Abstract
A wide range of taxa have been shown to display inducible, phenotypically plastic responses to known predators. Most studies of inducible defenses include only known predators but not non-predatory species in experimental designs, precluding tests of specificity for these responses. We tested the specificity of predator-induced defenses in the marine snail Nucella lamellosa, when exposed to chemical cues from potential crab predators as well as more distantly related non-predatory crabs that co-occur with this snail. Surprisingly, all crabs tested, even those that are not predators, triggered the common induced response of a reduction of soft-tissue mass relative to control animals, likely reflecting a reduction in snail feeding activity. In contrast, only N. lamellosa’s major predator, Cancer productus, triggered the production of a thicker apertural lip. Increased thickening of the apertural lip may be an adaptive response specific to C. productus, which uses shell-breaking at the apertural lip (i.e., shell-peeling) as their main form of attack. Apertural lip thickening appeared to be due to reallocation of shell material (i.e., a change in shell shape) rather than an increase in shell deposition. Our findings demonstrate the importance of determining the specificity of cues triggering inducible responses in prey, and the mechanisms that underlie these plastic responses, as the responses to general versus specific cues may limit the adaptive value of an inducible defense.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predator-induced changes in prey behavior, morphology, and life history can have important ecological consequences at the level of species interactions, population and community dynamics, and ecosystem function (Miner et al. 2005). Therefore, it is important to understand the environmental conditions that favor inducible defenses. Theory predicts that these conditions include: spatial and/or temporal variation in predation risk, costs associated with the expression of the defense, reliable cues that indicate the presence of predators or risk of predation (reviewed in Tollrian and Harvell 1999), and the ability of prey to respond with an effective defense within a short time frame relative to environmental change (Padilla and Adolph 1996; Gabriel et al. 2005). Although each of these conditions has been examined to some extent, we generally know very little about the specificity of cues that indicate predation risk and trigger inducible defenses.
In aquatic systems, water-borne chemical cues released by predators often induce defensive responses in prey (Dodson et al. 1994; Chivers and Smith 1998; Bronmark and Hansson 2000). If highly correlated with predation risk, these cues can provide reliable information about the level of threat in the environment. Previous studies of inducible defenses have examined the role of predator cue concentrations (e.g., Harvell 1998; Hawkins et al. 2007), the role of cues associated with damaged and consumed conspecific prey (e.g., Trussell and Nicklin 2002; Schoeppner and Relyea 2005; Laforsch et al. 2006; Bourdeau 2010a), and cues from multiple predatory species (e.g., Relyea 2003; Teplitsky et al. 2004; Bourdeau 2009). However, few studies have considered whether prey may respond to cues from other, non-predatory species (but see Iyengar and Harvell 2002; Langerhans and DeWitt 2002 for two notable exceptions). Yet, in nature, prey are exposed to a variety of water-borne cues from both predatory and non-predatory species alike. Thus, understanding the specificity of inducible responses to these different cues will be critical to our understanding of both the evolution of plastic traits and their ecological consequences.
In marine systems, a large number of shelled molluscs (gastropods and bivalves) produce thicker shells in response to water-borne chemical cues from predatory crabs, which has been suggested to be a predator-induced defense specific to crabs that attack these molluscs by crushing and/or peeling their shell (Appleton and Palmer 1988; Palmer 1990; Trussell 1996; Leonard et al. 1999; Caro and Castilla 2004; Dalziel and Boulding 2005; Bourdeau 2009). Previous studies of these inducible shell defenses in molluscs have only examined responses to chemical cues from large predatory species of crabs (Appleton and Palmer 1988; Trussell 1996; Leonard et al. 1999; Caro and Castilla 2004; Dalziel and Boulding 2005; Freeman and Byers 2006; Edgell and Neufeld 2008; Bourdeau 2009), but molluscs often co-exist with, and are likely exposed to, a variety of predatory and non-predatory crab species (Vermeij 1987). Furthermore, many predatory crabs select relatively small molluscan prey (Juanes 1992). Some molluscs survive by growing rapidly until they reach a size refuge at which predator-induced mortality declines significantly (e.g., Paine 1976; Whetstone and Eversole 1981). Thus, other inducible responses, such as rapid growth, might be effective defenses. In addition, smaller decapod crustaceans (e.g., hermit crabs and shore crabs) that attack molluscs via shell entry or ‘winkling’ (Rochette et al. 2007; Edgell and Rochette 2009) may cause substantial mortality to early juvenile stages (Gosselin and Chia 1995; Gosselin and Rehak 2007), and thus, select for inducible responses like shell elongation and increased retraction depth (Bourdeau 2009; Edgell et al. 2009; Miner et al. 2013).
At present, it is not known whether there are specific cues associated with predatory crab species, or generalized cues associated with all crabs that trigger inducible defenses in molluscs (but see Hooks and Padilla 2014). Here, we tested whether inducible shell defenses and associated changes in soft-tissue growth in Nucella lamellosa, one of the most well-studied marine species displaying inducible defenses (Padilla and Sevedo 2013; Bourdeau et al. 2015), are specific to its most common predator, the rock crab Cancer productus, or if other co-occurring predatory and non-predatory crabs can induce similar or different responses.
Methods
Study organisms and collection sites
Nucella lamellosa is an intertidal zone snail that produces characteristic inducible defenses, a heavy shell with a thickened apertural lip and apertural teeth, in the presence of water-borne chemical cues from its major crab predator, the red rock crab, C. productus (Appleton and Palmer 1988; Edgell and Neufeld 2008; Bourdeau 2009). In addition to co-occurring with C. productus, N. lamellosa lives sympatrically with a suite of predatory and non-predatory crabs throughout much its range in the eastern north Pacific, from Alaska to California (Kozloff 1987; Jensen 1995; Collins et al. 1996).
We used five of these sympatric crab species in our study, all of which are common on protected rocky shores in the eastern north Pacific, but which differ in their diets and their ability to eat hard-shelled prey (i.e., durophagy; Kozloff 1987; Jensen 1995). C. productus (hereafter Cancer), and the pygmy rock crab, Glebocarcinus oregonensis (formerly Cancer oregonensis), are primarily durophagous and possess relatively large, strong chelae capable of producing powerful crushing forces (Yamada and Boulding 1998; Taylor et al. 2000). Whereas Cancer is large, highly mobile, and actively accesses the intertidal zone at high tide and consumes a large number of prey per unit body weight, Glebocarcinus is relatively sedentary, is found mostly in shelters in the low intertidal and subtidal zones, and has a small effect on intertidal zone gastropod prey compared to Cancer (Robles et al. 1989; Yamada and Boulding 1996, 1998). The purple shore crab, Hemigrapsus nudus, is a generalist omnivore; it has smaller, weaker claws than Glebocarcinus or Cancer, and consumes only the smallest snail species (Yamada and Boulding 1998). We also included two anomuran crabs, neither of which regularly consume hard-shelled prey. The grainyhand hermit crab, Pagurus granosimanus, is mainly a scavenger and detritivore; although it can feed opportunistically on Nucella hatchlings, it does so only rarely (Gosselin 1997). The porcelain crab, Petrolisthes eriomerus, is primarily a suspension feeder and detritivore, and does not use its chelae to feed (Jensen 1995).
We collected 180 juvenile (< 25 mm) N. lamellosa from the Westside Preserve, a current-swept shore on the west side of San Juan Island, WA, USA (48°30′26.76″N, 123°8′35.20″W). All crab species used in this study can be found at this site, but are rare (Bourdeau, personal observation). This ensured that experimental snails had little prior field exposure to cues from any of the test crab species. Cancer was trap-collected from the pier at the University of Washington’s Friday Harbor Laboratories (FHL). All other crabs used in the experiment were collected by hand approximately 16 km from FHL in areas on the south and west sides of San Juan Island, which are exposed to wave action during winter storms (Dayton 1971; Menge 1972).
Experimental design
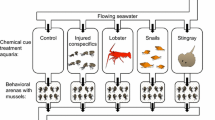
All experiments were conducted at FHL. Snails were exposed to six treatments: a ‘no cue’ control (no crab), and water-borne cues from each of the five different crab species: Cancer, Glebocarcinus, Hemigrapsus, Pagurus, and Petrolisthes. Because of the relatively large number of treatments (six), two replicate aquaria (30.5W × 19.1D × 20.3H cm) were used for each treatment. Although more replicate aquaria per treatment would have given us more statistical power, a previous study (Appleton and Palmer 1988) used only two replicates per treatment. They found that N. lamellosa reared in the presence of chemical cues from C. productus fed fish grew only 78% as much as snails in the absence of these cues (Appleton and Palmer 1988). Furthermore, subsequent studies (Bourdeau 2010a; Bourdeau 2012) have documented that N. lamellosa grow roughly 67% (between 45 and 90%) as much as snails in the absence of these cues, indicating an approximate effect size that should be statistically detectable with only two replicates. Fifteen snails, individually numbered with bee tags, were randomly allocated to each replicate aquarium. Snails had the same somatic mass (ANOVA, F5,6 = 1.19, P = 0.41) and shell mass (ANOVA, F5,6 = 1.90, P = 0.23) across treatments at the beginning of the experiment. Because predator biomass has been shown to determine the magnitude of inducible prey responses to predatory crabs (Hill and Weissburg 2013), and because the crab species which we used differed greatly in size, we used different numbers of crabs of each species in an effort to keep the biomass of the crabs used similar among treatments [1 Cancer (mean carapace width = 117.33 mm), 3 Glebocarcinus (maximum carapace width = 53 mm), 4 Hemigrapsus (max. width = 34 mm), 6 Pagurus (max. width = 19 mm), and 5 Petrolisthes (max. width = 19 mm)]. Crabs were placed in a plastic chamber (1.9 L) fastened to the underside of the lid of each experimental aquarium. Gravity-fed seawater flowed from a header tank into each chamber through a feeding hatch in the lid. Overflow from the chamber provided each replicate aquarium with seawater. This design allowed constant flow-through of crab chemical cues while preventing physical contact between crabs and snails. Snails were fed ad libitum their preferred barnacle prey, Balanus glandula, encrusted on small stones. Barnacle-depleted stones were replaced with new barnacle-covered stones as needed, such that the snails were never food limited. Crabs were fed frozen fish (Pacific Dover sole, Microstomus pacificus), so that experimental snails were not exposed to the scent of injured snails, which, in conjunction with cues from crabs, can enhance induced shell defenses (Bourdeau 2010a). This was necessary to allow us to isolate the specific effect of each crab species from the effects of a general alarm cue (i.e., crushed or consumed snails) on the inducible responses of the experimental snails. Thawed frozen fish does not induce shell defenses in N. lamellosa (Bourdeau 2010a).
Soft-tissue growth and shell morphology
Snails were measured and weighed prior to the beginning and at the end of the experiment, which lasted 60 days. A nondestructive method was used to separate shell mass from soft-tissue mass whereby snails are weighed in air and then weighed submerged in water (Palmer 1982). Shell mass was calculated as 1.572 × (submerged weight) + 0.0162, a species-specific regression equation derived from N. lamellosa populations collected near FHL (Palmer 1982). Soft-tissue mass was calculated by subtracting shell mass from the total damp weight in air. Shell length, width, and apertural lip thickness were measured to the nearest 0.01 mm with digital calipers (Fig. 1). Apertural lip thickness was measured at the mid-point of the apertural lip and at the lip suture, and these two values were averaged. A total of three snails died during the course of the experiment.
Statistical analysis
All data were additively coded (+ 1) and then log10-transformed to better meet the assumptions of normality and homoscedasticity for parametric tests (Sokal and Rohlf 1995).
To examine treatment effects on final snail size, we analyzed final shell length with a nested analysis of variance (ANOVA) with cue treatment as a fixed factor and aquarium as a random factor nested within treatment (as snails within a aquarium are not independent). We tested for treatment effects on somatic growth by analyzing final soft-tissue mass with a nested analysis of covariance (ANCOVA) with cue treatment as a fixed factor, aquarium as a random factor nested within treatment, and initial body mass as a covariate to control for initial snail size.
To test for treatment effects on shell thickening, we used a nested ANCOVA on apertural lip thickness with treatment as a fixed factor, aquarium as a random factor nested within treatment, and final shell length as a covariate. To further examine cue treatment effects on shell thickening, we analyzed final shell mass with a nested ANCOVA with cue treatment as a fixed factor and aquarium as a random factor nested within cue treatment, and final shell length as a covariate to account for differences in snail size. For all ANCOVA models, treatment × covariate interactions with P > 0.10 were removed from the models (Hendrix et al. 1982).
We made post hoc comparisons of covariate-adjusted means with Fisher’s protected least significant difference test (PLSD). Analyses of variance and post hoc comparison of means were conducted with Statistica (v 6.1) and R 2.14.1 (R Development Core Team 2011), and the Wilcox procedure was performed with the program WILCOX (Quinn and Keough 2002).
Results
Cue treatment had no significant effect on final shell length (ANOVA, F5,6 = 1.18, P = 0.42; Table 1). There was no significant effect of the treatment × initial body mass interaction on final soft-tissue mass (ANCOVA, treatment × initial body mass: F5,160 = 1.71, P = 0.14), so this interaction term was dropped from the model. Treatment did have a significant effect on final soft-tissue mass (Table 2). Post hoc analyses of initial size-adjusted mean soft-tissue mass revealed significant differences between the control and each crab cue treatment, but not among crab cue treatments (Fig. 2).
Final soft-tissue mass (g) of N. lamellosa raised under six treatment conditions: no cue control (no crab), grainyhand hermit crab (Pagurus), porcelain crab (Petrolisthes), purple shore crab (Hemigrapsus), pygmy rock crab (Glebocarcinus), and red rock crab (Cancer). Values are back-transformed least-squares means and 95% confidence intervals of log10 + 1 transformed data computed for the covariate initial body mass (g) at its mean. All treatment groups were significantly different from the control (PLSD, P < 0.05), but not from each other (PLSD, P > 0.05)
Apertural lip thickness did not meet the assumption of equal variances (Levene’s, P = 0.027), even after transformation; but we report the results of the ANCOVA, because it is generally robust to violations of the assumption as long as group sizes are equal and variances are not dramatically different from each other (Sokal and Rohlf 1995). For apertural lip thickness, we found a significant interaction between treatment and the covariate, shell length (Table 3). As a result, we were not able to compare length-adjusted mean shell thickness, because the scaling between it and shell length was not the same across our experimental treatments. We therefore used the Wilcoxon modification of the Johnson–Neyman procedure to determine the range of covariates over which response variables were significantly different among treatments (Huitema 1980). However, only the regression line for Cancer differed in slope from the control (P < 0.05; Table 3; Fig. 3). Snails exposed to Cancer had significantly thicker apertural lips than snails in the no crab treatment when the covariate, final shell length, varied between 27.49 and 30.94 mm (Wilcoxon Johnson–Neyman test; Fig. 3).
Relationship between log(apertural lip thickness + 1) and log(final shell length (mm) + 1) of N. lamellosa raised under six treatment conditions: no cue control (no crab), grainyhand hermit crab (Pagurus), porcelain crab (Petrolisthes), purple shore crab (Hemigrapsus), pygmy rock crab (Glebocarcinus), and red rock crab (Cancer). To facilitate visualization, best-fit lines from simple linear regression are shown without associated data points. Best-fit lines are restricted to the range of observed shell lengths
ANCOVA revealed that the slopes of regressions for final shell mass as a function of shell length were equal for all the treatments (ANCOVA, treatment × final shell length: F5,160 = 1.17, P = 0.32). Hence, the treatment × final shell length interaction term was dropped from the model and we were able to compare size-adjusted mean shell mass, because the scaling between it and shell length was the same across our experimental treatments. There was no significant effect of cue treatment on the final shell mass (Table 4), although responses were highly variable within most treatments (Fig. 4; Table 4).
Final shell mass (g) of N. lamellosa raised under six treatment conditions: no cue control (no crab), grainyhand hermit crab (Pagurus), porcelain crab (Petrolisthes), purple shore crab (Hemigrapsus), pygmy rock crab (Glebocarcinus), and red rock crab (Cancer). Values are back-transformed least-squares means and 95% confidence intervals of log10 + 1 transformed data computed for the covariate shell length (mm) at its mean. None of the treatments were significantly different from one another (PLSD P > 0.05)
Discussion
Some aspects of the phenotypic response of N. lamellosa were highly specific, restricted to chemical cues from the snail’s most dangerous predator, while other aspects of the snail’s phenotype changed in response to chemical cues from all of the crabs, independent of the risk which they posed. A common response of snails exposed to increased risk of predation is a significant reduction in soft-tissue mass, and we observed this across all crab treatments, independent of the predation risk posed by the crab species. Reductions in somatic growth are usually attributed to greater refuge use and reduced feeding under predation risk (Bourdeau 2010b; Bourdeau and Johansson 2012), which would reduce the exposure of snails to potential predators. That all crabs, even those that are omnivores or scavengers, seemed to induce a response in N. lamellosa was surprising. This result may indicate that there is some chemical cue associated with all of these sympatric crabs that indicates a habitat with higher risk of predation.
Although size-corrected final shell mass was the same across all experimental treatments, thicker apertural lips in snails exposed to Cancer indicate a different geometric allocation of shell mass in those snails. For snails in the control treatment (absence of any crab cues) and in the presence of lower risk or non-predatory crab species, increases in shell mass during the course of the experiment were not concentrated at the apertural lip, whereas snails exposed to the high-risk predator, Cancer, allocated shell material preferentially at the apertural lip.
Because theory predicts that inducible defenses are costly (either due to energetic, developmental, or opportunity costs), they should only be deployed in a risk-sensitive manner (i.e., when the risk of predation is high). Therefore, snails were expected to show a strong, specific induced defensive response to predatory crabs, especially in response to Cancer, their most important predator, and a weak response or no response to non-predatory species. Although N. lamellosa grew less in response to all crab species tested, even those that pose no risk of predation, it only thickened its apertural lip in response to chemical cues from Cancer, the species posing the greatest threat of predation. The lip thickening response to Cancer is consistent with theoretical expectations, as it would reduce vulnerability to crabs (like Cancer) that peel shells from the aperture; however, such shells would still be vulnerable to crushing attacks (Vermeij 1978), a tactic that is also employed by Cancer (Zipser and Vermeij 1978). The shell mass of snails exposed to chemical cues from different crabs was not different, and was not different than that of control snails. This result suggests that snails that allocated more shell to the apertural lip in the presence of Cancer, sacrificed an overall reinforcement of the shell.
Because an overall thickening of the shell may developmentally constrain soft-tissue growth (Palmer 1981), we suggest that allocating shell material to the apertural lip may be a way for N. lamellosa to mount an ‘intermediate’ defense while minimizing soft-tissue growth costs associated with overall shell-thickening (e.g., Bourdeau 2010b). Our finding that apertural lip thickening in response to Cancer did not incur any reduction in soft-tissue growth beyond that observed in snails exposed to less threatening, or non-predatory crabs supports this hypothesis. Furthermore, although not statistically significant, there was a trend for snails exposed to Cancer to develop shells with relatively lower aspect ratio than snails exposed to chemical cues from other crabs (Online Appendix A); that is, they were shorter and more rotund. Such relatively rotund shells have been hypothesized to spread crushing forces more evenly over a given amount of shell material, increasing crushing resistance without investing in additional shell material (DeWitt et al. 2000). Thus, re-allocating shell material to change the overall shape of the shell to one that is more resistant to crab attacks without adding more shell material and thus limiting somatic growth (i.e., low aspect ratio with thicker apertural lip) may itself be an adaptive, low-cost strategy against crabs like Cancer that attack via both shell-peeling and shell-crushing.
Observational and experimental evidences also support a cost-minimization strategy for apertural lip thickening in Nucella spp. For example, N. lapillus from habitats where predation risk from crabs is high, thicken just one microstructural layer of their shell (the homogeneous layer)—a relatively weak but energetically inexpensive shell material with low organic content (Avery and Etter 2006)—and Cancer-induced lip thickening in N. lamellosa appears to show a similar pattern (Bourdeau 2010b). Enhancing the weaker shell microstructural layer may be energetically cheaper, reducing the cost to soft-tissue growth and allowing snails to grow rapidly to a size refuge, which may ultimately be more important for reducing the risk of predation than maximizing shell strength. Further experimentation is needed to assess the relative importance of lip thickening, shell shape (i.e., aspect ratio), and shell size for protection from crab predation for Nucella.
Two different mechanisms could lead to increased lip thickness in N. lamellosa exposed to Cancer: (1) increased deposition rate of shell material at the apertural lip with constant linear shell translation (i.e., increase of shell length along the axis of coiling; Brookes and Rochette 2007), or (2) reduced linear translation of the shell in conjunction with constant deposition of shell material (Bourdeau 2010b). Our study was not designed to distinguish between these two mechanisms, but our results are partially consistent with the ‘reduced linear translation and constant shell deposition’ model. For example, shell length (linear shell growth) did not differ between crab-exposed and control snails and, for a given shell length, the amount of shell material deposited (shell mass) also did not differ. Thus, snails exposed to Cancer produced a thicker apertural lip without increasing shell deposition relative to the control or other crab cue treatments. It should be noted, however, that there was also no reduction in linear shell translation in snails exposed to Cancer relative to the other treatments. Thus, in this study, N. lamellosa appeared to thicken the shell by modifying the geometric allocation of shell material (i.e., changing its shell shape), rather than depositing more shell material (e.g., Appleton and Palmer 1988; Palmer 1990) or simply passively accreting shell at the apertural lip via reduced linear shell growth (Bourdeau 2010b).
Surprisingly, N. lamellosa exposed to lower risk and non-predatory crabs exhibited reduced growth similar to those snails exposed to Cancer. It is likely that this result is closely linked to snail feeding rates. Although we did not quantitatively document snail feeding behavior (e.g., barnacle drilling), mid-experiment visual scans of experimental snails indicated a marginally significant trend (F5,6 = 4.28, P = 0.053) for snails in the presence of crab (predatory or otherwise) cues to be on the underside of stones in experimental aquaria, whereas snails in the control treatment were not. Many studies have documented similar increases in refuge use and reduced feeding activity in gastropods exposed to the presence of predatory crab cues (Palmer 1990; Richardson and Brown 1992; Trussell et al. 2003, 2006; Bourdeau 2009, 2013; but see Hooks and Padilla (2014) where some snails exposed to a nonnative predatory crab did not show this response). The use of refugia (e.g., hiding) reduces the risk of encountering predators, but often comes at the cost of reduced foraging and, therefore, reduced somatic growth.
For increased refuge use and reduced feeding to be adaptive for snails when exposed to cues of crabs that do not pose a risk, costs must be balanced by or outweighed by the benefits of the response (Levins 1968; Lively 1986). Snails experience a growth cost with reduced feeding, increasing the time to attain a size refuge from many predators (e.g., Harding 2003) and a smaller overall body size with reduced fecundity (e.g., Harding et al. 2007). Given these opportunity costs, responding to cues from low-risk or non-predatory crabs when predatory crabs are absent would seem maladaptive (Langerhans and DeWitt 2002). Two possible alternative hypotheses could explain these results.
First, the overall abundance of crabs (including both predatory and non-predatory species) on rocky shores may be highly correlated with the abundance of Cancer. Both predatory and non-predatory crabs tend to be more abundant on wave-protected than wave-exposed shores, and thus, chemical signals from any crab may indicate a risky environment, stimulating a defensive response. There is precedent for such ‘indirect’ cues in freshwater systems, where the water flea Daphnia undergoes a diel vertical migration as a predator avoidance response to chemical cues released by both planktivorous and piscivorous fish, even though piscivores pose no risk for Daphnia. However, because piscivores co-exist with planktivores, their presence indirectly indicates the presence of planktivores (von Elert and Pohnert 2000). Extensive field data will be needed to test the hypothesis that the presence of non-predatory crabs is positively correlated with the presence of predatory crabs across N. lamellosa’s range.
Alternatively, even low-risk crabs may pose enough risk that reduced feeding in their presence is advantageous for N. lamellosa. For example, thicker-lipped shells may not protect snails from attacks by Pagurus, Hemigrapsus, and Glebocarcinus, which are likely to use shell-entry attacks (where crabs insert their claw through the shell aperture and pull out the soft parts of the snails) on snails. Indeed, snails exposed to Hemigrapsus, and Glebocarcinus showed a trend of developing higher aspect ratio (i.e., longer, narrower) shells relative to control snails and snails exposed to Cancer cues (Online Appendix A). Predator-induced increases in shell aspect ratio have been observed in response to other shell-entering predators, and provide more room for snails to withdraw into, better protecting them against shell-entry attacks (e.g., DeWitt et al. 2000; Bourdeau 2009). However, entry-resistant shells can be more susceptible to crushing attacks, indicating an inherent survival trade-off between entry-resistant and crush-resistant shells (e.g., Bourdeau 2009). Thus, a shell elongation response could represent an adaptive response specific to shell-entry attacking crabs that is balanced by a survival trade-off with the shell-thickening response induced by shell-breaking crabs.
Given that snail behavior can track temporal changes in predation risk more rapidly than changes in shell morphology, snails that respond over-cautiously or even inappropriately to a general crab cue could quickly reverse their behavior and compensate for periods of inactivity with increased feeding and growth during periods when cues associated with predation risk are absent (Arendt 1997; Stachowicz and Hay 1999). Thus, while opportunity costs of responding to general cues from crabs might exist over short time scales, long-term costs may be relatively minor and outweighed by the survival benefits of responding cautiously in risky habitats.
Finally, it is also possible that relatives of the non-predatory crab species are predators on juvenile N. lamellosa. Snails may then respond to non-predatory species due to cue similarity with closely related predatory species (Sih et al. 2010). For example, although P. granosimanus rarely feeds on Nucella hatchlings (Gosselin 1997), its congener P. hirsutiusculus may be a significant source of mortality for the early juvenile Nucella (Gosselin and Chia 1995). If the chemical signature of P. granosimanus and P. hirsutiusculus are similar enough, N. lamellosa may respond to the non-predatory congener as if it was a predatory threat.
Our results indicate that N. lamellosa can distinguish between chemical cues released by high-risk predators and low-risk and non-predatory species and respond phenotypically in a risk-sensitive manner. Fully factorial experiments that cross gradients of risk and resources will be necessary to fully determine the precise nature and magnitude of integration among snail feeding activity, soft tissue and shell growth, and changes in shell mass and shell shape in N. lamellosa and other marine gastropods capable of modifying their phenotype in response to risk cues from crabs. More information is needed about indirect environmental cues that may signal risky environments to fully understand the role of chemical signaling on inducible responses that we presently interpret as adaptive.
References
Appleton RD, Palmer AR (1988) Water-borne stimuli released by predatory crabs and damaged prey induce more predator-resistant shells in a marine gastropod. Proc Natl Acad Sci USA 85:4387–4391
Arendt JD (1997) Adaptive intrinsic growth rates: an integration across taxa. Q Rev Biol 72:149–177
Avery R, Etter RJ (2006) Microstructural differences in the reinforcement of a gastropod shell against predation. Mar Ecol Prog Ser 323:159–170
Bourdeau PE (2009) Prioritized phenotypic responses to combined predators in a marine snail. Ecology 90:1659–1669
Bourdeau PE (2010a) Cue reliability, risk sensitivity and inducible morphological defense in a marine snail. Oecologia 162:987–994
Bourdeau PE (2010b) An inducible morphological defence is a passive by-product of behaviour in a marine snail. Proc R Soc B 277:455–462
Bourdeau PE (2012) Intraspecific trait cospecialization of constitutive and inducible morphological defences in a marine snail from habitats with different predation risk. J Anim Ecol 81:849–858
Bourdeau PE (2013) Morphological defense influences absolute, not relative, nonconsumptive effects in marine snails. Behav Ecol 24:505–510
Bourdeau PE, Johansson F (2012) Predator-induced morphological defences as by-products of prey behaviour: a review and prospectus. Oikos 121:1175–1190
Bourdeau PE, Butlin RK, Brönmark C, Edgell TC, Hoverman JT, Hollander J (2015) What can aquatic gastropods tell us about phenotypic plasticity? A review and meta-analysis. Heredity 115(4):312
Brookes JI, Rochette R (2007) Mechanism of a plastic phenotypic response: predator‐induced shell thickening in the intertidal gastropod Littorina obtusata. J Evol Biol 20(3):1015–1027
Bronmark C, Hansson LA (2000) Chemical communication in aquatic systems: an introduction. Oikos 88:103–109
Caro AU, Castilla JC (2004) Predator-inducible defences and local intrapopulation variability of the intertidal mussel Semimytilus algosus in central Chile. Mar Ecol Progr Ser 276:115–123
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5:338–352
Collins TM, Frazer K, Palmer AR, Vermeij GJ, Brown WM (1996) Evolutionary history of northern hemisphere Nucella (Gastropoda, Muricidae): molecular, morphological, ecological, and paleontological evidence. Evolution 50:2287–2304
Dalziel B, Boulding EG (2005) Water-borne cues from a shell-crushing predator induce a more massive shell in experimental populations of an intertidal snail. J Exp Mar Biol Ecol 317:25–35
Dayton PK (1971) Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr 41:351–389
DeWitt TJ, Robinson BW, Wilson DS (2000) Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evol Ecol Res 2:129–148
Dodson SI, Crowl TA, Peckarsky BL, Kats LB, Covich AP, Culp JM (1994) Nonvisual communication in freshwater benthos—an overview. J N Am Benthol Soc 13:268–282
Edgell TC, Neufeld CJ (2008) Experimental evidence for latent developmental plasticity: intertidal whelks respond to a native but not an introduced predator. Biol Lett 4:385–387
Edgell TC, Rochette R (2009) Prey-induced changes to a predator’s behaviour and morphology: implications for shell–claw covariance in the northwest Atlantic. J Exp Mar Biol Ecol 382:1–7
Edgell TC, Lynch BR, Trussell GC, Palmer AR (2009) Experimental evidence for the rapid evolution of behavioral canalization in natural populations. Am Nat 174:434–440
Freeman AS, Byers JE (2006) Divergent induced responses to an invasive predator in marine mussel populations. Science 313:831–833
Gabriel W, Luttbeg B, Sih A, Tollrian R (2005) Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am Nat 166:339–353
Gosselin LA (1997) An ecological transition during juvenile life in a marine snail. Mar Ecol Progr Ser 157:185–194
Gosselin LA, Chia FS (1995) Distribution and dispersal of early juvenile snails: effectiveness of intertidal microhabitats as refuges and food sources. Mar Ecol Progr Ser 128:213–223
Gosselin LA, Rehak R (2007) Initial juvenile size and environmental severity: influence of predation and wave exposure on hatching size in Nucella ostrina. Mar Ecol Progr Ser 339:143–155
Harding JM (2003) Predation by blue crabs, Callinectes sapidus, on rapa whelks, Rapana venosa: possible natural controls for an invasive species? J Exp Mar Biol Ecol 297:161–177
Harding JM, Mann R, Kilduff CW (2007) The effects of female size on fecundity in a large marine gastropod Rapana venosa (Muricidae). J Shellfish Res 26:33–42
Harvell CD (1998) Genetic variation and polymorphism in the inducible spines of a marine bryozoan. Evolution 52:80–86
Hawkins LA, Magurran AE, Armstrong JD (2007) Innate abilities to distinguish between predator species and cue concentration in Atlantic salmon. Anim Behav 73:1051–1057
Hendrix LJ, Carter MW, Scott DT (1982) Covariance analyses with heterogeneity of slopes in fixed models. Biometrics 38:641–650
Hill JM, Weissburg MJ (2013) Predator biomass determines the magnitude of non-consumptive effects (NCEs) in both laboratory and field environments. Oecologia 172(1):79–91
Hooks AP, Padilla DK (2014) Prey responses to the presence of a native and nonnative predator. J Exp Mar Biol Ecol 461:209–215
Huitema BE (1980) The analysis of covariance and alternatives. Wiley, New York
Iyengar EV, Harvell CD (2002) Specificity of cues inducing defensive spines in the bryozoan Membranipora membranacea. Mar Ecol Prog Ser 225:205–218
Jensen GC (1995) Pacific Coast crabs and shrimps. Sea Challengers, Monterey
Juanes F (1992) Why do decapod crustaceans prefer small-sized molluscan prey? Mar Ecol Progr Ser 87:239–249
Kozloff EN (1987) Marine invertebrates of the Pacific Northwest. University of Washington Press, Seattle
Laforsch C, Beccara L, Tollrian R (2006) Inducible defenses: the relevance of chemical alarm cues in Daphnia. Limnol Oceanogr 51:1466–1472
Langerhans RB, DeWitt TJ (2002) Plasticity constrained: over-generalized induction cues cause maladaptive phenotypes. Evol Ecol Res 4:857–870
Leonard GH, Bertness MD, Yund PO (1999) Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology 80:1–14
Levins R (1968) Evolution in changing environments. Princeton University Press, Princeton
Lively CM (1986) Canalization versus developmental conversion in a spatially-variable environment. Am Nat 128:561–572
Menge BA (1972) Foraging strategy of a starfish in relation to actual prey availability and environmental predictability. Ecol Monogr 42:25–50
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692
Miner BG, Donovan DA, Portis LM, Goulding TC (2013) Whelks induce an effective defense against sea stars. Mar Ecol Progr Ser 493:195–206
Padilla DK, Adolph SC (1996) Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol Ecol 10:105–117
Padilla DK, Savedo MM (2013) A systematic review of phenotypic plasticity in marine invertebrate and plant systems. In: Advances in marine biology, vol 65. Academic Press, pp 67–94
Paine RT (1976) Size-limited predation: an observational and experimental approach with the Mytilus–Pisaster interaction. Ecology 57:858–873
Palmer AR (1981) Do carbonate skeletons limit the rate of body growth? Nature 292:150–152
Palmer AR (1982) Growth in marine gastropods—a non-destructive technique for independently measuring shell and body-weight. Malacologia 23:63–73
Palmer AR (1990) Effect of crab effluent and scent of damaged conspecifics on feeding, growth, and shell morphology of the Atlantic dogwhelk Nucella lapillus (L). Hydrobiologia 193:155–182
Quinn GP, Keough MJ (2002) Experimental design and data analysis. Cambridge University Press, New York
R Development Core Team, RFFSC (2011) R: a language and environment for statistical computing
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Richardson TD, Brown KM (1992) Predation risk and feeding in an intertidal predatory snail. J Exp Mar Biol Ecol 163:169–182
Robles C, Sweetnam DA, Dittman D (1989) Diel variation of intertidal foraging by Cancer productus L in British Columbia. J Nat Hist 23:1041–1049
Rochette R, Doyle SP, Edgell TC (2007) Interaction between an invasive decapod and a native gastropod: predator foraging tactics and prey architectural defenses. Mar Ecol Progr Ser 330:179–188
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. State University of New York at Stony Brook, New York
Stachowicz JJ, Hay M (1999) Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs. Mar Ecol Progr Ser 188:169–178
Taylor GM, Palmer AR, Barton AC (2000) Variation in safety factors of claws within and among six species of Cancer crabs (Decapoda : Brachyura). Biol J Linn Soc 70:37–62
Teplitsky C, Plenet S, Joly P (2004) Hierarchical responses of tadpoles to multiple predators. Ecology 85:2888–2894
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
Trussell GC (1996) Phenotypic plasticity in an intertidal snail: the role of a common crab predator. Evolution 50:448–454
Trussell GC, Nicklin MO (2002) Cue sensitivity, inducible defense, and trade-offs in a marine snail. Ecology 83:1635–1647
Trussell GC, Ewanchuk PJ, Bertness MD (2003) Trait-mediated effects in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology 84:629–640
Trussell GC, Ewanchuk PJ, Matassa CM (2006) The fear of being eaten reduces energy transfer in a simple food chain. Ecology 87:2979–2984
Vermeij GJ (1978) Biogeography and adaptation: patterns of marine life. Harvard University Press, Cambridge
Vermeij GJ (1987) Evolution and escalation: an ecological history of life. Princeton University Press, Princeton
von Elert E, Pohnert G (2000) Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach. Oikos 88:119–128
Whetstone JM, Eversole AG (1981) Effects of size and temperature on mud crab, Panopeus herbstii, predation on hard clams, Mercenaria mercenaria. Estuaries 4:153–156
Yamada SB, Boulding EG (1996) The role of highly mobile crab predators in the intertidal zonation of their gastropod prey. J Exp Mar Biol Ecol 204:59–83
Yamada SB, Boulding EG (1998) Claw morphology, prey size selection and foraging efficiency in generalist and specialist shell-breaking crabs. J Exp Mar Biol Ecol 220:191–211
Zipser E, Vermeij GJ (1978) Crushing behavior of tropical and temperate crabs. J Exp Mar Biol Ecol 31:155–172
Acknowledgements
We thank the director and staff of Friday Harbor Laboratories for logistical support, M. Mach for help monitoring the experiment and drawing the snail in Fig. 1, and M. Dethier for graciously surrendering lab space to accommodate the experiment. G. Trussell and 2 anonymous reviewers provided constructive criticism on earlier versions of the manuscript. This is contribution number 1254 from the Department of Ecology and Evolution at Stony Brook University. The experiments comply with the current laws of the country in which they were performed.
Funding
A Stephen and Ruth Wainwright Fellowship supported PEB. DKP was supported by NSF IOS 0920032 during the writing of this paper and acknowledges the Helen C. Whitley Center at the Friday Harbor Laboratories.
Author information
Authors and Affiliations
Contributions
PEB conceived, designed, and performed the experiment, and analyzed the data. PEB and DKP wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for sampling, care, and experimental use of organisms for the study were followed. Research was completed under permits from the Washington Department of Fish and Wildlife (WDFW).
Additional information
Responsible Editor: G. Chapman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bourdeau, P.E., Padilla, D.K. Cue specificity of predator-induced phenotype in a marine snail: is a crab just a crab?. Mar Biol 166, 84 (2019). https://doi.org/10.1007/s00227-019-3526-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3526-0