Abstract
Studies of putatively adaptive plasticity, such as inducible defenses, frequently explore the fitness consequences of expressing alternative phenotypes in alternative environments, but few studies examine how and why the pattern of selection changes in relation to trait induction. We induced snails in the presence/absence of nonlethal predatory crayfish, exposed both phenotypes (alone and combined) to selection by lethal crayfish, and quantified linear and nonlinear selection differentials. Crayfish induced an increase in mass, shell thickness, and absolute (but not relative) shell dimensions. Crayfish predation on uninduced snails was rapid, accomplished via shell-crushing and revealed strong selection for increased size (i.e., mass and shell dimensions). Conversely, crayfish predation on predator-induced snails was slower, often accomplished using an alternative mode of predation (shell-crushing 70% of the time, but shell-extraction 30% of the time), and revealed selection for wide apertures and thick shells. Crayfish selection on uninduced snails in the presence of predator-induced snails was stronger than predation on uninduced snails alone demonstrating that selection can be frequency dependent. Therefore, predator-induced changes in size and shell thickness appear to be adaptive and, along with reciprocal adjustments in the mode of predation, result in altered patterns of selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past quarter-century, a tremendous number of examples of inducible defenses have been demonstrated in a great variety of taxa (Karban and Baldwin 1997; Tollrian and Harvell 1999). Such inducible defenses are typically viewed as a form of adaptive phenotypic plasticity where an organism can express a condition-specific phenotype in response to environmental cues (Gotthard and Nylin 1995). Phenotypic trade-offs are predicted to play a major role in favoring inducible expression of certain phenotypes over constitutive expression (Harvell and Tollrian 1999; Berrigan and Scheiner 2004). Across-environment phenotypic trade-offs in defensive phenotypes can emerge when defended phenotypes experience increased survival in the presence of a predator but reduced fitness in the absence of a predator (e.g., Ågren and Schemske 1993; Hoverman et al. 2005) or when a defense produced in response to one predator increases vulnerability to a different predator (e.g., Smith and Jennings 2000; Relyea 2003).
Demonstrating that plasticity is an adaptive strategy requires evidence of cause and effect underlying the induction and fitness consequences of trait changes, not simply evidence that the traits are under selection (Wade and Kalisz 1990; Gotthard and Nylin 1995; Doughty and Reznick 2004). One method that can been used to establish the adaptive nature of plasticity is to conduct a selection experiment to demonstrate that induced changes in the phenotype increase fitness in the inducing environment (e.g., Van Buskirk et al. 1997; Van Buskirk and Relyea 1998). Subsequently, one can examine the pattern of selection on a set of inducible traits and evaluate whether the direction of induction and the direction of selection are congruent.
The pattern of selection on inducible traits may change among environments, and this can have several causes. First, the pattern of selection on induced traits may be altered directly in response to a change in trait values. Alternatively, the pattern of selection on induced traits may change due to trait changes in other organisms (e.g., predators, competitors). Such a situation may arise when species interactions result in reciprocal plasticity (e.g., if the predator changes its foraging mode in response to the expression of a defense in its prey). While many studies have explored these ideas independently, we lack examples of how induction of defensive traits, selection on these traits, and the type of selection (e.g., the mode of predation) are linked.
Here, we use a predator-prey system to understand how induced prey phenotypes alter rates of predation, modes of predation, and patterns of selection. Our target organism, the freshwater snail Physa acuta (Basommatophora), has been widely used as a model system for studying predator-induced plasticity (DeWitt 1998; DeWitt et al. 1999, 2000; Turner et al. 2000; Auld and Relyea 2008). Physa detects predators via water-borne chemical cues (Covich et al. 1994) and previous work has demonstrated the potential for trade-offs in response to different predators (DeWitt et al. 2000; DeWitt and Langerhans 2003). Snails with elongate shells experience increased survival by restricting shell entry by predatory crayfish while snails with rotund, crush-resistant shells experience increased survival with predatory, shell-crushing fish (DeWitt et al. 2000). However, the relationship between predator induction and its selective benefits has not been demonstrated. Indeed, studies demonstrating the relationship between induction of and selection on inducible defenses are rare across all systems, which makes it difficult to understand the adaptive nature of many inducible defenses. Here, we specifically address the question of whether predator-induced morphological plasticity represents as adaptive response to the presence of predators. We predict that the induction of morphological defenses will correspond to the pattern of selection (i.e., that the expression of inducible defenses is an adaptive response to predation risk), but we need to explore the relationship between trait induction and selection to reveal the adaptive nature of this plasticity. Moreover, variation in the pattern of selection may be related to flexible predator foraging tactics that change in response to altered prey phenotypes and we explore this potentially important interaction.
Methods
Animal collection and rearing
All snails used in this experiment were descendents of >100 wild-caught Physa acuta from Geneva pond #3 in northwest Pennsylvania (41°, 35′N; 80°, 14′W). Ovipositing snails were placed in plastic containers filled with carbon-filtered, UV-irradiated water in the lab at the Pymatuning Laboratory of Ecology (PLE; Linesville, PA), and fed ground Spirulina (OSI Marine Lab, Inc., Burlingame, CA) ad libitum. The experimental room was held at 22°C with 12-h light/dark cycles during hatching and the subsequent experiment. Crayfish (Procambarus acutus) were collected from the Thompson gravel pit (41°, 40′N; 80°, 30′W) in May 2006, held in 200-L pools outside, and fed P. acuta snails and rabbit chow ad libitum until needed.
Induction experiment
To produce predator-induced and “uninduced” (i.e., no predator exposure) snails, we set up 20 200-L plastic wading pools outside at PLE (hereafter, we refer to snails that were never exposed to predator cues as “uninduced” as a convenient shorthand; we do not mean to assert that they were not induced by anything). On 22 May 2006, these pools were filled with well water, supplemented with 5 g rabbit chow as an initial nutrient source and an aliquot of pond water containing zooplankton and phytoplankton from 3 nearby, natural ponds. These pools were covered to prevent colonization by insects and amphibians and aged for 2 weeks to allow periphyton to grow in the pools as a food source for the snails. Each pool was equipped with a predator cage composed of a 10-cm section of corrugated PVC pipe covered with window screen at both ends. These cages allow chemical cues from predators to diffuse into the pools without allowing the predators to kill the focal animals. On 5–6 June, 100 hatchling (i.e., ~2 week old) snails were added to each pool. These snails represent a random sample among all of the offspring of the wild-caught snails (described above). Ten of these pools had empty predator cages while the other 10 pools had a crayfish placed into the predator cage. These crayfish were fed ~250 mg of P. acuta 3 times/week. Note that P. acuta can not only detect and differentially respond to a variety of predators, but also distinguish among various predator diets (e.g., DeWitt et al. 2000; Turner 2008). When feeding the predators, the cages in the predator-free pools were lifted to equalize disturbance. Approximately 5 g of additional rabbit chow was added to each pool weekly to provide adequate food for the snails. On 9 July, all predator cages were removed. On 10 July, the 20 snail pools were drained and all snails were collected. To randomize any idiosyncratic effects among pools assigned the same treatment, all snails from the 10 predator-free pools were mixed; snails from the predator-cue pools were mixed as well.
Selection experiment
To examine the strength and direction of selection on predator-induced and uninduced traits, we exposed predator-induced and uninduced snails to selection by lethal predators and estimated selection differentials by comparing the phenotypic distribution before and after selection. To accomplish this, we set up a selection experiment using 3 snail-phenotype treatments. All selection trials took place in 10-L plastic tubs filled with 3 L of water. To these containers, we added either 10 predator-induced snails, 10 uninduced snails, or 5 predator-induced snails and 5 uninduced snails. The latter treatment was included to compare the pattern and intensity of selection on uninduced snails in the presence of induced snails (i.e., to evaluate whether selection is frequency-dependent). To keep track of the predator induction, we marked snails with nail polish, which is harmless to the snails (Henry and Jarne 2007). To control for any potential effects of marking, we marked one-half of the induced snails and one-half of the uninduced snails; these were distinguished by recording which tubs received which snails. We did not individually mark the snails and therefore our estimates of linear and nonlinear selection represent total selection on traits (i.e., selection differentials; Brodie et al. 1995).
We had enough snails to set up 143 tubs of 10 snails each. From these, 102 tubs were selected for exposure to a lethal crayfish (34 tubs for each of the 3 treatments). All snails in the selection trials were fed and allowed to acclimate for 20 h. After adding snails to these tubs, we collected 102 crayfish from our outdoor pools, isolated the crayfish in 1-L containers in the lab, and left them overnight. Crayfish were not fed during this period. On 11 July, 1 randomly selected crayfish (mean carapace length ± SD: 2.49 cm ± 0.29; range 1.89–3.47 cm) was added to each tub and allowed to begin consuming the snails. The total duration of the experiment was 96 h because we expected predation to be rapid. All containers were checked every 1.5 h for the first 24 h and every 3 h for the subsequent 72 h. A tub was terminated and the surviving snails preserved in 10% formalin when the crayfish had consumed at least 5 snails or when 96 h had passed (mean duration of the tubs (hours) ± SE: uninduced: 33.4 ± 4.9, combination: 59.2 ± 5.9, induced: 75.7 ± 5.5). We recorded the number of snails consumed at each checkpoint to assess predation rate. As predation rate on uninduced snails was rapid, it is unlikely that much induction took place during the selection phase of the experiment. Based on previous observations, we knew that crayfish would kill the snails in one of two ways. Crayfish can either crush the shell or reach into the shell and extract the flesh. We quantified how the crayfish killed the snails by recording whether snails were crushed or extracted when we checked survivorship throughout the experiment. Emptied shells were collected and preserved separately from the survivors. Therefore, we could examine how induction by predators affected predation rate (the number of snails killed per hour) and the mode of predation (the proportion of killed snails that were crushed).
The remaining 41 tubs (from our original 143) were placed in the lab in the same manner as the experimental tubs to assess mortality due to our handling. There were 14 tubs of the uninduced and combination treatments and 13 tubs of the predator-induced treatment. Survival at 24 h was 100% and these snails were subsequently preserved in 10% formalin to assess induction and provide a sample of the phenotypes exposed to selection. Therefore, both the initial-sample snails and the snails exposed to selection were held in the lab under the same conditions for 1 day before being preserved or exposed to selection, respectively. Both marking schemes (uninduced marked and induced marked) were represented equally in all samples.
Traits measured
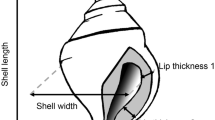
We measured the following 6 traits on each snail: mass, shell length, shell width, aperture length, aperture width, and shell thickness. Preserved snails were placed in a drying oven for 24 h to remove any liquid. Subsequently, individual snails were massed (0.01 mg resolution) and photographed with a digital camera. Images were viewed in Optimas (Bothell, WA) and used to measure the length and width of the shell and aperture. Each dimension was measured at its maximum; aperture dimensions were measured on the inside of the aperture (Fig. 1). Shell thickness was measured with calipers (0.01 mm resolution) at the growing edge of the shell.
Predator induction and selection on mass and five shell variables are shown by plotting the means (±SE) before and after selection for uninduced (i.e., predator-naïve; open symbols) and predator-induced (“induced”; closed symbols) phenotypes. Dashed lines show the pattern of selection on uninduced snails exposed to selection with predator-induced snails. Inset figures show the shell dimensions that were measured; lines are not drawn to exact scale. The arrow in b points to the location where shell thickness was measured
Statistical analysis
We conducted separate analyses to assess the effects of induction and selection. These analyses were conducted on both untransformed and size-corrected data. The strength and direction of selection was examined by comparing trait means and (co)variances before and after selection. We also explored the effects of induction on predation rate and the mode of predation.
Analysis of induction
To assess the effects of predator induction on the 6 traits, we calculated the tub means using the 14 uninduced and 13 predator-induced initial-sample tubs. These tub means served as response variable in a MANOVA with predator induction as a factor. Univariate comparisons were examined when the multivariate effect was significant. We also analyzed the aspect ratio (i.e., length/width) of the shell and aperture (as in DeWitt et al. 1999, 2000). All statistical analyses were performed using SPSS (v.11 for Mac) and EXCEL.
Because the 5 shell traits were correlated to mass, we also size adjusted our data to make these values mass-independent. Size adjustment was done on individual trait values and subsequently, tub means of mass-adjusted trait values were calculated to serve as response variables. To make shell measurements mass-independent, we conducted a MANCOVA with log-transformed mass as a covariate, predator induction as a factor, and the five log-transformed shell traits as response variables. We verified the assumptions of the MANCOVA model including the absence of treatment-by-response variable interactions (i.e., all treatment slopes were parallel). Additionally, we verified that the uninduced and predator-induced treatments shared scaling relationships through common principal components analysis using Phillips’ CPC program (see Phillips and Arnold 1999; McCoy et al. 2006). This program detected no difference in the variance–covariance matrices between the induced and uninduced treatments. We saved the residuals from the MANCOVA analyses as well as the estimated mass-independent means for each treatment. Subsequently, we added each individual’s residual to these estimated means to obtain estimates of shape variables for individuals of equal mass, a technique we have used in previous studies (e.g., Auld and Relyea 2008). The effects of predator induction on these mass-adjusted shell traits were examined using MANOVA as described above.
Analysis of selection experiment
We calculated linear and nonlinear selection differentials to assess the change in the mean and (co)variance due to selection (Brodie et al. 1995). Selection differentials were calculated based on standardized tub means; tub means were standardized based on the mean and standard deviation of individual trait values from the initial samples (i.e., the phenotypic distribution prior to selection). Selection differentials were calculated for all 3 treatments, where only the uninduced snails in the combination treatment were used to calculate the tub mean (i.e., selection on uninduced snails in the presence of induced snails). We did not calculate the reciprocal selection differentials (i.e., for induced snails in the presence of uninduced snails) because these values would arise from the same experimental units (and thus lack statistical independence). Linear selection differentials measure total selection on a trait mean as the difference before and after selection. Nonlinear selection differentials measure the change in variance (univariate) or covariance (bivariate) in after selection and reveal stabilizing/disruptive and correlational selection (Brodie et al. 1995). To asses the statistical significance of these selection differentials, we estimated 95% confidence intervals on each differential based on the standard deviation of 1,000 bootstrap samples taken across tubs. Significance was inferred based on the exclusion of 0 from the C.I.
We also calculated selection differentials based on mass-adjusted trait values to determine if controlling for selection on mass (i.e., overall size) revealed a different pattern of selection. Because the snails that survived predation represent a phenotypic subset of the snails that were exposed to predators, we used the same regression coefficients estimated in the mass-adjustment analysis described above. With our estimates of the slope and intercept for each treatment-specific regression, we calculated the residual for each individual’s traits as e = y – a − bx where e is the residual for a regression of y (log-transformed trait value) on x (log-transformed mass), a is the intercept, and b is the regression coefficient. By adding the estimated residual to the estimated mass-adjusted mean for each treatment, we obtained mass-adjusted values of each shell trait for snails that survived predation. All mass-adjusted variables were then averaged within a tub to provide response variables, as described above.
Analysis of predation rate and mode of predation
We also examined treatment effects on predation rate and the mode of predation (i.e., the proportion of killed snails that were crushed, as opposed to being extracted from their shells). Preliminary analyses demonstrated heteroscedasticity-of-error variances and deviations from normality in these variables, so the data were ranked and analyzed using Kruskal–Wallis tests. Predation rate from one tub in the uninduced treatment was excluded from this analysis as an outlier (based on Dixon’s test; Sokal and Rohlf 1995, p. 406); the predation rate in this tub was more than an order of magnitude greater than the average for this treatment. Additionally, even though crayfish size (carapace length) did not differ among the treatments (ANOVA, F 2,99 = 1.893, P = 0.156), we examined the effect of crayfish size on our measures of predation through regressions of carapace length on predation rate and the proportion crushed. Crayfish size did not affect predation rate or the proportion crushed and these results are not discussed further.
Results
Induction experiment
Predator induction significantly affected shell size and shape (F 6,20 = 44.7; P < 0.001, Fig. 1). Predator cues induced a 78% increase in mass (F 1,25 = 123.7, P < 0.001), a 14% increase in shell length (F 1,25 = 59.9, P < 0.001), a 13% increase in shell width (F 1,25 = 61.0, P < 0.001), an 11% increase in aperture length (F 1,25 = 43.9, P < 0.001), a 10% increase in aperture width (F 1,25 = 22.4, P < 0.001), and a 108% increase in shell thickness (F 1,25 = 163.8, P < 0.001). We found no effect of predator induction on either shell aspect ratio or aperture aspect ratio (P > 0.1). Predator cues also had a significant multivariate effect on mass-adjusted shell variables (F 5,21 = 17.2, P < 0.001); all 5 univariate tests on mass-adjusted traits were significant (Supplementary Fig. 1). After adjusting for mass, predator-induced snails still had relatively thicker shells compared to uninduced snails, but predator induction resulted in relatively shorter and narrower shell and aperture dimensions. This discrepancy between predator induction of increased overall size but relatively smaller shell dimensions is probably a product of changes in soft body tissue.
Selection experiment
Crayfish imposed significant linear and nonlinear selection on predator-induced and uninduced snails (Fig. 1, Table 1). Selection on uninduced snails was stronger in the presence of predator-induced snails than alone. Selection on predator-induced snails was, on average, weaker than selection on uninduced snails.
For uninduced snails exposed to selection alone, we detected positive linear selection differentials on all traits, with especially strong selection on aperture width (Table 1A). All nonlinear selection differentials were positive and significant with the exception of the univariate coefficient for mass. Thus, after selection by predators, there was an increase in the variance for all shell traits as well as an increase in the covariance among shell traits.
For predator-induced snails, linear selection differentials on aperture width and shell thickness were significant, but not for other traits (Table 1B). Thus, among predator-induced snails, the survivors of predation had wide apertures and thick shells, but there was no change in the mean for other traits. Nonlinear univariate selection differentials were significant for mass, aperture width and shell thickness. We also observed significant correlational selection between mass and all traits except shell thickness as well as between shell length and all traits except shell thickness. Significant correlational selection was also observed between aperture width and aperture length and between aperture width and shell thickness.
For uninduced snails exposed to selection with predator-induced snails, all linear and nonlinear selection differentials were significant and all were greater in magnitude than for uninduced snails exposed to selection alone (Table 1C). For some selection differentials these confidence intervals overlap, but for many they do not. This demonstrates that, on average, selection on uninduced snails was stronger in the presence of predator-induced snails than alone.
We also quantified selection differentials on mass-adjusted trait values in order to determine if, by removing selection on mass, the pattern of selection on other traits was altered (i.e., given that large snails have an increased chance of surviving in the presence of a lethal predator, is there still selection on shell shape and thickness?). In general, a similar pattern of selection on mass-adjusted traits was observed compared to selection on untransformed trait values, but there were also clear changes (Supplementary Table 1). After removing the effects of mass, it was clear that the uninduced snails that survived exposure to predation (both alone and in the presence of predator-induced snails) did so by means of relatively wide apertures and thick shells. There was also evidence for nonlinear selection on mass-adjusted traits, but this was not as extensive as when the traits were untransformed. The pattern of nonlinear selection was largely similar for uninduced snails that were exposed to selection alone and with predator-induced snails (Supplementary Table 1A, C). After removing the effects of mass, we observed significant, positive linear selection differentials on all shell traits for predator-induced snails, with especially strong selection on relative aperture width (Supplementary Table 1B; note that the linear selection differential for shell thickness was borderline significant—within rounding error of the 95% CI). Furthermore, strong correlational selection was also observed among all shell and aperture dimensions. Thus, by controlling for mass, we revealed that predator selection also operated on relative shell shape.
Predation rate and mode of predation
Crayfish exhibited a different predation rate and mode of predation based on whether their prey were predator-induced or not. The predation rate on uninduced snails was about 5-times faster than on predator-induced snails (Χ 2 = 28.631, P < 0.001; Fig. 2). Of the snails that were killed, 100% of the uninduced snails were crushed, but only 70% of the predator-induced snails were crushed (Χ 2 = 23.210, P < 0.001; Fig. 3). The remaining 30% were extracted from their shells leaving the shells completely intact. This change in the mode of predation may explain the altered pattern of selection between predator-induced and uninduced snails.
The proportion crushed for uninduced and predator-induced snails is the number of snails that were crushed divided by the number of snails killed in each tub. Snails that were killed without being crushed were killed by shell-extraction; the predator reached in and removed the body leaving the shell completely intact
Discussion
Predation is a potent agent of selection known to operate in nature (e.g., Vermeij and Covich 1978; Osenberg and Mittelbach 1989; Crowl 1990; Trussell 2000a). Many prey organisms have evolved the ability to alter their phenotypes in response to predators in ways that increase survival (Tollrian and Harvell 1999). Thus, inducible defenses are often predicted to be adaptive, but evidence demonstrating the adaptive nature of predator-induced responses is relatively rare. Experimental manipulation of the environmental factor that both affects the distribution of phenotypes exposed to selection and imposes the covariance between phenotype and fitness that results in selection is critical to evaluate the cause of selection on any phenotype (Wade and Kalisz 1990). In particular, inducible defenses represent adaptive plasticity if induction by predator cues results in increased survival with lethal predators (e.g., Van Buskirk et al. 1997; Van Buskirk and Relyea 1998). We have shown that chemical cues from crayfish induced morphological changes in Physa acuta that decreased predation rate resulting in increased survival with lethal predators. As the direction of trait induction and the direction favored by selection were the same for shell size and shell thickness, this pattern of induction represents adaptive plasticity. Importantly, we found that selection on shell shape and thickness was significant even when we controlled for selection on overall size. Further, our results show that the pattern of selection depends on induction and highlight a mechanism for this effect—flexibility in the predator’s foraging mode.
In our study, predators caused selection for large size when snails were small and more fine-scale selection on shape when snails were large. Crayfish induced an increase in overall size (i.e., mass and absolute shell dimensions), but overall size was only under selection in the uninduced treatment. This pattern most likely results because predator-induced snails were overall larger than uninduced snails and crayfish are small-size-selective predators that cannot consume prey that have reached a size refuge (Juanes 1992; J. R. Auld, personal observation). DeWitt and Langerhans (2003) also reported that crayfish selection on Physa results in increased size. We know from previous studies that this predator-induced increase in size is attained by increasing growth rate and delaying reproduction (Auld and Relyea 2008), but it remains unclear how much of this increase in mass is due to shell versus soft body tissue. These results correspond to other studies on Physa, other genera of freshwater snails, and other organisms (Crowl 1990; Crowl and Covich 1990; Riessen 1999; Hoverman et al. 2005) demonstrating a trade-off between growth and reproduction that depends on predation risk (and other environmental factors).
Shell thickness and size played an apparently large role in affecting the mode of predation; while 100% of uninduced snails were crushed, crayfish switched to shell-entry to kill 30% of the predator-induced snails. Potentially, possessing a wide aperture may confer some crush-resistance (DeWitt et al. 2000). However, while larger snails gain some size advantage, the overall increase in shell size results in a wider aperture which can facilitate the shell-entry mode of predation (DeWitt et al. 2000; Trussell 2000b; Edgell et al. 2008). Previous results show that a different species of predatory crayfish selects for relatively narrow, entry-resistant shells (DeWitt et al. 2000). Our results for shell thickness are similar to those obtained in a marine decapod-gastropod predator-prey interaction (Trussell 2000a, b; Rochette et al. 2007; Edgell and Rochette 2007; Edgell et al. 2008). In these studies, predatory crabs (Carcinus maenus) induce an increase in shell thickness in snails (Littorina obtusata) that is an effective defense against predation. Additionally, these crab predators utilize multiple foraging modes, crushing the snails if they are small or using a complex, shell-entry tactic termed “winkling” when the snails are large and thick-shelled (Rochette et al. 2007). Furthermore, evidence from this and other decapod-mollusc systems suggests that predators may not only change behavior in response to prey defenses but also morphology allowing more efficient prey capture (Edgell et al. 2008; Baldridge and Smith 2008).
Understanding how and why the pattern of selection on a set of inducible traits changes due to induction of traits themselves and the mode of predation has made the adaptive nature of these predator-induced defenses more clear. Interestingly, we found that selection on uninduced snails was even stronger when they were exposed to selection in the presence of predator-induced snails. This suggests that any variation in the inducibility of traits (i.e., variation in plasticity) should be under strong selection as well. Unfortunately, our experimental design does not permit a distinction between direct selection operating on a trait and indirect selection operating on a correlated trait (Lande and Arnold 1983), but we can deduce that certain trait combinations increase the chance of survival in an encounter with a lethal predator consistent with the observation of correlational selection. Future studies that separate the direct and indirect targets of selection will facilitate a greater understanding of how the suite of traits that are induced by predators are integrated into a functional response that can be favored by selection (DeWitt and Langerhans 2003; Relyea 2004). Furthermore, it is important to bear in mind that in nature prey can use additional means of defending themselves other than those we have quantified (e.g., behavior; DeWitt et al. 1999; Turner et al. 2000). Importantly, selection on other types of traits such as behavior can alter the pattern of selection as well and reveal different targets of selection (DeWitt et al. 1999, 2000; DeWitt and Langerhans 2003). Considering that prey must often respond to multiple types of predators simultaneously and that predators themselves can alter traits like the mode of predation, the adaptive benefits of maintaining plasticity in these traits are clear.
Future studies that further consider the predator response to prey defenses will be important for understanding the coevolution of plasticity and how this may escalate into an arms race (e.g., inducible offenses and defenses). Prey-induced offenses have been observed in other species (e.g., Kopp and Tollrian 2003; Kishida et al. 2006) and the coevolution of plasticity in defense and offense may be a general pattern in numerous systems. One example highlighting the potential generality of such coevolutionary interactions comes from a study demonstrating that a salivary enzyme produced by an herbivorous caterpillar functions to inhibit the anti-herbivore secondary compounds produced by tobacco and tomato plants (Musser et al. 2005). In this system, it is not clear whether the change in the herbivore phenotype will alter the pattern of selection on the plants, but if this does occur (e.g., different physiological or morphological traits are exposed to selection following the induction of an inducible offense) we can hypothesize that an escalatory arms race between enemy and victim may occur. Generally, an increased focus on how all the members of an ecological interaction respond to environmental changes will facilitate a deeper evolutionary understanding of plasticity (Lima 2002).
References
Ågren J, Schemske DW (1993) The cost of defense against herbivores: an experimental study of trichome production in Brassica rapa. Am Nat 141:338–350
Auld JR, Relyea RA (2008) Interacting effects of mate availability and predation risk on life history and defense in a simultaneous hermaphrodite. J Evol Biol 21:1371–1378
Baldridge AK, Smith LD (2008) Temperature constraints on phenotypic plasticity explain biogeographic patterns in predator trophic morphology. Mar Ecol Prog Ser 365:25–34
Berrigan D, Scheiner SM (2004) Modeling the evolution of phenotypic plasticity. In: DeWitt TJ, Scheiner SM (eds) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, New York, pp 82–97
Brodie ED III, Moore AJ, Janzen FJ (1995) Visualizing and quantifying natural selection. Trends Ecol Evol 10:313–318
Covich AP, Crowl TA, Alexander JE, Vaughn CC (1994) Predator-avoidance responses in freshwater decapod-gastropod interactions mediated by chemical stimuli. J North Am Benthol Soc 13:283–290
Crowl TA (1990) Life-history strategies of a freshwater snail in response to stream permanence and predation: balancing conflicting demands. Oecologia 84:238–243
Crowl TA, Covich AP (1990) Predator-induced life-history shifts in a freshwater snail. Science 247:949–950
DeWitt TJ (1998) Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J Evol Biol 11:465–480
DeWitt TJ, Langerhans RB (2003) Multiple prey traits, multiple predators: keys to understanding complex community dynamics. J Sea Res 49:143–155
DeWitt TJ, Sih A, Hucko JA (1999) Trait compensation and cospecialization in a freshwater snail: size, shape and antipredator behaviour. Anim Behav 58:397–407
DeWitt TJ, Robinson BW, Wilson DS (2000) Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evol Ecol Res 2:129–148
Doughty P, Reznick DN (2004) Patterns and analysis of adaptive phenotypic plasticity in animals. In: DeWitt TJ, Scheiner SM (eds) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, New York, pp 126–150
Edgell TC, Rochette R (2007) Geographic correlation between reciprocally adaptive traits of an exotic decapod predator and native gastropod prey: evidence of an arms race? Evol Ecol Res 9:579–597
Edgell TC, Brazeau C, Grahame JW, Rochette R (2008) Simultaneous defense against shell entry and shell crushing in a snail faced with the predatory shore crab Carcinus maenas. Mar Ecol Prog Ser 371:191–198
Gotthard K, Nylin S (1995) Adaptive plasticity and plasticity as an adaptation: a selective review of plasticity in animal morphology and life history. Oikos 74:3–17
Harvell CD, Tollrian R (1999) Why inducible defenses? In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, pp 3–19
Henry P-Y, Jarne P (2007) Marking hard-shelled gastropods: tag loss, impact on life-history traits, and perspectives in biology. Invert Biol 126:138–153
Hoverman JT, Auld JR, Relyea RA (2005) Putting prey back together again: integrating predator-induced behavior, morphology, and life history. Oecologia 144:481–491
Juanes F (1992) Why do decapod crustaceans prefer small-sized molluscan prey? Mar Ecol Prog Ser 87:239–249
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kishida O, Mizuta Y, Nishimura K (2006) Reciprocal phenotypic plasticity in a predator-prey interaction between larval amphibians. Ecology 87:1599–1604
Kopp M, Tollrian R (2003) Reciprocal phenotypic plasticity in a predator-prey system: inducible offences against inducible defenses? Ecol Lett 6:742–748
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226
Lima SL (2002) Putting predators back into behavioral predator-prey interactions. Trends Ecol Evol 17:70–75
McCoy MW, Bolker BM, Osenberg CW, Miner BG, Vonesh JR (2006) Size correction: comparing morphological traits among populations and environments. Oecologia 148:547–554
Musser RO, Cipollini DF, Hum-Musser SM, Williams SA, Brown JK, Felton GW (2005) Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in Solanaceous plants. Archiv Insect Biochem Physiol 58:128–137
Osenberg CW, Mittelbach GG (1989) Effects of body size on the predator-prey interaction between pumpkinseed sunfish and gastropods. Ecol Monogr 59:405–432
Phillips PC, Arnold SJ (1999) Hierarchical comparison of genetic variance-covariance matrices. I. Using the Flury hierarchy. Evolution 53:1506–1515
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Relyea RA (2004) Integrating phenotypic plasticity when death is on the line: insights from predator-prey systems. In: Pigliucci M, Preston K (eds) The evolutionary biology of complex phenotypes. Oxford University Press, New York, pp 176–194
Riessen HP (1999) Predator-induced life history shifts in Daphnia: a synthesis of studies using meta-analysis. Can J Fish Aquat Sci 56:2487–2494
Rochette R, Doyle SP, Edgell TC (2007) Interaction between an invasive decapod and a native gastropod: predator foraging tactics and prey architectural defenses. Mar Ecol Prog Ser 330:179–188
Smith LD, Jennings JA (2000) Induced defensive responses by the bivalve Mytilus edulis to predators with different attack modes. Mar Biol 136:461–469
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman & Company, New York
Tollrian R, Harvell CD (eds) (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
Trussell GC (2000a) Phenotypic clines, plasticity, and morphological trade-offs in an intertidal snail. Evolution 54:151–166
Trussell GC (2000b) Predator-induced plasticity and morphological trade-offs in latitudinally separated populations of Littorina obtusata. Evol Ecol Res 2:803–822
Turner AM (2008) Predator diet and prey behaviour: freshwater snails discriminate among closely related prey in a predator’s diet. Anim Behav 76:1211–1217
Turner AM, Bernot RJ, Boes CM (2000) Chemical cues modify species interactions: the ecological consequences of predator avoidance by freshwater snails. Oikos 88:148–158
Van Buskirk J, Relyea RA (1998) Selection for phenotypic plasticity in Rana sylvatica tadpoles. Biol J Linn Soc 65:301–328
Van Buskirk J, McCollum SA, Werner EE (1997) Natural selection for environmentally induced phenotypes in tadpoles. Evolution 51:1983–1992
Vermeij GJ, Covich AP (1978) Coevolution of freshwater Gastropods and their predators. Am Nat 112:833–843
Wade MJ, Kalisz S (1990) The causes of natural selection. Evolution 44:1947–1955
Acknowledgments
We gratefully acknowledge funding from the Pymatuning Laboratory of Ecology’s McKinley Research Grant (JRA) and the National Science Foundation (RAR). JRA was supported by fellowships from the A.W. Mellon Foundation, a post-doc from the French C.N.R.S. (awarded to P. David and A. Charmantier), and the National Evolutionary Synthesis Center (NESCent), NSF #EF-0905606. We thank A. Stoler for help with the experiment and especially D. Jones for assistance with the experiment and for measuring the snails. T.-L. Ashman, A. Biere, M. Groner, J. Hammond, J. Hoverman, P. Jarne, S. Kalisz, A. Stoler, S. Tonsor, J. Wolf, and several anonymous reviewers provided comments that improved this work. This is a contribution from the Pymatuning Laboratory of Ecology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Auld, J.R., Relyea, R.A. Adaptive plasticity in predator-induced defenses in a common freshwater snail: altered selection and mode of predation due to prey phenotype. Evol Ecol 25, 189–202 (2011). https://doi.org/10.1007/s10682-010-9394-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-010-9394-1