Abstract
Fusarium spp. and Verticillium spp. are widespread soil pathogens responsible for vascular wilts causing heavy yield losses in eggplant (Solanum melongena) as well as in many other crops. Here we report on the identification of QTLs affecting the resistance to Fusarium and Verticillium in an F2 intraspecific population of 156 individuals bred from the cross ‘305E40’ × ‘67/3’, we previously characterized for key agronomic and biochemical traits. The female parent (‘305E40’) is an androgenetic introgressed line carrying the resistance locus Rfo-Sa1 derived from Solanum aethiopicum. The line is fully resistant to Fusarium and also displays a previously uncharacterized partial resistance to Verticillium. The male parent (‘67/3’) is an F8 selection from the eggplant intra-specific cross cv. ‘Purpura’ x cv. ‘CIN2’ which, unexpectedly, revealed a not previously characterized partial resistance to Fusarium, but it is highly susceptible to Verticillium. The degree of resistance of the F2 population was assessed following artificial inoculation in greenhouse (Fusarium) or growth chamber (Verticillium) of F2:3 progenies obtained by selfing each F2 individual. Other than a major QTL for the resistance to Fusarium, which lies in the genomic region of the Rfo-Sa1 locus, major and minor QTL influencing the response to both Fusarium and Verticillium were spotted, and putative tomato orthologous genes were identified as well. The markers linked to the spotted QTL may find application in the context of marker-assisted breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eggplant, also known as brinjal or aubergine (Solanum melongena L., 2n = 2x = 24) is a member of the Solanaceae, a large plant family including around 2700 plant species among which tomato (S. lycopersicum L.), potato (S. tuberosum L.), pepper (Capsicum annuum L.) and tobacco (Nicotiana tabacum L.). Unlike most of the other major Solanaceous crops, which are native of the New World (Fukuoka et al. 2010; Albert and Chang 2014; Hirakawa et al. 2014), eggplant has a uniqueness phylogeny due to its Old World domestication (Lester and Hasan1991), which occurred in Asia as a result of at least two separate events (Daunay 2012; Meyer et al. 2012; Cericola et al. 2013; Knapp et al. 2013).
Eggplant is cultivated worldwide, with a total production of more than 50.2 M tons in 2014, however, more than 90% of its production is concentrated in Asia, where it represents a staple food in countries such as China and India (FAOSTAT 2014, http://faostat3.fao.org/browse/Q/QC/E). Because of its importance for food security, eggplant is included, with 34 other crops, in the Annex 1 of the International Treaty on Plant Genetic Resources for Food and Agriculture (Fowler et al. 2003).
Eggplant is susceptible to many diseases, which cause yield losses both in greenhouse and in open field cultivations (Sihachakr et al. 1994), and the fungal wilts caused by Verticillium dahliae (Vd) Kleb., V. albo-atrum (McKeen 1972; Bhat and Subbarao 1999; Diwan et al. 1999; Karagiannidis et al. 2002) as well as Fusarium oxysporum f. sp. melongenae (Fom) (Stravato et al. 1993; Urrutia Herrada et al. 2004; Altinok 2005) are among the most common and serious. As occurs in many other domesticates, anthropogenic selection has caused a drastic reduction of the genetic variation in cultivated germplasm, thus hampering the identification of resistance traits and their exploitation in breeding programs (Daunay et al. 1991; Rotino et al. 2014). On the other hand, the other two inter-fertile eggplant cultivated species, i.e. S. aethiopicum L. and S. macrocarpon L., as well as wild and allied relatives which share a relatively recent common ancestry with eggplant, represent a reservoirs of potential useful resistance traits exploitable for breeding purposes (Plazas et al. 2016; Syfert et al. 2016). Wild and cultivated relatives have been employed through both conventional (sexual crosses) and un-conventional (protoplast fusion, embryo rescue) strategies for the introgression of resistance traits in cultivated eggplant (Rotino et al. 2014; Kaushik et al. 2016), such as partial resistance to Vd (Acciarri et al. 2004; Liu et al. 2015) or complete resistance to Fom (Rizza et al. 2002; Rotino et al. 2005).
In eggplant, a few studies aimed at identifying QTL/genes affecting resistance to fungal wilts as well as elucidating the defense responses and signaling pathways activated upon infection have been conducted. Toppino et al. (2008) demonstrated that Fom resistance trait introgressed into eggplant from S. aethiopicum and S. integrifolium was controlled by a single dominant locus (named Rfo-sa1), which was mapped on CH02 by Barchi et al. (2010, 2012). Furthermore, a number of candidate genes involved in early defence responses or signalling pathways activated upon infection have been recently identified in Fom-resistant ILs carrying the Rfo-sa1 locus (Barbierato et al. 2016). Boyaci et al. (2010, 2011) performed a phenotypic characterization and genetic analysis of eggplant lines subjected to Fom inoculation. Recently, Miyatake et al. (2016) mapped two Fusarium semi-dominant inherited resistance loci (Fukuoka et al. 2012; Hirakawa et al. 2014) on chromosomes E02 and E04 in linkage maps developed from Asian Fom-resistant lines (Monma et al. 1996), and the locus on E02 was reported to be orthologous to the Rfo-sa1 locus already mapped on E02.
Few studies are available on Verticillium resistance. A first attempt of mapping the resistance to Verticillium dahliae was carried out using an interspecific cross between eggplant and the highly tolerant S. sodomeum (= S. linneanum) by developing a first RAPD-AFLP map (Sunseri et al., 2003). The Ve homologous gene of the potato StVe was isolated from the wild eggplant species S. torvum (Fei et al. 2004), and following deep sequencing of small RNAs, some miRNAs acting in response to Vd infection were spotted (Yang et al. 2013). A most recent de novo sequencing and transcriptome analysis of roots from Solanum aculeatissimum in response to Vd (Zhou et al. 2016) revealed the presence of a great number of differentially expressed genes participating in 128 metabolite pathways, among which those related to plant-pathogen interactions, plant-hormone signal transduction and phenylpropanoid biosynthesis are the most represented.
Inter-specific eggplant linkage maps have been constructed and used for the identification of QTL affecting agronomical and fruit quality traits (Doganlar et al. 2002a, b; Frary et al. 2003, 2014; Wu et al. 2009, Gramazio et al. 2014), however studies aimed at mapping QTL affecting fungal resistance traits to date have been very limited. The first eggplant intra-specific genetic map published by Nunome et al. (2001), and afterwards integrated with various sets of molecular markers (Nunome et al. 2003, 2009; Fukuoka et al. 2012), was used for mapping two QTL underpinning parthenocarpy and Fusarium resistance (Miyatake et al. 2012, 2016). We also developed a densely populated RAD-tag derived markers map (Barchi et al. 2011) based on an F2 intra-specific population, which made it possible to identify QTL affecting anthocyanin content (Barchi et al. 2012), key agronomic traits (Portis et al. 2014) as well as biochemical and morphological fruit properties (Toppino et al. 2016). Furthermore, through a GWAs approach, we validated marker/trait associations previously detected and new ones were identified (Cericola et al. 2014; Portis et al. 2015).
The present work aims to locate QTL affecting resistance to Fusarium oxysporum f. sp. melongenae and Verticillium dahliae Kleb in the previously developed intraspecific map, as the female parent ‘305E40’ of the F2 mapping population bears the Rfo-sa1 locus conferring complete resistance to Fom and displays also a partial resistance to Vd. On the other hand the male parent (‘67/3’), although lacks the Rfo-sa1 locus, revealed a not previously characterized partial resistance to Fom while is highly susceptible to Verticillium. The tomato orthologous genes falling within the confidence interval (C.I.) of the discovered QTL were also identified.
Materials and methods
Plant material
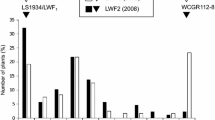
The starting material was a population of 156 F2 plants, obtained by selfing the F1 hybrid derived from the cross between the two eggplant breeding lines ‘305E40’ and ‘67/3’, contrasting for a wide number of key agronomic and metabolic traits (Barchi et al. 2012; Portis et al. 2014; Toppino et al. 2016). The line ‘305E40’ (female parent) is a double haploid derived from an interspecific somatic hybrid Solanum aethiopicum gr. gilo(+)S. melongena cv. Dourga (Rizza et al. 2002), which was repeatedly backcrossed with the recurrent lines DR2 and Tal1/1, prior to selfing and anther culture. This line carries the locus Rfo-sa1 from S. aethiopicum, which confers complete resistance to the soil-borne fungus Fusarium oxysporum f. sp. melongenae (Fom) (Toppino et al. 2008). Moreover, on the basis of our preliminary evaluations, assessed in resistance tests in which the ‘305E40’ was compared to the male parental line ‘67/3’ as well as to eggplant lines which are routinely used in our lab as reference, as they show major or minor manifestations of symptoms (supplemental Fig. 1), the line ‘305E40’ shows a partial resistance to Verticillium dahliae (Vd). Otherwise, the line ‘67/3’ is an F8 selection from the intra-specific cross between cv. ‘Purpura’ x cv. ‘CIN2’, which lacks the Rfo-sa1 locus and is fully susceptible to Vd.
In order to assess Fom and Vd resistance of the F2 progeny, each of the 156 individuals was selfed and as many F2:3 progenies obtained. Forty-eight plantlets of each F2:3 progeny as well as of each parental line and the F1 hybrid, for a total of 7,632 plantlets, were grown in greenhouse to assess the resistance to Fom. Side by side, forty plantlets of each F2:3 progeny plus the parental lines and the F1 hybrid, for a total of 6,360 plantlets, were grown in growth chambers to assess the resistance to Vd.
Assessment of Fusarium oxysporum f.s. melongenae resistance
Fom inoculation of the F2:3 progenies as well as parental lines and the F1 hybrid progenies was performed at Montanaso Lombardo (45°20′12″N 9°28′11″E, Italy), according to the dip-root method reported by Cappelli et al. (1995). Plantlets at 2–3th true leaf stage, grown in pasteurized peat in 104 holes plastic trays, were gently removed, their roots washed under running tap water and, after inoculation with Fom isolate (the same used by Cappelli et al. 1995) at concentration of 1.5 × 106 conidia/ml for 15 min, were transferred in 54 holes plastic trays filled with pasteurized peat (1:1, v/v). Plantlets were arranged in two randomized complete blocks in greenhouse, with 24 plants for each F2:3 progeny per block. At 30 Days After Inoculation (DAI), each plantlet was assessed and its degree of Fom infection was scored according to a scale ranging from 1 to 0, where 1 corresponds to “fully resistant plant with complete absence of symptoms”, 0 to “dead plant” with the intermediate values as follows: 0.9 = some spot of yellowing in basal leaves, absence of symptoms in intermediate and upper ones; 0.8 = extended yellowing in basal leaves; 0.7 = extended yellowing in basal leaves and some spot of yellowing in intermediate ones; 0.6 = extended yellowing in both basal and intermediate leaves; 0.5 = some spot of necrosis in basal leaves, extended yellowing in basal and intermediate leaves and some spot of yellowing in upper ones; 0.4 = partial necrosis in basal leaves, extended yellowing in intermediate and upper ones; 0.3 = necrosis in basal leaves and some spot of necrosis in intermediate ones; 0.2 = necrosis in basal and intermediate leaves, falling of basal leaves; 0.1 = complete necrosis in all the leaves, falling of basal and intermediate leaves.
For each block, the resistance ratio was calculated as follows:
Assessment of Verticillium dahliae resistance
Vd inoculation of the F2:3 progenies as well as parental lines and the F1 hybrid was performed according to a root-dip method at Carmagnola (44°53′N; 7°41′E, Italy). Plantlets at 2–3th true leaf stage, grown as previously described, were inoculated with Verticillium dahliae Kleb isolate (eggplant isolate V7) by dipping roots in a conidial suspension at a concentration of 5 × 105 conidia/ml for 15 min, and transferred in 15 cm-diam plastic pots filled with a pasteurized mixture of sand and soil (1:1, v/v) containing NPK fertilizer granules. Plantlets were then arranged in two randomized complete blocks in two separates growth chambers, with 20 plants per each entry (F2 individual) per block and kept at 25 °C ± 2 °C day, 20 °C ± 2 °C night, 50 µEm−2 s−1 with a 12-h photoperiod. The severity of Vd symptoms was evaluated on each leaf of each plant after 20 (early response) and 40 (late response) DAI. The symptomatic leaves were classified in a scale ranging from 0 to 5 as follows: 0 = no necrosis or chlorosis; 1 = asymmetry and/or chlorosis on ¼ of a leaf, 2 = chlorosis on ½ of a leaf, 3 = chlorosis on more than ½ of a leaf, 4 = wilted or partially necrotic leaf, 5 = necrotic or dead leaf.
Statistical analyses, molecular mapping and QTL detection
Statistical analyses were performed using R software (Team 2009). A conventional analysis of variance was applied to estimate genotype and environment effects based on the linear model Yij = μ + gi + bj + eij, where μ, g, b and e represent, respectively, the overall mean, the genotypic effect, the block effect and the error. Broad-sense heritability values were given by \( {{\sigma_{\text{G}}^{2} } \mathord{\left/ {\vphantom {{\sigma_{\text{G}}^{2} } {\left[ {\sigma_{\text{G}}^{2} + \left( {\sigma_{\text{E}}^{2} } \right)/{\text{n)}}} \right]}}} \right. \kern-0pt} {\left[ {\sigma_{\text{G}}^{2} + \left( {\sigma_{\text{E}}^{2} } \right)/{\text{n)}}} \right]}} \), where \( \sigma_{\text{G}}^{2} \) represents the genetic variance, \( \sigma_{\text{E}}^{2} \) the residual variance and n the number of blocks. Correlations between traits were estimated using the Spearman coefficient, and normality, kurtosis and skewness were assessed with the Shapiro-Wilks test (α = 0.05). Segregation was considered as transgressive when at least the scoring of one F2 individual was higher or lower by at least two standard deviations than the higher or lower scoring of a parental line.
Sequence comparison between orthologs of the tomato candidate genes falling within the C.I. of the FM1 QTL as reported in Miyatake et al. (2016) yielded polymorphisms between ‘305E40’ and ‘67/3’, which allowed to develop three new HRM markers from the tomato genes Solyc02g032030.1, Solyc02g032200.2 and Solyc02g037540.1 (primers sequence in Supplementary Table 1). These three markers were added to our previously developed genetic map (Barchi et al. 2012), using the software JoinMap v4.0 (van Ooijen 2006) and mapped on E02 (see Fig. 1b). This newly developed map, which includes 418 makers (339 SNPs, 5 HRMs, 3 CAPSs, 11 RFLPs, 33 SSRs and 27 COSII) and spans 1,390 cM., was the basis for QTL analyses.
a Eggplant chromosomes containing the Fom, Vd20 and Vd40 QTL detected. Each line inside the chromosome represents a molecular markers, with map distances (in cM) shown outside each chromosome. The width of the bars represents the confidence interval of the QTL (LODmax−1 interval). b Detailed regions of Fom, Vd20 and Vd40 QTL, with marker information, including the position of the three new HRM markers developed, and genetic distances. The width of the bars represents the confidence interval of the QTL (LODmax−1 interval), while the graphs on right side of each region represent the plot of LOD values for the regions considered, together with the GW thresholds for declaring a QTL to be significant at the 5% genome-wide probability level
QTL analyses for Fom resistance was based on the values of the resistance ratios while Vd resistance data were treated as adjusted line means (best linear unbiased predictors). Several multivariate linear mixed models were tested using a combination of the F-test (for the fixed component) and the Akaike test (for the random component). For both Fom and Vd resistance the best fit model was: pib = rb + gi + e, where pib represents the phenotype of the bth replicate of the ith genotype; rb the fixed effect of the bth replicate; gi the random effect of the ith genotype, and e the residual.
Putative QTL location was determined by both interval (Lander and Botstein 1989) and MQM (Jansen 1993; Jansen and Stam 1994) mapping, as implemented in MapQTL v5 software (Van Ooijen 2004). QTL were initially identified using interval mapping, afterwards one linked marker per putative QTL was treated as a co-factor in the approximate multiple QTL model. Co-factor selection and MQM analysis were repeated until no new QTL could be identified. LOD thresholds for declaring a QTL to be significant at the 5% genome-wide probability level were established empirically by applying 1,000 permutations per trait (Churchill and Doerge 1994). Additive and dominance genetic effects, as well as the percentage of the phenotypic variance (PV) explained by each QTL, were obtained from the final multiple QTL model. Individual QTL were prefixed by a trait abbreviation (Fom or Vd), followed by the chromosome designation and, according to the scoring for Vd inoculation, were suffixed as “20 DAI” or “40 DAI”. Circos reporting the resulting map based on the Krzywinski et al. (2009) software was drawn. Syntenic regions of the tomato genome (sequence build 2.50; http://solgenomics.net/organism/Solanum_lycopersicum/genome) were accessed by blastN to identify candidate genes co-localizing within the Interval Mapping (IM) of eggplant QTL. Initial searches were conducted using 20 kb intervals, then narrowed to 10 kb for intervals of interest. Putative tomato orthologous of the eggplant genes were identified by Blast search in the tomato gene indices at DFCI (http://compbio.dfci.harvard.edu/tgi/).
Results
Phenotypic variation and inter-trait correlations
Trait codes, their correspondent values, broad sense heritability and the number of transgressive individuals in respect to the two parental lines are shown in Table 1. As expected, the line ‘305E40’ which carries the locus Rfo-Sa1, displayed a complete resistance against Fom but also a partial resistance against Vd, as at both 20 and 40 days after Vd inoculation, plantlets showed low or moderate symptoms of infection. Unexpectedly, the ‘67/3’ plantlets survived to Fom inoculation and, although showed a reduced growth and yellowish leaves, displayed a partial resistance which was not described before; on the other hand they exhibited a high sensitivity to Vd at both 20 and 40 DAI.
The F1 plantlets revealed a resistance analogous to the female parent ‘305E40’in respect to Fom, while intermediate between the parental lines in respect to Vd. Based on the assessment of the F2:3 progenies, some F2 individuals resulted highly sensitive to Vd inoculation and transgressive even in respect to the most sensitive parent ‘67/3’, both at 20 and 40 DAI (10 and 8 plants respectively), since their progenies were unable to survive after 30 DAI. As regard resistance to Fom, transgressive segregation in respect to the most sensitive parent ‘67/3’ was detected in 51 F2 individuals.
The broad sense heritability values were high for all the three traits in study, ranging from 0.985 (Vd40) to 1 (Fom) (Table 1). Significant positive inter-trait correlations (p < 0.05) were detected for Vd20/Vd40 (0.287) and Vd20/Fom (0.25) (Table 2).
QTL affecting Fusarium resistance in eggplant
Two QTL related to Fom resistance were mapped in the F2 population (Table 3, Fig. 1). A major QTL (FomE02.01), explaining ~ 68% of the PV and derived from the female parental line ‘305E40’ lies on E2. A further major QTL (FomE011.01), which explains about 11% of the PV, but derives from the male parental line ‘67/3’, was located on E11.
QTL affecting Verticillium resistance in eggplant
A major QTL involved in resistance to Vd at 20 DAI (Vd20E08.01) was mapped on E08 (top segment), which explains ~ 11% of the PV. At 40 DAI, a major QTL, (Vd40E05.01), explaining 20.7% of PV, was located on E05. Furthermore, a minor QTL (Vd40E09.01) explaining 7.3 of the PV mapped on E09. All of the identified QTL derived from the female parental line ‘305E40’ (Table 3 and Fig. 1).
Candidate genes identification based on orthology with tomato
The tomato (build 2.50; http://solgenomics.net/organism/Solanum_lycopersicum/genome) orthologous sequences, syntenic to the eggplant regions underlying the identified QTL, were mined for NLR (also known as NB-LRR or NBS-LRR) and/or any other conserved domain associated to resistance (R) genes, as well by entering the keyword “resistance”, in order to identify candidate genes putatively involved in the control of the traits in study. The identified tomato candidate genes are reported in Table 4.
The QTL FomE02.1 was found to be bounded by markers Solyc02g037540.1 and Solyc02g032200.2, which are located at 0.3 cM from each other. This interval is orthologous to a region of 3Mbp on T2, with the former marker mapping at 31,080,754 and the latter at 28,002,925. Within this interval ± 0.5 Mb (27.5 Mb to 31.5 Mb), 10 genes associated to resistance in tomato were identified. The QTL FomE011.1 was linked to the marker C2_At3g51010, which maps to its orthologous T4 region at 2,426,283 Mb. In a neighbour-hood of 1 Mb, 21 tomato orthologous genes associated to resistance were spotted.
The QTL Vd20E08.1 was found associated to the marker 18202_PstI_L304, which maps at 1,902,945 on T8. At 1 Mb around these coordinates, 5 genes associated to NLR or to other annotated ‘R domain’ were identified. The QTL Vd40E05.1 was bounded by two markers: 10016_PstI_L402 and 12391_PstI_L355, mapping on T12 at 66,653,563 and 66,128,270 respectively. Ten genes annotated as associated to resistance were detected in a 1 Mb around these coordinates. The QTL Vd40E09.1 was associated to the marker 32063_PstI_L393, which maps at 7,0144,642 on T9 and in the 1 Mb around this coordinate, three genes associated to resistance were spotted.
Discussion
The two fungi Fusarium oxysporum f. sp. melongenae (Fom) and Verticillium dahliae (Vd) are among the most serious and widespread diseases in eggplant, also due to the fact can persist in the soil for many years. Currently, there is no robust single control measure by which to manage both pathogens, even because diverse isolates express different levels of virulence (Michielse and Rep 2009; Altinok and Can 2010; Altinok et al. 2013, 2014). Crop rotation, fumigation and fungicide applications can reduce the risk of infection, however they are not highly effective, consistent and are environmentally impactful (Fradin et al. 2009; King et al. 2010). A possible alternative is represented by grafting cultivated eggplant varieties on resistant rootstocks, which has become a common practice mostly in greenhouse cultivation. However, this practice is time consuming, expensive and may influence plant vigor, yield and product quality (Villneuve et al. 2014). An ideal alternative is represented by the development of genetically resistant cultivars; thus, the identification of the QTL affecting resistance and candidate genes playing a key role in the plant response to the infection, combined with the development of molecular markers strictly linked to the resistance trait, can substantially speed up breeding programs aimed at the obtainment of improved resistant varieties.
Verticillium and Fusarium resistance traits have been extensively studied in the Solanaceous crop tomato. With regard to Verticillium, the resistance genes Ve1 and Ve2 were cloned, but only Ve1 was found to provide resistance against race 1 strains of V. dahliae and V. albo-atrum (Diwan et al. 1999; Kawchuk et al. 2001; Fradin et al. 2009). The sequence information of the two genes was also used to amplify candidate Ve orthologs in potato, and markers to track resistance in potato germplasm developed (Bae et al. 2008; Uribe et al. 2014). Ve1 encodes an extracellular leucine-rich repeat (eLRR) receptor-like protein (RLP), that serves as a cell surface receptor for recognition of the secreted Verticillium effector Ave1 (Fradin et al. 2009). Several other Ve-similar genes from different species have been identified in the last years (Bae et al. 2008; Vining and Davis 2009; Hayes et al. 2011; Zhang et al. 2012), suggesting a common role of these genes in resistance against Verticillium wilt. QTL and four resistance genes against Fom were identified in the tomato relatives Solanum pennellii and Solanum pimpinellifolium (Sarfatti et al. 1989, 1991; Bournival et al. 1990; Segal et al. 1992; Ori et al. 1997; Hemming et al. 2004; Lim et al. 2008) and the I2 gene, a member of the tomato I2C multigenic cluster coding for a NBS-LRR protein, was found to confer full resistance to Fusarium oxysporum f.sp. lycopersici race 2 (Simons et al. 1998).
At present no effective resistance gene(s) against Verticillium wilt have been detected in the S. melongena germplasm, although some allied species, such as S. linnaeanum, S. aculeatissimum, S. sisymbrifolium and S. torvum, exhibit different levels of resistance to the pathogen (Daunay 2008). The latter may thus represent a key source for the introgression of the resistance traits into cultivated varieties. Verticillium resistance was transferred from the wild species S. linneanum (syn. S. sodomaeum) to cultivated eggplants; advanced breeding lines carrying partial resistance to Vd from this wild species were also established (Acciarri et al. 2001, 2004), and a gene specific marker for the Ve homolog developed (Liu et al. 2015).
We identified three new QTL involved in the early and late response to Vd inoculation, namely Vd20E08, Vd40E05.1 and Vd40E09, which are located on E8, E5 and E9 respectively. These results demonstrate that presumably different genomic regions are involved in the interaction between Vd and S. melongena during the time-course of their interaction offering the possibility to better steer both the study and the breeding activity to improve the resistance to Verticillium. The QTL analysis revealed larger CI for these QTL when compared to the ones we detected to be involved in resistance to Fom, presumably because of a minor phenotypic variation detected between the parents of our mapping population in respect to the one observed for Fom. Notwithstanding, synteny analyses with tomato revealed that the regions corresponding to all the eggplant QTL for resistance to Vd contain clusters of genes associated to “resistance” or “LRR”, suggesting that also these regions may play an important role in the defence mechanism in tomato, and presumably also in eggplant. However the identified eggplant Vd40E09 QTL, which maps at ~ 7.7 Mb on the tomato chromosome T9, plays only a small role in resistance to Vd and from our synteny analysis it does not correspond to the region containing the tomato Ve1 (reported as major resistance gene) and Ve2 genes, which are located on the top of chromosome T9.
Although the resistant parent of our mapping population did not display a marked resistance against Vd, we identified a major resistance QTL on chromosome E5, which explains about 20% of the PV. The latter represents the first major QTL involved in resistance to Verticillium spp. in eggplant and, although deserving a more detailed characterization, it can be considered a starting point for dissecting the resistance trait against the pathogen.
The first sources of resistance to Fom in eggplant were identified in wild and allied species (Daunay et al. 1991). An example is represented by the resistance trait to Fom introgressed into cultivated eggplant from S. aethiopicum and S. integrifolium through somatic hybridization followed by anther culture of the tetraploid somatic hybrids for obtaining di-haploid plants (Rizza et al. 2002). Advanced introgression lines were then developed through backcrosses with recurrent S. melongena lines, followed by selfing and/or anther culture to obtain pure lines (Rotino et al. 2014). Genes conferring partial resistance to Fom were also detected in Asian landraces (Komochi et al. 1996; Monma et al. 1996, 1997), which were associated with genetic markers and introgressed in European eggplant genotypes (Mutlu et al. 2008). One of these lines (LS1934) was employed to develop “Daitaro” and “Daizaburou”, two resistant eggplant rootstocks (Yoshida et al. 2004).
The female parent (line ‘305E40’) of our mapping population is an introgression line carrying the resistance locus Rfo-sa1 derived from Solanum aethiopicum and is fully resistant to Fom, as it grows vigorously and symptomless after Fusarium inoculation. On the other side, the male parental line ‘67/3’, which was thought to be fully sensitive to the pathogen, unexpectedly displayed a partial resistance to Fom, as when inoculated showed evident symptoms and reduced growth, but survived at 30 DAI and beyond.
We detected a QTL on chromosome E2, namely FomE02.1, which explains about 70% of the phenotypic variation and derived from the resistant parent ‘305E40’. This QTL is located at just 1.2 cM away from the locus Rfo-sa1 and co-maps with resistance locus FM1 at the end of chromosome 2 (Miyatake et al. 2016).
Due to the availability of the eggplant genome sequence (Hirakawa et al. 2014), Miyatake et al. (2016) explored the synteny between eggplant and tomato and identified 25 tomato resistance genes syntenic with the eggplant genomic region of FM1. The latter spans a tomato genomic region of about 29 Mb, suggesting that additional knowledge on eggplant genome is required, also in view of exploiting the resistance genes present in allied species such as S. aethiopicum (Gramazio et al. 2016), S. torvum (Yang et al. 2014) and S. aculeatissimum (Zhou et al. 2016). By comparing the eggplant homologous sequences of these tomato genes in our parental lines, we developed three new molecular markers which were added to our genetic map (Barchi et al., 2012). In this new map, the FusE02.1 QTL shows a much smaller CI in respect to FM1, and corresponds to a tomato syntenic region of approximately 1.8 Mb, which is 16 fold narrower than the one previously identified by Miyatake et al. (2016) and contains 10 genes annotated as ‘NLR’ or ‘resistance’ or both.
The activation of defense mechanisms requires pathogen detection using either cell surface or intracellular receptors. Most disease resistance (R) genes encode for proteins belonging to the nucleotide-binding, leucine-rich repeat protein families, which directly or indirectly recognize pathogen effectors and activate a range of defence responses through different signalling domains at their N termini (TIR-NLRs or CC-NLRs). It is well known that plant genomes contain hundreds of NLR-encoding genes and genes encoding for proteins having a role in diverse signalling pathways, leading to plant defence responses. Indeed, clusters of genes involved in redox and lipid metabolism as well as transcription factors were identified in the FusE02.1 region (data not shown). This result is in accordance with the recent finding that, following F. oxysporum infection, a basal molecular response to pathogens occurs, involving recognition of the pathogen in the cell surface and/or the modulation of genes related to both redox state maintenance and cell wall modification and composition (Barbierato et al. 2016). Presumably, the gene responsible for resistance mediates a rapid response at the site of infection and activates a defensive pathway, which in turn protects the plant from further diffusion of the pathogen (Goggin et al. 2006). This hypothesis is supported by the evidence that the Rfo-Sa1 mediated response activated upon inoculation with Fusarium was able to improve the reaction of eggplant plantlets also against Verticillium attacks (Barbierato et al. 2016).
The resistance trait associated to the QTL FomE02.1 segregated as a single Mendelian dominant trait in our F2 mapping population (χ2 = 1.44 P = 0.05) as well as in crosses between introgression lines holding the resistance locus and fully susceptible eggplant genotypes (Toppino et al. 2008; data not shown), and the same does the locus FM1. This seems to confirm that in both RfoSa1 and FM1 loci only one gene is responsible for the resistance trait against Fusarium. However, it must be stressed that R genes commonly reside in complex clusters making it difficult the dissection of clustered genes conferring resistance (Kawashima et al. 2016). Moreover, as previously reported (Portis et al. 2014), the line ‘305E40’ carries, in the region hosting the FomE02.1 QTL, an introgressed portion of the S. aethiopicum genome, which might represent an additional source of resistance genes compared to the orthologous eggplant region.
We also detected on E11 a new major QTL, namely FomE11.1, which is involved in the Fusarium resistance and explains about 11% of the PV. This resistance trait derives from the male parental line ‘67/3’, which showed partial resistance following Fusarium inoculation. The partial resistance was also detected in several F3 progenies derived from F2 plants that were heterozygous or lacked the Rfo-sa1 locus (see supplementary Table 2). The response mediated by this trait seems to act differently, by avoiding the lethal outcome of the infection but with an evident manifestation of symptoms. A total of 21 genes annotated as ‘NLR’ or ‘resistance’ or both were identified in the FomE11.1 CI; however, none of them corresponds to the tomato I2 gene, which is located at the bottom of T11. Unfortunately we were not able to study the pattern of inheritance of this locus, as its effect was masked in the F1 and the employed population by the dominant allele of the major QTL FusE02.1 derived from ‘305E40’, thus further studies are needed to better elucidate its genetic bases in an ad hoc developed segregant population. The availability of multiple functional polymorphisms associated to a single resistance gene as well as the presence of independent genes conferring resistance to Fom is of great interest for breeding (Fukuoka et al. 2014), thus the deep mining of allelic variation is of substantial importance for the development of superior lines carrying a durable resistance conferred by pyramided traits of resistance (Fukuoka et al. 2015).
References
Acciarri N, Rotino GL, Valentino D, Vitelli G, Sunseri F, Martelli G, Tamietti G (2001) Genetic improvement of eggplant for resistance to Verticillium through interspecific hybridization with Solanum sodomeum L. In Proceedings of the 11th Eucarpia meeting on genetics and breeding of capsicum and eggplant, pp. 298–302
Acciarri N, Rotino GL, Sabatini E, Valentino D, Sunseri F, Mennella G, Tamietti G (2004). Improvement of eggplants for resistance to Verticillium. In: Proceedings of the 12th Eucarpia meeting on genetics and breeding of capsicum and eggplant, p 178
Albert VA, Chang TH (2014) Evolution of a hot genome. Proc Natl Acad Sci USA 111(14):5069–5070. https://doi.org/10.1073/pnas.1402378111
Altinok HH (2005) First report of fusarium wilt of eggplant caused by Fusarium oxysporum f. sp. melongenae in Turkey. Plant Pathol 54:577
Altınok HH, Can C (2010) Characterization of Fusarium oxysporum f. sp. melongenae isolates from eggplant in Turkey by pathogenicity, VCG and RAPD analysis. Phytoparasitica 38(2):149–157
Altinok HH, Can C, Çolak H (2013) Vegetative compatibility, pathogenicity and virulence diversity of Fusarium oxysporum f. sp. melongenae recovered from eggplant. J Phytopathol 1619:651–660
Altinok HH, Can C, Boyaci HF, Topcu V (2014) Genetic variability among breeding lines and cultivars of eggplant against Fusarium oxysporum f. sp. melongenae from Turkey. Phytoparasitica 421:75–84
Bae J, Halterman D, Jansky SH (2008) Development of a molecular marker associated with Verticillium wilt resistance in diploid interspecific potato hybrids. Mol Breed 22:61–69
Barbierato V, Toppino L, Rinaldi P, Sala T, Bassolino L, Valè G, Ferrarini A, Delledonne M, Bagnaresi P, Rotino GL (2016) Phenotype and gene expression analyses of the Rfo-sa1 resistant aubergine interaction with Fusarium oxysporum f. sp. melongenae and Verticillium dahliae. Plant Pathol 5(8):1297–1309. https://doi.org/10.1111/ppa.12518
Barchi L, Lanteri S, Portis E, Stagel A, Valè G, Toppino L, Rotino GL (2010) Segregation distortion and linkage analysis in eggplant (Solanum melongena L.). Genome 53:805–815
Barchi L, Lanteri S, Portis E, Acquadro A, Valè G, Toppino L, Rotino GL (2011) Identification of SNP and SSR markers in eggplant using RAD tag sequencing. BMC Genomics. https://doi.org/10.1186/1471-2164-12-304
Barchi L, Lanteri S, Portis E, Valè G, Volante A, Pulcini L, Ciriaci T, Acciarri N, Barbierato V, Toppino L, Rotino G (2012) A RAD Tag derived marker based eggplant linkage map and the location of QTLs determining anthocyanin pigmentation. PLoS ONE 7:e43740
Bhat RG, Subbarao KV (1999) Host range specificity in Verticillium dahliae. Phytopathology 8912:1218–1225
Bournival BL, Vallejos CE, Scott JW (1990) Genetic analysis of resistances to races 1 and 2 of Fusarium oxysporum f. sp. lycopersici from the wild tomato Lycopersicon pennellii. Theor Appl Genet 79(5):641–645
Boyaci F, Unlu A, Abak K (2010) Screening for resistance to Fusarium wilt of some cultivated eggplants and wild Solanum accessions. In XXVIII International Horticultural Congress on Science and Horticulture for People IHC2010: International Symposium on New, pp 23–27
Boyaci HF, Unlu A, and Abak K (2011) Genetic analysis of resistance to wilt caused by Fusarium (Fusarium oxysporum melongenae) in eggplant (Solanum melongena). The Indian Journal of Agricultural Sciences, p 819
Cappelli C, Stravato VM, Rotino GL, Buonaurio R (1995) Sources of resistance among Solanum spp., to an Italian isolate of Fusarium oxysporum f. sp. melongenae, In: Andràsfalvi A, Moòr A, Zatykò (eds) EUCARPIA, 9th Meeting on Genet Breed Capsicum Eggplant, SINCOP, Budapest, pp 221–224
Cericola F, Portis E, Toppino L, Barchi L, Acciarri N, Ciriaci T, Sala T, Rotino GL, Lanteri S (2013) The population structure and diversity of eggplant from Asia and the Mediterranean Basin. PLoS ONE 8:e73702
Cericola F, Portis E, Lanteri S, Toppino L, Barchi L, Acciarri N, Pulcini L, Sala T, Rotino GL (2014) Linkage disequilibrium and genome-wide association analysis for anthocyanin pigmentation and fruit color in eggplant. BMC Genomics. https://doi.org/10.1186/1471-2164-15-896
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Daunay (2008). Eggplant. Prohens J, Nuez F, editors. Handbook of Plant Breeding—Vegetables II. Springer, New York, pp 163–220
Daunay MC (2012) Eggplant. In: Peter KV, Hazra P (eds) Handbook of vegetables. Studium Press, Houston, pp 257–322
Daunay MC, Lester RN, Laterrot H (1991). The use of wild species for the genetic improvement of Brinjal eggplant Solanum melongena and tomato Lycopersicon esculentum, In: Hawkes JC, Lester RN, Nee M, Estrada N (Eds). Solanaceae III: Taxonomy, Chemistry, Evolution, Vol 27. Royal Botanic Gardens Kew and Linnean Soc London, pp 389–413
Diwan N, Fluhr R, Eshed Y, Zamir D, Tanksley SD (1999) Mapping of Ve in tomato: a gene conferring resistance to the broad-spectrum pathogen, Verticillium dahliae race 1. Theor Appl Genet 982:315–319
Doglanar S, Frary A, Daunay MC, Lester RN, Tanksley SD (2002a) A comparative genetic linkage map of eggplant (Solanum melongena) and its implication for genome evolution in the Solanacee. Genetics 161:1697–1711
Doglanar S, Frary A, Daunay MC, Lester RN, Tanksley SD (2002b) Conservation of gene function in the solanaceae as revealed by comparative mapping of domestication trait in eggplant. Genetics 161:1713–1726
Fei J, Chai Y, Wang J, Lin J, Sun X, Sun C, Zuo K, Tang K (2004) cDNA cloning and characterization of the Ve homologue gene StVe from Solanum torvum Swartz. Mitochondrial DNA 15(2):88–95
Fowler C, Moore GK, Hawtin G (2003) The international treaty on plant genetic resources for food and agriculture. Bioversity International, Rome
Fradin EF, Zhang Z, Ayala JCJ, Castroverde CD, Nazar RN, Robb J, Liu CM, Thomma BP (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150(1):320–332
Frary A, Doganlar S, Daunay MC, Tanksley SD (2003) QTL analysis of morphological traits in eggplant and implications for conservation of gene function during evolution of solanaceous species. Theor Appl Genet 107:359–370
Frary A, Frary A, Daunay MC, Huvenaars K, Mank R, Doğanlar S (2014) QTL hotspots in eggplant (Solanum melongena) detected with a high resolution map and CIM analysis. Euphytica 197(2):211–228. https://doi.org/10.1007/s10681-013-1060-6
Fukuoka H, Yamaguchi H, Nunome T, Negoro S, Miyatake K, Ohyama A (2010) Accumulation, functional annotation, and comparative analysis of expressed sequence tags in eggplant (Solanum melongena L.), the third pole of the genus Solanum species after tomato and potato. Gene 450:76–84. https://doi.org/10.1016/j.gene.2009.10.006
Fukuoka H, Miyatake K, Nunome T, Negoro S, Shirasawa K, Isobe S, Asamizu E, Yamaguchi H, Ohyama A (2012) Development of gene-based markers and construction of an integrated linkage map in eggplant by using Solanum orthologous (SOL) gene sets. Theor Appl Genet 125(1):47–56. https://doi.org/10.1007/s00122-012-1815-9
Fukuoka S, Yamamoto SI, Mizobuchi R, Yamanouchi U, Ono K, Kitazawa N, Yasuda N, Fujiita Y, Thanh Nguyen TT, Koizumi S, Sugimoto K, Matsumoto T, Yano M (2014) Multiple functional polymorphisms in a single disease resistance gene in rice enhance durable resistance to blast. Scientific Reports 4, (article number 4550) https://doi.org/10.1038/srep04550
Fukuoka S, Saka N, Mizukami Y, Koga H, Yamanouchi U, Yoshioka Y, Hayashi N, Ebana K, Mizobuchi R, Yano M (2015) Gene pyramiding enhances durable blast disease resistance in rice. Sci Rep 5:7773
Goggin FL, Jia L, Shah G, Hebert S, Williamson VM, Ullman DE (2006) Heterologous expression of the Mi-1.2 gene from tomato confers resistance against nematodes but not aphids in eggplant. Mol Plant Microbe Interact 19(4):383–388
Gramazio P, Prohens J, Plazas M, Andújar I, Herraiz FJ, Castillo E, Knapp S, Meyer RS, Vilanova S (2014) Location of chlorogenic acid biosynthesis pathway and polyphenol oxidase genes in a new interspecific anchored linkage map of eggplant. BMC Plant Biol 14(1):1
Gramazio P, Blanca J, Ziarsolo P, Herraiz FJ, Plazas M, Prohens J, Vilanova S (2016) Transcriptome analysis and molecular marker discovery in Solanum incanum and S. aethiopicum, two close relatives of the common eggplant (Solanum melongena) with interest for breeding. BMC Genomics 17(1):1
Hayes RJ, McHale LK, Vallad GE, Truco MJ, Michelmore RW, Klosterman SJ, Maruthachalam K, Subbarao KV (2011) The inheritance of resistance to Verticillium wilt caused by race 1 isolates of Verticillium dahliae in the lettuce cultivar La Brillante. Theor Appl Genet 123:509–517
Hemming MN, Basuki S, McGrath DJ, Carroll BJ, Jones DA (2004) Fine mapping of the tomato I-3 gene for fusarium wilt resistance and elimination of a co-segregating resistance gene analogue as a candidate for I-3. Theor Appl Genet 109:409–418
Hirakawa H, Shirasawa K, Miyatake K, Nunome T, Negoro S, Ohyama A, Yamaguchi H, Sato S, Isobe S, Tabata S, Fukuoka H (2014) Draft genome sequence of eggplant (Solanum melongena L): the representative solanum species indigenous to the old world. DNA Res 216:649–660
Jansen R (1993) Interval mapping of multiple quantitative trait loci. Genetics 135:205–211
Jansen R, Stam P (1994) High-resolution of quantitative traits into multiple loci via interval mapping. Genetics 136:1447–1455
Karagiannidis N, Bletsos F, Stavropoulos N (2002) Effect of Verticillium wilt (Verticillium dahliae Kleb.) and mycorrhiza (Glomus mosseae) on root colonization, growth and nutrient uptake in tomato and eggplant seedlings. Sci Hort. 94:145–156
Kaushik P, Prohens J, Vilanova S, Gramazio P, Plazas M (2016) Phenotyping of eggplant wild relatives and interspecific hybrids with conventional and phenomics descriptors provides insight for their potential utilization in breeding. Front Plant Sci 7:677
Kawashima CG, Guimarães GA, Nogueira SR, MacLean D, Cook DR, Steuernagel B, Baek J, Bouyioukos C, do VA Melo B, Tristão G, de Oliveira JC, Rauscher G, Mittal S, Panichelli L, Bacot K, Johnson J, Iyer G, Tabor G, Wulff BBH, Ward E, Rairdan GJ, Broglie KE, Wu G, van Esse1 HP, Jones JDJ, Brommonschenkel SH (2016). A pigeonpea gene confers resistance to Asian soybean rust in soybean. Nat Biotechnol 34:661–665
Kawchuk LM, Hachey J, Lynch DR, Kulcsar F, Van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, Fischer R, Prüfer D (2001) Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci 98(11):6511–6515
King SR, Davis AR, Zhang X, Crosby K (2010) Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Sci Hortic 127(2):106–111
Knapp S, Vorontsova MS, Prohens J (2013) Wild relatives of the eggplant (Solanum melongena L.: Solanaceae): new understanding of species names in a complex group. PLoS ONE. https://doi.org/10.1371/journal.pone.0057039
Komochi S, Monma S, Narikawa T, Sakata Y (1996) Evaluation of resistance to bacterial wilt and verticillium wilt in eggplants (Solanum melongena L.) collected in Malaysia. J Jpn Soc Hortic Sci 65:81–88
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19(9):1639–1645
Lander E, Botstein D (1989) Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Lester RN and Hasan SMZ (1991). Origin and domestication of the brinjal eggplant, Solanum melongena, from S. incanum, in Africa and Asia. In Hawkes JG, Lester RN, Nee M, Estrada N eds. Solanaceae III: taxonomy, chemistry, evolution. R Bot Gard: Kew & Linnean Soc., London, pp 369–387
Lim GT, Wang GP, Hemming MN, McGrath DJ, Jones DA (2008) High resolution genetic and physical mapping of the I-3 region of tomato chromosome 7 reveals almost continuous microsynteny with grape chromosome 12 but interspersed microsynteny with duplications on Arabidopsis chromosomes 1, 2 and 3. Theor Appl Genet 118:57–75
Liu J, Zheng Z, Zhou X, Feng C, Zhuang Y (2015) Improving the resistance of eggplant Solanum melongena to Verticillium wilt using wild species Solanum linnaeanum. Euphytica 2013:463–469
McKeen CD (1972) Observations on the invasion and subsequent development of Verticillium albo-atrum and Verticillium dahliae in eggplant, tomato and potato. Proc Can Phytopathol Soc 39:21
Meyer RS, Karol KG, Little DP, Nee MH, Litt A (2012) Phylogeographic relationships among Asian eggplants and new perspectives on eggplant domestication. Mol Phylogenet Evol 63:685–701
Michielse CB, Rep M (2009) Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol 103:311–324
Miyatake K, Saito T, Negoro S, Yamaguchi H, Nunome T, Ohyama A, Fukuoka H (2012) Development of selective markers linked to a major QTL for parthenocarpy in eggplant Solanum melongena L. Theor Appl Genet 124:1403–1413
Miyatake K, Saito T, Negoro S, Yamaguchi H, Nunome T, Ohyama A, Fukuoka H (2016) Detailed mapping of a resistance locus against Fusarium wilt in cultivated eggplant Solanum melongena. Theor Appl Genet 129(2):357–367. https://doi.org/10.1007/s00122-015-2632-8
Monma S, Sato T, Matsunaga H (1996) Evaluation of resistance to bacterial Fusarium and Verticillium wilt in eggplant and eggplant-related species collected in Ghana. Capsicum Eggplant Nwsl 15:71–72
Monma S, Akazawa S, Simosaka K, Sakata Y, Matsunaga H (1997) ‘Daitaro’, a bacterial wilt- and Fusarium wilt-resistant hybrid eggplant for rootstock. Bull Natl Inst Veg Ornam Plants Tea 12:73–83 (in Japanese with English summary)
Mutlu N, Boyaci FH, Göçmen M, Abak K (2008) Development of SRAP, SRAP-RGA, RAPD and SCAR markers linked with a Fusarium wilt resistance gene in eggplant. Theor Appl Genet 117:1303–1312
Nunome T, Ishiguro K, Yoshida T, Hirai M (2001) Mapping of fruit shape and color development traits in eggplant (Solanum melongena L.) based on RAPD and AFLP markers. Breed Sci 51:19–26
Nunome T, Suwabe K, Iketani H, Hirai M (2003) Identification and characterization of microsatellites in eggplant. Plant Breed 122:256–262
Nunome T, Negoro S, Kono I, Kanamori H, Miyatake K, Yamaguchi H, Ohyama A, Fukuoka H (2009) Development of SSR markers derived from SSR-enriched genomic library of eggplant (Solanum melongena L.). Theor Appl Genet 119:1143–1153
Ori N, Eshed Y, Paran I, Presting G, Aviv D, Tanksley S, Zamir D, Fluhr R (1997) The I2C family from the wilt disease resistance locus I2 belongs to the nucleotide binding, leucine-rich repeat superfamily of plant resistance genes. Plant Cell 9:521–532
Plazas M, Vilanova S, Gramazio P, Rodríguez-Burruezo A, Fita A, Herraiz FJ, Ranil R, Fonseka R, Niran L, Fonseka H, Kouassi B, Kouassi A, Kouassi A, Prohens J (2016) Interspecific hybridization between eggplant and wild relatives from different genepools. J Am Soc Hort Sci 141(1):34–44
Portis E, Barchi L, Toppino L, Lanteri S, Acciarri N, Felicioni N, Fusari F, Barbierato V, Cericola F, Valè GP, Rotino GL (2014) QTL mapping in eggplant reveals clusters of yield-related loci and orthology with the tomato genome. PLoS ONE 9:e89499
Portis E, Cericola F, Barchi L, Toppino L, Acciarri N, Pulcini L, Sala T, Lanteri S, Rotino GL (2015) Association mapping for fruit, plant and leaf morphology traits in eggplant. PLoS ONE 108:e0135200
Rizza F, Mennella G, Collonnier C, Shiachakr D, Kashyap V, Rajam MV, Prestera M, Rotino GL (2002) Androgenic dihaploids from somatic hybrids between Solanum melongena and S. aethiopicum group gilo as a source of resistance to Fusarium oxysporum f. sp. melongenae. Plant Cell Rep 2011:1022–1032
Rotino GL, Sihachakr D, Rizza F, Vale’ GP, Tacconi MG, Alberti P, Mennella G, Sabatini E, Toppino L, D’alessandro A, Acciarri N (2005) Current status in production and utilization of dihaploids from somatic hybrids between eggplant (Solanum melongena L), and its wild relatives. Acta Physiol Plant 274B:723–733
Rotino GL, Sala T, Toppino L (2014). Eggplant. Book chapter 16. In Pratap A, Kumar J (eds) Alien gene transfer in crop plants, vol 2, 381, Achievements and impacts. Springer, New York. https://doi.org/10.1007/978-1-4614-9572-7_16
Sarfatti M, Katan J, Fluhr R, Zamir D (1989) An RFLP marker in tomato linked to the Fusarium oxysporum resistance gene I2. Theor Appl Genet 78:755–759
Sarfatti M, Abu-Abied M, Katan J, Zamir D (1991) RFLP mapping of I1, a new locus in tomato conferring resistance against Fusarium oxysporum f. sp. lycopersici race 1. Theor Appl Genet 82:22–26
Segal G, Sarfatti M, Schaffer MA, Ori N, Zamir D, Fluhr R (1992) Correlation of genetic and physical structure in the region surrounding the I2 Fusarium oxysporum resistance locus in tomato. Mol Gen Genet 231:179–185
Sihachakr D, Daunay MC, Serraf L, Chaput MH, Mussio I, Haicour R, Rossignol L, Ducreux G (1994). Somatic hybridization of eggplant (Solanum melongena L), with its close and wilt relatives. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, somatic hybridization in crop improvement. Springer, Berlin
Simons G, Groenendijk J, Wijbrandi J, Reijans M, Groenen J, Diergaarde P, Van der Lee T, Bleeker M, Onstenk J, de Both M, Haring M, Mes J, Cornelissen B, Zabeau M, Vos P (1998) Dissection of the fusarium I2 gene cluster in tomato reveals six homologs and one active gene copy. Plant Cell 10:1055–1068
Stravato VM, Cappelli C, Polverari A (1993) Attacchi di Fusarium oxysporum f, sp, melongenae agente della tracheofusariosi della melanzana in Italia centrale. Inf Fitopatol 4310:51–54
Sunseri F, Sciancalepore A, Martelli G, Rotino GL, Acciarri N, Valentino D, Tamietti G (2003) Development of RAPD-AFLP map of eggplant and improvement of tolerance to Verticillium Wilt. Acta Hortic 625:107–115
Syfert M, Castañeda-Álvarez NP, Khoury CK, Särkinen T, Sosa CC, Achicanoy HA, Bernau V, Prohens J, Daunay MC, Knapp S (2016) Crop wild relatives of the brinjal eggplant (Solanum melongena): poorly represented in genebanks and many species at risk of extinction. Am J Bot 103:635-651
Team R (2009) R: a language and environment for statistical computing
Toppino L, Valè GP, Rotino GL (2008) Inheritance of Fusarium wilt resistance introgressed from Solanum aethiopicum Gilo and Aculeatum groups into cultivated eggplant S. melongena and development of associated PCR-based markers. Mol Breed 222:237–250. https://doi.org/10.1007/s11032-008-9170-x
Toppino L, Barchi L, Lo Scalzo R, Palazzolo E, Francese G, Fibiani M, D’Alessandro A, Papa V, Laudicina VA, Sabatino L, Pulcini L, Sala T, Acciarri N, Portis E, Lanteri S, Mennella G, Rotino GL (2016). Mapping Quantitative Trait Loci Affecting Biochemical and Morphological Fruit Properties in Eggplant (Solanum melongena L.) Front plant sci 7 (2016)
Uribe P, Jansky S, Halterman D (2014) Two CAPS markers predict Verticillium wilt resistance in wild Solanum species. Mol Breed 33(2):465–476
Urrutia Herrada MT, Gomez Garcia VM, Tello Marquina J (2004) Fusarium wilt on eggplant in Almeria (Spain). Boletin de Sanidad Vegetal, Plagas 30:85–92
Van Ooijen JW (2004) MapQTL 5, software for the mapping of quantitative trait loci in experimental populations
van Ooijen J (2006) JoinMap H 4, Software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen, Netherlands
Villeneuve F, Latour F, Théry T, Steinberg C, Edel-Hermann V, Pitrat M, Daunay MC (2014) The control of soil borne vascular diseases: limits of genetic resistance of cultivars and rootstocks for controlling Fusarium oxysporum f, sp, melonis (melon) and Verticillium sp, (eggplant). Acta Hortic 1044:57–65
Vining K, Davis T (2009) Isolation of a Ve homolog, mVe1, and its relationship to verticillium wilt resistance in Mentha longifolia (L.) Huds. Mol Genet Genomics 282:173–184
Wu F, Eannetta N, Xu Y, Tanksley S (2009) A detailed synteny map of the eggplant genome based on conserved ortholog set II (COSII) markers. Theor Appl Genet 118:927–935
Yang L, Jue De, Li W, Zhang R, Chen M, Yang Q (2013) Identification of MiRNA from eggplant (Solanum melongena L), by small RNA deep sequencing and their response to Verticillium dahliae infection, PloS ONE vol, 8 8 p, e72840
Yang X, Cheng YF, Deng C, Ma Y, Wang ZW, Chen XH, Xue LB (2014) Comparative transcriptome analysis of eggplant (Solanum melongena L.) and turkey berry (Solanum torvum Sw.): phylogenomics and disease resistance analysis. BMC Genomics 15(1):412
Yoshida T, Monma S, Matsunaga H, Sakata Y, Sato T, Saito T (2004) Development of a new rootstock eggplant cultivar ‘Daizaburou’ with high resistance to bacterial wilt and Fusarium wilt. Bull Natl Inst Veg Tea Sci 3:199–211 (in Japanese with English summary)
Zhang B, Yang Y, Chen T, Yu W, Liu T, Li H, Fan X, Ren Y, Shen D, Liu L, Dou D, Chang Y (2012) Island cotton Gbve1 gene encoding a receptor-like protein confers resistance to both defoliating and non-defoliating isolates of Verticillium dahliae. PLoS ONE 7(12):e51091
Zhou X, Bao S, Liu J, Zhuang Y (2016) De novo sequencing and analysis of the transcriptome of the wild eggplant species Solanum Aculeatissimum in response to Verticillium dahliae. Plant Mol Biol Rep. https://doi.org/10.1007/s11105-016-0998-7
Acknowledgements
This work has been funded in part by European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No 677379 (G2P-SOL project: Linking genetic resources, genomes and phenotypes of Solanaceous crops).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barchi, L., Toppino, L., Valentino, D. et al. QTL analysis reveals new eggplant loci involved in resistance to fungal wilts. Euphytica 214, 20 (2018). https://doi.org/10.1007/s10681-017-2102-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-017-2102-2