study showing that extra-pair mating is a less frequent reproductive strategy in a far northern Great Tit population in comparison to southern populations is in line with the results from recent comparative studies documenting a latitudinal decline in the frequency of extra-pair paternity. The surprisingly low variation in mean sperm total length among samples of different males, however, runs counter to established knowledge and suggests more passerine populations inhabiting extreme latitudes should be probed for both patterns of paternity and variation in sperm phenotype.
Data availability
The data used in this study are provided in the electronic supplementary information (ESM1).
References
Abbey-Lee RN, Araya-Ajoy YG, Mouchet A, Moiron M, Stuber EF, Kempenaers B, Dingemanse NJ (2018) Does perceived predation risk affect patterns of extra-pair paternity? A field experiment in a passerine bird. Funct Ecol 32:1001–1010. https://doi.org/10.1111/1365-2435.13052
Anmarkrud JA, Kleven O, Bachmann L, Lifjeld JT (2008) Microsatellite evolution: mutations, sequence variation, and homoplasy in the hypervariable avian microsatellite locus HrU10. BMC Evol Biol 8:138. https://doi.org/10.1186/1471-2148-8-138
Bensch S, Price T, Kohn J (1997) Isolation and characterization of microsatellite loci in a Phylloscopus warbler. Mol Ecol 6:91–92. https://doi.org/10.1046/j.1365-294X.1997.00150.x
Birkhead TR, Montgomerie R (2020) Three decades of sperm competition in birds. Philos Trans R Soc B 375:20200208. https://doi.org/10.1098/rstb.2020.0208
Birkhead TR, Hosken DJ, Pitnick S (2009) Sperm biology: an evolutionary perspective. Academic Press, Oxford, UK
Briskie JV, Montgomerie R (1992) Sperm size and sperm competition in birds. Proc R Soc B 247:89–95. https://doi.org/10.1098/rspb.1992.0013
Brouwer L, Griffith SC (2019) Extra-pair paternity in birds. Mol Ecol 28:4864–4882. https://doi.org/10.1111/mec.15259
Calhim S, Immler S, Birkhead TR (2007) Postcopulatory sexual selection is associated with reduced variation in sperm morphology. PLoS ONE 2:e413. https://doi.org/10.1371/journal.pone.0000413
Charmantier A, Perret P (2004) Manipulation of nest-box density affects extra-pair paternity in a population of Blue Tits (Parus caeruleus). Behav Ecol Sociobiol 56:360–365. https://doi.org/10.1007/s00265-004-0794-5
Cramer ERA, Yilma ZB, Lifjeld JT (2023) Selection on sperm size in response to promiscuity and variation in female sperm storage organs. J Evol Biol 36:131–143. https://doi.org/10.1111/jeb.14120
Cramp S, Perrins CM (1993) Handbook of the birds of Europe, the Middle East and North Africa: flycatchers to shrikes, vol 7. Oxford University Press, New York
Dadhania A, Nelson M, Caves G, Santiago R, Podini D (2013) Evaluation of Copan 4N6FLOQSwabs™ used for crime scene evidence collection. Forensic Sci Int Genet Suppl 4:e336–e337. https://doi.org/10.1016/j.fsigss.2013.10.171
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol Ecol 17:1170–1188. https://doi.org/10.1111/j.1365-294X.2007.03659.x
Edme A, Zobač P, Korsten P, Albrecht T, Schmoll T, Krist M (2019) Moderate heritability and low evolvability of sperm morphology in a species with high risk of sperm competition, the collared flycatcher Ficedula albicollis. J Evol Biol 32:205–217. https://doi.org/10.1111/jeb.13404
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567. https://doi.org/10.1111/j.1755-0998.2010.02847.x
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212. https://doi.org/10.1046/j.1365-294X.2002.01613.x
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x
Handel CM, Pajot LM, Talbot SL, Sage GK (2006) Use of buccal swabs for sampling DNA from nestling and adult birds. Wild Soc Bull 34:1094–1100. https://doi.org/10.2193/0091-7648(2006)34[1094:UOBSFS]2.0.CO;2
Kleven O, Laskemoen T, Fossøy F, Robertson RJ, Lifjeld JT (2008) Intraspecific variation in sperm length is negatively related to sperm competition in passerine birds. Evolution 62:494–499. https://doi.org/10.1111/j.1558-5646.2007.00287.x
Kleven O, Fossøy F, Laskemoen T, Robertson RJ, Rudolfsen G, Lifjeld JT (2009) Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution 63:2466–2473. https://doi.org/10.1111/j.1558-5646.2009.00725.x
Kleven O, Rudolfsen G, Schmoll T (2020) Extra-pair paternity in the boreal socially monogamous Grey-headed Chickadee (Poecile cinctus). Ornis Fenn 97:38–44
Laskemoen T, Kleven O, Fossøy F, Lifjeld JT (2007) Intraspecific variation in sperm length in two passerine species, the Bluethroat Luscinia svecica and the Willow Warbler Phylloscopus trochilus. Ornis Fenn 84:131–139
Laskemoen T, Kleven O, Johannessen LE, Fossøy F, Robertson RJ, Lifjeld JT (2013) Repeatability of sperm size and motility within and between seasons in the Barn Swallow (Hirundo rustica). J Ornithol 154:955–963. https://doi.org/10.1007/s10336-013-0961-4
Lifjeld JT, Laskemoen T, Kleven O, Albrecht T, Robertson RJ (2010) Sperm length variation as a predictor of extrapair paternity in passerine birds. PLoS ONE 5:e13456. https://doi.org/10.1371/journal.pone.0013456
Lifjeld JT, Gohli J, Albrecht T, Garcia-del-Rey E, Johannessen LE, Kleven O, Marki PZ, Omotoriogun TC, Rowe M, Johnsen A (2019) Evolution of female promiscuity in Passerides songbirds. BMC Evol Biol 19:169. https://doi.org/10.1186/s12862-019-1493-1
Losdat S, Helfenstein F (2018) Relationships between sperm morphological traits and sperm swimming performance in wild Great Tits (Parus major). J Ornithol 159:805–814. https://doi.org/10.1007/s10336-018-1539-y
Lubjuhn T, Gerken T, Brün J, Schmoll T (2007) Yearling male Great Tits, Parus major, suffer more strongly from cuckoldry than older males. Zoology 110:387–397. https://doi.org/10.1016/j.zool.2007.07.005
Macartney EL, Morrison K, Snook RR, Lagisz M, Nakagawa S (2023) Intra-specific correlations between ejaculate traits and competitive fertilization success: a meta-analysis across species and fertilization modes. Evolution 78:497–510. https://doi.org/10.1093/evolut/qpad229
Matysioková B, Remeš V (2022) Stronger negative species interactions in the tropics supported by a global analysis of nest predation in songbirds. J Biogeogr 49:511–522. https://doi.org/10.1111/jbi.14321
Parker GA, Begon ME (1993) Sperm competition games: sperm size and number under gametic control. Proc R Soc B 253:255–262. https://doi.org/10.1098/rspb.1993.0111
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics 28:2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Saladin V, Richner H (2012) A set of 48 microsatellite loci for the Great Tit Parus major including 15 novel markers. Mol Ecol Res 12:185–189
Saladin V, Bonfils D, Binz T, Richner H (2003) Isolation and characterization of 16 microsatellite loci in the Great Tit Parus major. Mol Ecol Notes 3:520–522. https://doi.org/10.1046/j.1471-8286.2003.00498.x
Santema P, Valcu M, Kempenaers B (2020) Exposure to predator models during the fertile period leads to higher levels of extra-pair paternity in Blue Tits. J Anim Ecol 89:647–657. https://doi.org/10.1111/1365-2656.13114
Schmoll T, Sanciprian R, Kleven O (2016) No evidence for effects of formalin storage duration or solvent medium exposure on avian sperm morphology. J Ornithol 157:647–652. https://doi.org/10.1007/s10336-015-1321-3
Schmoll T, Kleven O, Rusche M (2018) Individual phenotypic plasticity explains seasonal variation in sperm morphology in a passerine bird. Evol Ecol Res 19:561–574
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69:82–90. https://doi.org/10.1139/z91-013
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3d edn. Freeman, New York
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Svensson L (1992) Identification guide to European passerines. Lars Svensson, Stockholm
Svobodová J, Bauerová P, Eliáš J, Velová H, Vinkler M, Albrecht T (2018) Sperm variation in Great Tit males (Parus major) is linked to a haematological health-related trait, but not ornamentation. J Ornithol 159:815–822. https://doi.org/10.1007/s10336-018-1559-7
Valcu C-M, Valcu M, Kempenaers B (2021) The macroecology of extra-pair paternity in birds. Mol Ecol 30:4884–4898. https://doi.org/10.1111/mec.16081
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
Westneat DF (1990) The ecology and evolution of extra-pair copulations in birds. In: Power DM (ed) Current Ornithology, vol 7. Plenum Press, New York, pp 331–369
Yuta T, Koizumi I (2016) Does nest predation risk affect the frequency of extra-pair paternity in a socially monogamous passerine? J Avian Biol 47:153–158. https://doi.org/10.1111/jav.00713
R Core Team (2023) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. URL https://www.R-project.org/. Retrieved from http://www.R-project.org
Rasband WS (1997–2018). ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA, URL http://imagej.nih.gov/ij/
Acknowledgements
We are grateful to Paul Aspholm for assistance with erecting the nest boxes and to Finnmarkseiendommen (FeFo) for allowing us to work on their property. We thank two anonymous reviewers for constructive comments.
Funding
Open access funding provided by Norwegian institute for nature research. Financial support was received from the Wessel fund, Norwegian Institute for Nature Research (NINA), Nord University and UiT The Arctic University of Norway.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics declarations
Permits to capture, handle and ring the birds were issued by the Norwegian Environment Agency to Oddmund Kleven (A-license 1082).
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kleven, O., Østnes, J.E., Rudolfsen, G. et al. Extra-pair paternity and sperm length variation in a far northern Great Tit (Parus major) population. J Ornithol (2024). https://doi.org/10.1007/s10336-024-02199-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10336-024-02199-4