Abstract
Verticillium wilt of lettuce caused by Verticillium dahliae can cause severe economic damage to lettuce producers. Complete resistance to race 1 isolates is available in Lactuca sativa cultivar (cv.) La Brillante and understanding the genetic basis of this resistance will aid development of new resistant cultivars. F1 and F2 families from crosses between La Brillante and three iceberg cultivars as well as a recombinant inbred line population derived from L. sativa cv. Salinas 88 × La Brillante were evaluated for disease incidence and disease severity in replicated greenhouse and field experiments. One hundred and six molecular markers were used to generate a genetic map from Salinas 88 × La Brillante and for detection of quantitative trait loci. Segregation was consistent with a single dominant gene of major effect which we are naming Verticillium resistance 1 (Vr1). The gene described large portions of the phenotypic variance (R 2 = 0.49–0.68) and was mapped to linkage group 9 coincident with an expressed sequence tag marker (QGD8I16.yg.ab1) that has sequence similarity with the Ve gene that confers resistance to V. dahliae race 1 in tomato. The simple inheritance of resistance indicates that breeding procedures designed for single genes will be applicable for developing resistant cultivars. QGD8I16.yg.ab1 is a good candidate for functional analysis and development of markers suitable for marker-assisted selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Verticillium wilt caused by the soil borne fungus Verticillium dahliae Kleb. is a serious disease affecting a broad range of economically important crops (Pegg and Brady 2002). Lettuce (Lactuca sativa L.) was not considered a host until the mid-1990s, when it was discovered in coastal lettuce production districts of California (Subbarao et al. 1997). The disease has subsequently spread within coastal California (Atallah et al. 2010) and has also been described in the Mediterranean basin (Garibaldi et al. 2007; Ligoxigakis et al. 2002). Verticillium dahliae is seed transmitted in lettuce and other vegetable crops grown in rotation with lettuce, raising concerns regarding its spread to other lettuce production areas (Atallah et al. 2010; Vallad et al. 2005). While all types of lettuce are susceptible, Verticillium wilt is most damaging on iceberg type cultivars. Plants often remain symptomless until they near harvest maturity, when the symptoms develop over a short period of time. Basal or “wrapper leaves” that completely cover the outer part of the head wilt in infected susceptible plants and then collapse as the disease progresses, leading to early plant death and an unharvestable head.
Host resistance is the best long term control method in lettuce, as current cultural control methods are cost prohibitive, potentially damaging to the environment, or of limited feasibility (Subbarao et al. 1997). Within V. dahliae isolates from lettuce, two pathogenic races (race 1 and race 2) are known. The L. sativa Batavian type cultivar La Brillante and several other heirloom cultivars are resistant to race 1 isolates, while no known sources of resistance against race 2 isolates have been reported (Hayes et al. 2007; Vallad et al. 2006). This is similar to what has been described in the tomato-Verticillium pathosystem (Alexander 1962; Vallad et al. 2006) and the pathogenicity of isolates (race 1 or race 2) from lettuce and tomato is strongly correlated (Maruthachalam et al. 2010). Resistance to race 1 in La Brillante is complete (no symptom development) (Vallad and Subbarao 2008) and is currently effective in grower fields (Hayes et al. 2007). Consequently, breeding race 1 resistant iceberg cultivars has emphasized using La Brillante as a parent. Current breeding efforts use the pedigree method by selecting for asymptomatic and commercially acceptable iceberg type plants, families, or lines in infested field experiments. While this approach has resulted in resistant iceberg breeding lines (Hayes et al. 2006), it is fraught with several inefficiencies including disease escapes, non-uniform disease pressure, ambiguous diseases symptoms, and a long breeding cycle time. While improved evaluation methods have been developed (Hayes et al. 2007; Vallad et al. 2006), nothing was previously known regarding the genetics of resistance in La Brillante. Such knowledge will greatly aid the development of more efficient breeding schemes.
In other crop species, the genetics of resistance to Verticillium wilt has been described as either qualitative or quantitative (Pegg and Brady 2002; Fradin and Thomma 2006). Dominant resistance genes have been reported in tomato (Schaible et al. 1951), cotton (Mert et al. 2005), sunflower (Putt 1964), potato (Jansky et al. 2004) and wild relatives of potato (Lynch et al. 1997). In tomato, Ve1 confers resistance to race 1 isolates and it is inseparably linked and has sequence similarity to Ve2 (Diwan et al. 1999; Fradin et al. 2009; Kawchuk et al. 2001; Schaible et al. 1951). Both genes are found at the Ve locus in tomato and encode proteins that belong to the extracellular Leucine-rich repeat (eLRR) receptor-like protein (RLP) class of disease resistance proteins (Fradin et al. 2009; Kawchuk et al. 2001). Based on assumed conservation of gene function within Solanaceae, Ve orthologs were used to develop molecular markers linked to Verticillium wilt resistance genes in potato (Bae et al. 2008; Simko et al. 2004a, b). Similar work has not been done in other plant families such as Compositae, since less is known regarding the genes conferring resistance in this and other plant families. In this paper, we describe a single dominant gene for race 1 resistance in La Brillante, and position the resistance gene on a genetic map coincident with a Ve homolog from lettuce.
Materials and methods
Population development
Lettuce (Lactuca sativa L.) is a diploid (2n = 2x = 18) autogamous species and cultivars are highly homozygous and homogenous. All F1 seed was produced using the method of Ryder and Johnson (1974) and all F2 and later generations were produced through self-pollination. Seed from each plant was kept separate, unless otherwise noted. The parents used in crossing were the L. sativa cultivars Salinas 88, Tiber, La Brillante, and a line of the cultivar Salinas carrying the Male sterile-7 gene (Ryder 1971), here referred to as Ms7-Salinas. Ms7-Salinas has had greater than seven backcrosses to Salinas to incorporate the Ms7 gene and was morphologically identical to Salinas in all aspects except male fertility. Salinas 88 was developed from backcrossing the mo1 2 allele for Lettuce mosaic virus resistance into the cultivar Salinas. Salinas, Salinas 88, Ms7-Salinas, and Tiber are iceberg type cultivars adapted to California production conditions and are susceptible to race 1 isolates of V. dahliae. A recombinant inbred line (RIL) population from the cross Salinas 88 × La Brillante were inbred up to the F7 generation using single seed descent (Fehr 1991). F8 seed lots of each RIL were produced from massing seed from approximately 20 field grown F7 plants.
Inoculation and disease evaluation
To inoculate plants with V. dahliae, seeds were sown in 200-well plug trays, incubated at 10°C in the dark for 48 h in a growth chamber and subsequently germinated and grown at 20°C with a 16-h photoperiod. Seedlings were inoculated at 2, 3 and 4 weeks after sowing by saturating the soil in each plug tray well with a 3 ml suspension containing 2 × 106 conidia/ml of V. dahliae in sterile, distilled water. Seedlings were incubated for another 1–2 weeks after the third inoculation before transplanting. The race 1 V. dahliae isolate VdLs16 was used in all experiments and was maintained and prepared according to Vallad et al. (2006). For greenhouse experiments, seedlings were transplanted into 0.5 L foam-insulated cups filled with a pasteurized sand:potting soil mixture (3:1 v/v). The plants were watered as needed and no supplemental lighting was supplied. Unless otherwise stated, greenhouse temperature was controlled with radiant heat when temperatures were below 18°C and with an exhaust fan with evaporative cooling when temperatures were above 24°C. Plants were maintained in the greenhouse for approximately 8–10 weeks after transplanting at which time foliar symptoms were evident on the susceptible cultivars. Each plant was then evaluated for disease severity (DS) on a 0–5 scale where 0 = no vascular discoloration, 1 = 1–25% of the vascular tissue exhibiting discoloration, 2 = 26–50%; 3 = 51–75%, and 4 = 76–100% discoloration in the absence of foliar symptoms, and 5 = 100% discoloration and the presence of foliar symptoms typical of Verticillium wilt. Disease incidence (DI) was calculated as the proportion of plants with DS ≥ 1.
Greenhouse evaluation of F1 and F2 families
Verticillium wilt DI was evaluated in F1 La Brillante × Salinas 88, F1 Ms7-Salinas × La Brillante, F2 Salinas 88 × La Brillante, and F2 Tiber × La Brillante in an April 16, 2006 transplanted greenhouse experiment. These same populations were evaluated again in a November 15, 2006 transplanted experiments with the exception of F2 Tiber × La Brillante. Parents were included in both experiments. Plants of each family or parent treatment were distributed among three replications as a randomized complete block design. In all experiments, a non-replicated block of non-inoculated plants of each parent was included as the negative control.
Greenhouse evaluation of Salinas 88 × La Brillante RILs
Two greenhouse experiments were conducted with 95 F5 and F6 RILs from Salinas 88 × La Brillante. Greenhouse production, inoculation, and evaluation methods were the same as those used for F1 and F2 families except for the following difference. Greenhouse experiment 1 (GH1) using F5 RILs was conducted with four replications of six plants per replication of each RIL and arranged as a randomized complete block design. The seedlings for GH1 were transplanted on December 1, 2006, and the greenhouse temperature for GH1 was maintained between 20 and 25°C. Greenhouse experiment 2 (GH2) used F6 RILs and was conducted as a RCBD with five replications of six plants per replication of each RIL. The seedlings for GH2 were transplanted on March 16, 2006. In GH2, an additional inoculation was conducted by injecting plant crowns with approximately 100 μl of a 2 × 106 conidia/ml suspension of V. dahliae at market maturity. This method was employed as an attempt to further reduce the number of disease escapes. Previous pilot studies indicated that stem injection, without root inoculation, results in normal root and foliar symptoms in susceptible cultivars (data not shown).
Field evaluation of Salinas 88 × La Brillante RILs
A field site in Salinas, CA was artificially infested by transplanting race 1 V. dahliae isolate VdLs16 inoculated 4-week-old seedlings of Salinas to the field in 2006 and 2007 and then growing the crops to maturity. The method of seedling inoculation was identical to the procedures used for greenhouse experiments. A susceptible iceberg cultivar was subsequently direct seeded and grown to market maturity in 2008 to further propagate the pathogen. The crop residue was incorporated and mixed into the soil after all three crops.
Ninety-one F8 Salinas 88 × La Brillante RILs were direct seeded into the V. dahliae infested field site on July 15, 2009 as a randomized complete block design with three replications. Each RIL was planted to a single plot per replication that was 6 m long and consisted of a single seed line on a 1 m wide double seed line bed standard for lettuce production in coastal California. Plant spacing was approximately 28 cm between seed lines and 28 cm between plants within a seed line. All trials were maintained using standard cultural practices for coastal California lettuce production (Ryder 1999). Five plants per plot were evaluated for DS and DI on October 1, 2009 on the same 0–5 scale used in greenhouse experiments. This evaluation date was past market maturity; this strategy was chosen to reduce the occurrence of infected susceptible plants that were still non-symptomatic due to delayed maturity (Hayes et al. 2007). Root tissue was sampled and placed on a NP10 semi-selective medium as needed to confirm the presence or absence of the pathogen (Kabir et al. 2004).
Analysis of greenhouse and field data
The total number of plants and the number of symptomatic plants within F1 and F2 families was pooled across replications, tabulated, and tested against potential segregation ratios using chi-square. To analyze segregation within the RIL population, any RIL with at least one symptomatic plant was classified as susceptible and those with zero symptomatic plants were classified as resistant. Observed ratios were compared to an expected ratio of 1 resistant:1 susceptible (single gene segregating) using chi-square.
Molecular marker genotyping and QTL mapping
Molecular marker genotyping and genetic map construction was completed using 95 F6 plants from the Salinas 88 × La Brillante RIL population. DNA was extracted using the Qiagen DNeasy® Plant Mini Kit (Qiagen Inc., Valencia, CA) following the manufacturer’s instructions. Each RIL was genotyped using the Illumina Golden Gate® SNP assay using 384 SNPs previously identified between the cultivar Salinas and L. serriola accession UC96US23 or between Salinas and the romaine cultivar Valmaine (McHale et al. 2009; http://compgenomics.ucdavis.edu/compositae_SNP.php; Table S1). One hundred and four markers were polymorphic in the Salinas 88 × La Brillante population and used to construct a genetic map consisting of 16 linkage groups and four unlinked markers using JoinMap v 4.0 with default settings and Kosambi mapping function (Stam and Van Ooijen 1995). Chromosome numbers were assigned to linkage groups based on alignment to the Salinas × UC96US23 genetic map (McHale et al. 2009; http://chiplett.ucdavis.edu). QTL analysis was conducted using Win QTL Cartographer v 2.5 using Composite Interval Mapping. Significance thresholds were generated for each trait using permutation analysis based on 1,000 permutations. Where QTL mapped to regions with low marker density, PCR based markers were designed to unigenes which map within these genetic regions and were also shown to be polymorphic between iceberg and Batavia type cultivars based on hybridization data (http://chiplett.ucdavis.edu; Table S2). To assess polymorphism, PCR products amplified from Salinas 88 and La Brillante were assayed by electrophoresis on agarose gels and single stranded conformational polymorphism analysis as described in McHale et al. 2009. Polymorphic markers were assayed on the RIL population. Genetic map construction and QTL analysis was repeated with these seven additional markers as described above.
To identify homologs of the tomato Ve gene in lettuce, the full length protein sequence of Ve1 (GenBank accession AAK58681) from Solanum lycopersicum cultivar Craigella (Fradin et al. 2009; Kawchuk et al. 2001) was queried against the Compositae Genome Project Database (CGPDB; http://cgpdb.ucdavis.edu/database/) of lettuce ESTs with tBLASTn (Altschul et al. 1997).
Results
Segregation of resistance in F1, F2, and RILs
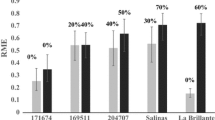
Greenhouse experiments produced high disease incidence in the susceptible lines Salinas 88, Tiber, and Ms7-Salinas (Table 1). No disease was observed in La Brillante and only a single symptomatic plant was found in the non-inoculated treatments (Ms7-Salinas in Experiment 2); plating the stem section on NP10 media determined that this plant was infected with V. dahliae, which likely resulted from contamination. In F1 La Brillante × Salinas 88, no symptomatic plants were found in either experiment, while 17 and 0.4% symptomatic plants were found in Ms7-Salinas × La Brillante in experiments 1 and 2, respectively. Among Salinas 88 × La Brillante F2 plants, 29% of plants were symptomatic in experiment one and 18% were symptomatic in experiment two. The data were consistent with a 3:1 segregation ratio for a single dominant resistance gene in both experiments (Table 1). F2 progeny from Tiber × La Brillante resulted in 33% symptomatic plants, which was not consistent with a 3:1 ratio (P = 0.002) (Table 1). However, the fit to a 9:7 (2 dominant genes, both needed for resistance) was less likely (P = 0.0002, data not shown).
Evaluation of Salinas 88 × La Brillante RILs in greenhouse and field experiments resulted in skewed or bimodal distributions for DI and DS data (Fig. 1). In all three experiments, La Brillante had no symptomatic plants, while the amount of disease in Salinas 88 varied between experiments (GH1: DI = 48%; DS = 2.4; GH2: DI = 90%; DS = 4.5; field experiment: DI = 36%; DS = 1.8). Using the criteria that susceptible RILs are those with at least one symptomatic plant while resistant RIL have no symptomatic plants, an acceptable fit to a 1:1 ratio for single gene segregation was found in GH1 (42 resistant:52 susceptible, P = 0.36). The segregation ratios for GH2 (18 resistant:81 susceptible, P < 0.001) and the field experiment (33 resistant:58 susceptible, P < 0.03) were a poor fit to a 1:1 ratio, however.
Frequency distribution of Verticillium wilt mean disease severity (0 = no disease through 5 = severe disease) and disease incidence (proportion plants with DS ≥ 1) for Salinas 88 × La Brillante recombinant inbred lines caused by race 1 Verticillium dahliae in replicated greenhouse and field experiments. Ninety-five RIL were evaluated in greenhouse experiment 1 (root drench inoculation) and greenhouse experiment 2 (root drench and stem injection inoculated). Ninety-one RIL were evaluated in the infested field experiment. P population mean, L La Brillante mean, S Salinas 88 mean
Quantitative trait locus analysis of RIL segregation data
Of the 384 SNPs assayed, we identified 104 polymorphic markers distributed through the genome between parental lines Salinas 88 and La Brillante. An additional six PCR-based markers were also developed. Segregation of all polymorphic markers was assayed in the 95 RILs and a genetic map comprising 106 polymorphic markers was constructed using Joinmap (Fig. 2); four markers remained unlinked. The map consisted of 16 linkage groups spanning 556 cM; chromosomal linkage groups 2, 3, 5, 8 and 9 were each represented by two groups and linkage group 7 by three. Genotyping information for all polymorphic markers can be viewed at http://cgpdb.ucdavis.edu/supplemental_data/.
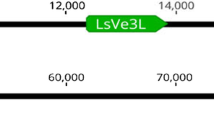
A large effect QTL was detected on linkage group 9 for DI and DS in all experiments. The peak LOD scores for DS were significant (P < 0.05) for GH1 (29.84), GH2 (25.06), and the field experiment (13.06). Nearly identical results were found for GH1 DI (28.99), GH2 DI (23.89), and field DI (13.08). The LOD score peaks lie between markers BLFW and BJGP-A for DI and DS in all environments and described large portions of variation (R 2 values: GH1 DS = 0.68; GH2 DS = 0.65; Field DS = 0.48; GH1 DI = 0.68; GH2 DI = 0.67; Field DI = 0.49). The allele for decreased disease was inherited from La Brillante. A single EST clone (QGD8I16.yg.ab1) of 721 nucleotides (GenBank accession BQ870252) was identified as a match (E value = 6e−57) to Ve1 from tomato. The amino acid sequence identity between the translated sequence of QGD8I16.yg.ab1 and a portion of the N-terminus of the tomato Ve1 (GenBank accession AAK58681) was 50% (E value = 6e−57). Also, a search of the National Center for Biotechnology Information (NCBI) database using the 721 nucleotide sequence of QGD8I16.yg.ab1 in a BLASTx query identified the Verticillium wilt disease resistance protein (Ve2) from S. lycopersicum as a match (E value = 5e−37) (Fradin et al. 2009; GenBank accession ACR33109). QGD8I16.yg.ab1 had been previously positioned to chromosome 9 as SNP marker QGD8I16 on a genetic map generated using a cross between Salinas and L. serriola accession UC96US23 containing candidate resistance genes (McHale et al. 2009). Marker QGD8I16 was not polymorphic between Salinas 88 and La Brillante but two other sequences in LG9 flanking QGD8I16 (CLX_S3_9182/BLFW and QGB24A18/BJGP-A) segregated in both populations, allowing for the alignment of LG9 in both maps. The QTL on LG9 for race 1 V. dahliae resistance is coincident with QGD8I16 (Fig. 3).
Position of Vr1 for race 1 resistance to Verticillium dahliae on linkage group 9 of Salinas 88 × La Brillante (LG9_SalxLB) and SNP marker QGD8I16 on linkage group 9 of L. sativa cv Salinas and L. serriola accession UC96US23 (LG9_SalxSer). QGD8I16 is based on a 721 nucleotide EST clone QGD8I16.yg.ab1, the translated sequence of which shares homology (E value = 5e−37) to the Verticillium wilt disease resistance protein (Ve2) from S. lycopersicum (Fradin et al. 2009; GenBank accession ACR33109). GH1 DI LOD0: Log odds score for disease incidence from greenhouse experiment 1. Vertical line is the genome wide threshold for significance (P < 0.05)
Discussion
We have detected a single gene of major effect in La Brillante conferring a high level of resistance to race 1 isolates of V. dahliae. We are naming this gene Verticillium resistance 1 (Vr1), for the first Verticillium resistance gene discovered in lettuce or its wild relatives. The dominant Vr1 allele from La Brillante confers resistance. However, the presence of additional minor genes cannot be excluded as indicated by the extensive variability between susceptible plants. Partial or incomplete resistance and dosage effects have been reported for major Verticillium resistance genes in potato (Bae et al. 2008; Jansky et al. 2004). In tomato, additional genes have been identified that make minor contributions to resistance in lines carrying the Ve1 gene (Fradin et al. 2009).
Mendelian analysis of F1, F2, and RIL population phenotypic data was consistent with a single major resistance gene in greenhouse experiments using root drench inoculation. In a field experiment and a greenhouse experiment using stem injection (GH2), deviations from expected segregation ratios were observed in the RIL population. Therefore, the environment can affect the observed phenotypic distribution of resistance and susceptible individuals. Regardless, quantitative trait locus mapping confirmed the presence of a single major locus in all the environments that the RIL population was evaluated. The inability to observe segregation consistent with a single gene in all experiments likely resulted from the challenges of working with a soil borne pathogen such as V. dahliae. In our experiments, symptoms were at times ambiguous with other diseases or physiological disorders. In particular, the field experiment had a high frequency of the disease corky root (van Bruggen et al. 1990) and a physiological disorder likely related to ammonium toxicity (Hartnett and Lorbeer 1971). Both of these problems can cause root symptoms that resemble those caused by V. dahliae under certain conditions. Root tissue plating on NP10 semi-selective media helped clarify many disease evaluations. However, such assays are time-consuming, and it was not possible to plate every plant that was evaluated. Furthermore, titer quantification methods using semi-selective media with serial dilutions of homogenized plant tissue (Vallad and Subbarao 2008), plant sap, or plating dried plant tissue (Jansky et al. 2004) are not feasible to conduct on a large scale with lettuce. Stem injection in GH2 resulted in localized discoloration at the crown, and while we opted to classify these plants as infected, the discoloration may have represented a wound response rather than disease symptoms. Consequently, incorrect scoring of some plants was inevitable in these studies. These complexities are not unique to lettuce and may explain why relatively few crops have described major resistance genes to V. dahliae, a pathogen with a broad host range.
Other sources of resistance to race 1 isolates of V. dahliae exist in lettuce and encompass a diversity of market types (Hayes et al. 2007). The allelic relationships between resistance in La Brillante and other cultivars needs to be determined, as these other resistance sources are important parents for developing new resistant leaf and romaine cultivars. In some cases, the resistance is phenotypically different from La Brillante, including a partial resistance (reduced disease incidence) to race 1 isolates (Hayes et al. 2007). Genetic analysis of these cultivars may improve our understanding of Vr1 and other genes that interact with Vr1, or possibly identify new loci for resistance to Verticillium wilt in lettuce.
We were able to detect Vr1 using either root infection by microsclerotia or conidia and by injection of plant crowns with conidia. The differences in V. dahliae colonization between La Brillante and Salinas was studied using confocal microscopy and a green fluorescent protein (GFP) expressing race 1 isolate in greenhouse experiments (Vallad and Subbarao 2008). Minimal differences in the initial infection and colonization of La Brillante and Salinas were observed. In resistant cultivars, however, the fungus was unable to grow into the taproots and never progressed into the leaves (Vallad and Subbarao 2008). While stem injection resulted in localized discoloration at the injection site, we were still able to detect Vr1 using this method. This indicates that Vr1 can contribute to host resistance outside of the root system.
Breeding iceberg cultivars for resistance to Verticillium wilt is an important goal for the lettuce industry. Molecular markers have been developed and used successfully in lettuce (Simko et al. 2009) and several authors working in a wide range of crops have acknowledged the value of molecular markers in selecting for Verticillium wilt resistance (Bae et al. 2008; Diwan et al. 1999; Simko et al. 2004c). The lettuce EST clone QGD8I16.yg.ab1 has sequence similarity to Ve1 and Ve2 from tomato and is in a coincident location with Vr1 on linkage group 9 of lettuce. While SNP marker QGD8I16 was not polymorphic between Salinas 88 and La Brillante, the strong correlation of pathogenicity between tomato and lettuce V. dahliae isolates (Maruthachalam et al. 2010) suggests that tomato and lettuce share similar race 1-specific genes for resistance to V. dahliae. Consequently, polymorphisms between the cultivars may exist in the remaining sequence of the gene, making this gene an excellent candidate for cloning, molecular marker development, and functional analysis.
Resistance to race 2 isolates of V. dahliae has not been reported for lettuce, although large scale germplasm collection and screening efforts are in progress. In tomato, race 2 isolates that overcame Ve resistance were quickly identified and now predominate in many tomato-production regions (Alexander 1962; Pegg and Brady 2002). The existence of race 2 isolates in California lettuce production regions is already known (Maruthachalam et al. 2010). Therefore, it is likely that lettuce cultivars possessing only race 1 resistance will eventually be rendered commercially ineffective. Regardless, race 1 resistance will likely be useful in combination with yet to be discovered sources of race 2 resistance.
References
Alexander LJ (1962) Susceptibility of certain Verticillium-resistant tomato varieties to an Ohio isolate of the pathogen. Phytopathology 52:998–1000
Anonymous (2005) Monterey County crop report 2004. Monterey County Agricultural Commissioner’s Office, Salinas, CA
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DL (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Atallah ZK, Maruthachalam K, du Toit L, Koike ST, Davis RM, Klosterman SJ, Hayes RJ, Subbarao KV (2010) Population analyses of the vascular plant pathogen Verticillium dahliae detect recombination and transcontinental gene flow. Fungal Genet Biol 47:416–422
Bae J, Halterman D, Jansky H (2008) Development of a molecular marker associated with Verticillium wilt resistance in diploid interspecific hybrids. Mol Breed 22:61–69
Diwan N, Fluhr R, Eshed Y, Zamir D, Tanksley SD (1999) Mapping of Ve in tomato: a gene conferring resistance to the broad-spectrum pathogen, Verticillium dahliae race 1. Theor Appl Genet 98:315–319
Fehr W (1991) Principles of cultivar development, v. 1. Theory and technique. Walter R Fehr, USA
Fradin EF, Thomma BPHJ (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol 7:71–86
Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CDM, Nazar RN, Robb J, Liu C-M, Thomma BPHJ (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150:320–332
Garibaldi A, Gilardi G, Gullino ML (2007) First report of Verticillium wilt caused by Verticillium dahliae on lettuce in Italy. Plant Dis 91:770
Hartnett JP, Lorbeer JW (1971) The production of a noninfectious lettuce root rot under controlled environmental and soil conditions. Phytopathology 61:1153–1158
Hayes RJ, Vallad GE, Ryder EJ, Subbarao KV (2006) Release of Iceberg lettuce germplasm with resistance to Verticillium Wilt. Agricultural Research Service, U.S. Dept. of Agriculture, USA
Hayes RJ, Vallad GE, Qin Q-M, Grube RC, Subbarao KV (2007) Variation for resistance to Verticillium wilt in lettuce (Lactuca sativa L.). Plant Dis 91:439–445
Jansky S, Rouse DI, Kauth PJ (2004) Inheritance of resistance to Verticillium dahliae in diploid interspecific potato hybrids. Plant Dis 88:1075–1078
Kabir Z, Bhat RG, Subbarao KV (2004) Comparison of media for recovery of Verticillium dahliae from soil. Plant Dis 88:49–55
Kawchuk LM, Hachey J, Lynch DR, Kulcsar F, van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, Fischer R, Prüfer D (2001) Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA 98:6511–6515
Ligoxigakis EK, Vakalounakis DJ, Thanassoulopoulos CC (2002) Weed hosts of Verticillium dahliae in Crete: susceptibility, symptomatology and significance. Phytoparasitica 30:511–518
Lynch DR, Kawchuk LM, Hachey J, Bains PS, Howard RJ (1997) Identification of a gene conferring high levels of resistance to Verticillium wilt in Solanum chacoense. Plant Dis 81:1011–1014
McHale LK, Truco MJ, Kozik A, Wroblewski T, Ochoa OE, Lahre KA, Knapp SJ, Michelmore RW (2009) The genomic architecture of disease resistance in lettuce. Theor Appl Genet 118:565–580
Maruthachalam K, Atallah Z, Vallad GE, Klosterman SJ, Hayes RJ, Davis RM, Subbarao KV (2010) Molecular variation among isolates of Verticillium dahliae and PCR-based differentiation of races. Phytopathology 100:1222–1230
Mert M, Kurt S, Gencer O, Akiscan Y, Boyaci K, Tok FM (2005) Inheritance of resistance to Verticillium wilt (Verticillium dahliae) in cotton (Gossypium hirsutum L.). Plant Breed 124:102–104
Pegg GF, Brady BL (2002) Verticillium wilts. CABI Publishing, New York
Putt E (1964) Breeding behavior of resistance to leaf mottle or Verticillium in sunflowers. Crop Sci 4:177–179
Ryder EJ (1971) Genetic studies in lettuce (Lactuca sativa L.). J Am Soc Hort Sci 96:826–828
Ryder EJ, Johnson AS (1974) Mist depollination of lettuce flowers. HortScience 9:584
Ryder EJ (1999) Lettuce, endive and chicory. Crop production science in horticulture series. CABI Publishing, New York
Schaible L, Cannon OS, Waddoups V (1951) Inheritance of resistance to Verticillium wilt in a tomato cross. Phytopathology 41:986–990
Simko I, Costanzo S, Haynes KG, Christ BJ, Jones RW (2004a) Linkage disequilibrium mapping of a Verticillium dahliae resistance quantitative trait locus in tetraploid potato (Solanum tuberosum) through a candidate gene approach. Theor Appl Genet 108:217–224
Simko I, Haynes KG, Ewing EE, Costanzo S, Christ BJ, Jones RW (2004b) Mapping genes for resistance to Verticillium albo-atrum in tetraploid and diploid potato populations using haplotype association tests and genetic linkage analysis. Mol Genet Genomics 271:522–531
Simko I, Haynes KG, Jones RW (2004c) Mining data from potato pedigrees: tracking the origin of susceptibility and resistance to Verticillium dahliae in North American cultivars through molecular marker analysis. Theor Appl Genet 108:225–230
Simko I, Pechenick D, McHale LK, Truco MJ, Ochoa OE, Michelmore RW, Scheffler BE (2009) Association mapping and marker-assisted selection of the lettuce dieback resistance gene Tvr1. BMC Plant Biol 9:135
Stam P, Van Ooijen JW (1995) Joinmap version 2.0: software for the calculation of genetic linkage maps. Plant Research International, Wageningen
Subbarao KV, Hubbard JC, Greathead AS, Spencer GA (1997) Verticillium wilt. In: Davis RM, Subbarao KV, Raid RN, Kurtz EA (eds) Compendium of lettuce diseases. The American Phytopathological Society, St. Paul, pp 26–27
Vallad GE, Bhat RG, Koike ST, Ryder EJ, Subbarao KV (2005) Weedborne reservoirs and seed transmission of Verticillium dahliae in lettuce. Plant Dis 89:317–324
Vallad GE, Qin Q-M, Grube RC, Hayes RJ, Subbarao KV (2006) Characterization of race-specific interaction among isolates of Verticillium dahliae pathogenic on lettuce. Phytopathology 96:1380–1387
Vallad GE, Subbarao KV (2008) Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 98:871–885
van Bruggen AHC, Brown PR, Greathead A (1990) Distinction between infectious and noninfectious corky root of lettuce in relation to nitrogen fertilizer. J Am Soc Hort Sci 115:762–770
Acknowledgments
This research was supported by the California Leafy Greens Research Program and the California Department of Food and Agriculture under the “Buy California Initiative” as well as an award to RWM from the National Research Initiative (NRI) Plant Genome Program of the USDA Cooperative State Research, Education and Extension Service (CSREES) Grant no. 04-35300-14601.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Havey.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hayes, R.J., McHale, L.K., Vallad, G.E. et al. The inheritance of resistance to Verticillium wilt caused by race 1 isolates of Verticillium dahliae in the lettuce cultivar La Brillante. Theor Appl Genet 123, 509–517 (2011). https://doi.org/10.1007/s00122-011-1603-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1603-y