Abstract
The most consumed food samples of cereals (rice, maize and wheat), vegetables (lentil, brinjal, carrot, bean, potato, tomato, onion and chili), fruits (banana, mango and jackfruit), fish (taki, rui, pangas and tilapia), egg (chicken and duck), milk (cow) and meat (chicken, duck, beef and mutton) were collected from some markets of Bogra district northern part of Bangladesh to evaluate the levels of arsenic (As) and associated health risk to the adult’s and child inhabitants. Arsenic is a highly toxic element, and its presence in food composites is a matter of concern to the world scientists. Target hazard quotients (THQs) and target carcinogenic risk were calculated to evaluate the non-carcinogenic and carcinogenic health risk from ingested arsenic. The highest and the lowest mean concentrations of arsenic were noted in the Tilapia fish [(0.94 mg/kg, wet weight (ww)] and beef (0.012 mg/kg, ww). The daily intakes of arsenic via foodstuffs were 1.92 and 3.30 µg/kg-bw/day for rural adults and children and 1.69 and 3.04 µg/kg-bw/day for urban adults and children, respectively. The result shows the highest THQs of arsenic in cereals and vegetables for both the rural and urban inhabitants which exceed the safe limit (>1) indicating that cereals and vegetables are the main food items contributing to the potential health risk. The estimated target cancer risks from ingesting dietary arsenic all exceeded 10−6, indicating increased risk of cancer for adults and children in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic is a ubiquitous element that can pollute soil, water and plants including other compartments of the ecosystem and the environment, and ultimately affects human health and well-being (Bundschuh et al. 2012; Islam et al. 2014a, b; Islam and Hoque 2014). Arsenic can be taken up by the terrestrial and aquatic biota which are either consumed by humans or incorporated into fodder for livestock (Bundschuh et al. 2012). As the most widely distributed toxic metal in food (Wang et al. 2013), there is no evidence that arsenic is essential in human bodies. Humans are exposed to many different inorganic and organic arsenic species present in food, water and other environmental media. The most common mode of arsenic toxicity in humans is the inactivation of an enzyme system by binding through various biologic ligands (Shen et al. 2013). Chronic arsenic poisoning can cause serious health effects including cancers, melanosis, hyperkeratosis, restrictive lung disease, peripheral vascular disease (black foot disease), gangrene, diabetes mellitus, hypertension and ischemic heart disease (Lindberg et al. 2008). Some of these human health effects are currently being observed in populations in the south and southeastern Asia, particularly in Bangladesh, India and Taiwan. Arsenic is recognized as a toxic element and has been classified as a human carcinogen to skin and lungs (WHO 2001).
Bangladesh is one of the developing countries in the world, and arsenic contamination is one of the most important issues concerning public health. In Bangladesh, human consequences of arsenic pollution of groundwater were expressed through exposure to drinking water and food where approximately 27 million people drank water with more than 50 μg/L and around 50 million people drank water with more than 10 μg/L (Jiang et al. 2013). A number of articles have reported on arsenic-contaminated foods in Bangladesh (Das et al. 2004; Islam et al. 2015a). Individuals could be exposed to arsenic through several sources, with food consumption and drinking water as the two most important ones. Although dermal and inhalation exposure is possible, food and drinking water are the principal routes of exposure to arsenic (WHO 2011a; IARC 2012; EFSA 2014). Organic arsenic species such as arsenobetaine and different arsenosugars are the most common forms in seafood while in foods of terrestrial origin the predominant arsenic forms are iAs [both As (V) and As (III)] and single methylated arsenic species (methylarsonate, methylarsenite and dimethylarsinate (DMA). Recently, diet studies that included evaluations of total arsenic in foods have been reported for many counties (Li et al. 2006; Wang et al. 2013). The total dietary study performed by US Food and Drug Administration (FDA) indicated that food contributes 93% of the total intake of arsenic (Adams et al. 1994). In addition, groundwater is continuously being used for irrigation to cultivate a variety of crops, vegetables and for fish culture including hatchery operation as well in Bangladesh. It is well known that levels of arsenic in foods generally are accumulated from the local environments which indicate that foodstuffs grown in arsenic-enriched rural areas are contaminated with arsenic in Bangladesh. In fact, most of the foodstuffs available in urban markets and commonly consumed by the urban residents also come from the rural areas. Hence, the identification of daily dietary intake of total arsenic and health risk assessment of arsenic based on the daily food consumption studies is urgent for both the rural and urban residents in Bangladesh.
In the present investigation, a micro level study was carried out to evaluate arsenic levels in food composites and daily dietary intake for both the rural and urban residents (adults and children) of the northern part in Bangladesh. The potential health exposure was assessed through dietary intakes of commonly consumed foods. The aim of this work is to measure the levels of arsenic in the commonly consumed foodstuffs and to assess the carcinogenic and non-carcinogenic health risks.
2 Materials and methods
2.1 Sample collection and preparation
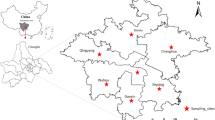
Daily intake of major food items and intakes of individual food items were used to characterize the food consumption pattern of both the rural and urban population in Bangladesh (HIES 2011; Islam et al. 2014a, b). Fresh 219 food samples of cereals (rice, maize and wheat), vegetables (lentil, brinjal, carrot, bean, potato, tomato, onion and chili), fruits (banana, mango and jackfruit), fish (taki, rui, pangas and tilapia), egg (chicken and duck), milk (cow) and meat (chicken, duck, beef and mutton) were collected from different markets of Bogra district in Bangladesh (Fig. 1). These foodstuffs are highly consumed by the Bangladeshi population as their daily diet (HIES 2011; Ahmed et al. 2016) and from the on spot survey during our sampling according to the procedure reported by Kelley et al. (2003). Briefly, a dietary questionnaire survey was conducted where the participants were asked for information about the type and quantity of water consumed, foods ingested and how the foods were prepared for consumption (raw or cooked), demographic characteristics (such as age, sex, place of residence and occupation). The 2010 Household Income and Expenditure Survey (HIES) was used to estimate food consumption rates. This survey included 12,240 households selected from rural and urban areas, and information on various socioeconomic and personal information, including the most widely consumed food items. A composite of at least 10 samples for each food item was prepared and homogenized in a food processor, and 50-g test portions was stored at −20 °C in the laboratory of the Institute of Nutrition and food Science (INFS), University of Dhaka, Bangladesh. The preprocessed samples were then shipped to the laboratory of environment and information sciences, Yokohama National University, Japan for chemical analysis. The measurements of arsenic in foods were taken at wet weight basis by calculating the moisture content from each food item. As people consume fresh food so, we converted the arsenic concentration in food as wet weight basis.
2.2 Reagents and sample digestion procedure
All solutions were prepared with analytical reagent-grade chemicals and ultrapure water. Standard stock solutions containing 10 µg/L of As (T As) and an internal standard solutions containing 1.0 mg/L of indium, yttrium, beryllium, telium, cobalt and titanium were purchased from Spex Certi Prep® USA. The connected tube of ICP-MS was inserted into the internal standard bottle and automatically added to the samples during analysis. A microwave digestion system (Berghof Microwave MWS-2, Germany) was used to extract arsenic from sample. Digestion reagents that were used included 5 ml HNO3 acid (69%) (Kanto Chemical Co, Japan) and 2 ml H2O2 (30%) (Wako Chemical Co, Japan). The weighed samples of 0.3 g were then placed into the digestion reagent in a Teflon vessel. The digest solution then filtered (DISMIC®—25HP PTFE syringe filter (pore size = 0.45 µm) Toyo Roshi Kaisha, Ltd., Japan), and stored in 50 mL polypropylene centrifuge tubes (Nalgene, New York). Samples were analyzed using inductively coupled plasma mass spectrometer (ICP-MS, Santa Clara, California, USA). The detection limit for the measurements of arsenic in foods was 0.4 ng/L.
2.3 Health risk assessment
2.3.1 Estimated daily intakes (EDI)
Estimated daily intakes (EDI) for As were calculated using the respective average concentration in composite food samples by the weight of food item consumed by an individual (body weight 60 kg for adults and 16 kg for children) in Bangladesh which was obtained from the “Report of the household income and expenditure survey 2010” (HIES 2011; Islam et al. 2014a, b), and calculated by using the formula:
where FIR is the food ingestion rate (g/person/day), C is the arsenic concentration in food samples (mg/kg), BW is the body weight. The EDI of inorganic arsenic (iAs) were calculated by using the fraction of iAs in the investigated food samples derived from the literature surveys. The fraction of iAs content of Bangladesh rice sample was 0.45 by Sun et al. (2008), and vegetable sample was accounted as 0.96 as reported by Smith et al. (2006). For freshwater fish samples, the fraction of iAs content was accounted as 0.26 (Piyawat et al. 2012). According to EFSA (2014), a fraction of 0.7 was applied to estimate iAs for the rest of foods of terrestrial origin. It should be mentioned here that, at the recent 72nd meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the previous provisional tolerable daily intake (PTDI) value for inorganic As intake (2.1 μg/kg-bw/day) has been withdrawn (JECFA 2011; EFSA 2014), because the value was in the lower range of the bench mark lower confidence limit for a 0.5% increased incidence of lung cancer (BMDL0.5) of 3.0 μg/kg-bw/day (2–7 μg/kg-bw/day based on the range of estimated total dietary exposure) (WHO 2011b). Thus currently, there is no established guideline to assess health risk due to dietary intake of inorganic As. However, the Codex Committee on Contaminants in Foods (CCCF) has argued that an EDI of iAs value below the BMDL0.5 does not necessarily indicate that there is no risk and cannot be regarded as a safety standard (JECFA 2011) which motivated us to compare the EDI of iAs with the previous PTDI value of 2.1 μg/kg-bw/day to assess the health risk of inorganic As exposure from dietary components along with drinking water.
2.4 Non-carcinogenic risk
For estimating the non-carcinogenic effects of iAs, the following equation is used for target hazard quotient (THQ) is as follows (US-EPA 1989, 1991),
where, THQ is the target hazard quotient (dimensionless); EFr is the exposure frequency (365 days/year); ED is the exposure duration (70 years) equivalent to the average human life time; RfD is the oral reference dose (mg/kg/day); BW is the average body weight; AT is the averaging time for non-carcinogens (365 days/year × number of exposure years, assuming 70 years). The oral reference dose was taken from Integrated Risk Information System (US-EPA 2010). If the THQ is less than 1, the exposed population is unlikely to experience obvious adverse effects. If the THQ is equal to or higher than 1, there is a potential health risk (Islam et al. 2014a, b, 2015b; Shaheen et al. 2016), and related interventions and protective measurements should be taken.
2.5 Target carcinogenic risk (TR)
The equation used for estimating the target cancer risk (lifetime cancer risk) is as follows (US-EPA 1989),
where, TR represents the target cancer risk or the risk of cancer over a lifetime; AT is the averaging time for carcinogens (365 days/year × ED); CSFo is the oral carcinogenic slope factor from the Integrated Risk Information System (US-EPA 2010) and database was 1.5 (mg/kg/day)−1 for arsenic.
3 Results and discussion
3.1 Arsenic concentration in foodstuffs
The exposure of arsenic from the food composites and drinking water in the rural and urban population at the northern part of Bangladesh and their health scenario due to intake of arsenic-contaminated foodstuffs is the main focus of this study. Arsenic concentrations in each food category showing individual food items are shown in Table 1. The mean range of arsenic concentrations (mg/kg, ww) in food categories on a wet weight basis was 0.26–0.37 (cereals), 0.17–0.31 (vegetables), 0.16–0.39 (fruits), 0.11–0.94 (fish), 0.07–0.11 (egg), 0.012–0.038 (meat) and 0.004–0.13 (milk) (Table 1). The highest and the lowest mean concentrations of arsenic were noted in the Tilapia fish (0.94 mg/kg) and beef (0.012 mg/kg). According to the study of Zhang et al. (2011), dietary uptake could be the primary route for arsenic bioaccumulation in fish. Generally, the mean concentrations of total arsenic from high to low were in the order of: fish > cereal > vegetable > fruit > egg > milk > meat. The results of the present study were in line with former studies conducted in Cambodia (Wang et al. 2013), UK (Al Rmalli et al. 2005), China (Li et al. 2011) and Bangladesh (Das et al. 2004; Islam et al. 2014b) that high arsenic contents were observed in fish and seafood comparing to other foodstuffs.
3.2 Dietary intake of arsenic
As there were large variations of arsenic levels detected in different types of food items even among the same food group, geometric means were used in the calculation of the mean levels of arsenic in the cereals, vegetables, fruits, fish and meat. The EDI of iAs were evaluated according to the average concentration of iAs in each foodstuffs and the respective consumption rate (Islam et al. 2014b; Santos et al. 2004) followed by average body weight of adults and children. The concentrations, consumption rates and estimated daily intakes of arsenic in different food groups are listed in Table 2. The daily intake of arsenic via studied food was 1.92 and 3.30 µg/kg-bw/day for adults and children in rural area and 1.69 and 3.04 µg/kg-bw/day for adults and children in urban area (Table 2). Former studies suggested the most two important sources for arsenic exposure were dietary intake (Alam et al. 2003). The estimations of total As intake were based on the sum of As ingested from different food items consumed by a normal adult (average body weight 60 kg) during the 24-h period. Our study revealed that daily intake of arsenic via foodstuffs in rural residents was slightly higher than that of resident in the urban areas. The estimated daily intake (EDI) for the rural (1.92 and 3.30 µg/kg-bw/day) and urban residents (1.69 and 3.04 µg/kg-bw/day) was higher than the recommended provisional tolerable intake value recommend by WHO benchmark dose (BMDL0.5 = 1.96 µg/kg.bw/day) (JECFA 2011). Seventeen dietary surveys from thirteen different countries across Europe showed that the mean dietary exposure to iAs ranged from 0.20 to 0.36 μg/kg-bw/day for children and 0.11 to 0.17 μg/kg-bw/day for adults (EFSA 2014). The high daily intake values of arsenic in rural residents were mainly due to the high intake of cereals and vegetables which contain high level of arsenic regarding this study.
The contributions of arsenic from different food items to the dietary intake are summarized in Fig. 2. Cereals consumption contributed the greatest proportion of total arsenic intake in the rural residents (about 66%) and urban residents (about 60%). It might be due to that cereal contained much higher concentrations of arsenic than other food items and consumption rate also higher than other food (Table 2). Previous studies revealed that an elevated accumulation of arsenic in rice (Xu et al. 2008) might be due to the high arsenic content in soil of crop fields and excessive use of contaminated irrigation water. In Bangladesh, Das et al. (2004) found the concentration of arsenic in soil was 15.68 ± 6.59 mg/kg, which was higher than the reported average of 10 mg/kg of arsenic naturally occurring in the soil worldwide. They also found a positive correlation (r = 0.74, p = 0.01) of arsenic in contaminated water and arsenic in soils. Vegetables contributed to the second largest proportion of total arsenic intake in rural (20%) and urban (21%) areas. This might be the transportation of arsenic from soil to cereal and vegetable results in the relatively high arsenic concentrations in vegetable and cereal (Wang et al. 2006; Zheng et al. 2007). Fish consumption contributed a considerable proportion of total arsenic intake in rural (about 8%) and urban (about 12%) areas. It might be due to that fish contained slight higher concentrations of arsenic than other food items (Tables 1, 2).Our results were in line with previous studies showing that fish accounted 75–90% of the total arsenic intakes via dietary source (Das et al. 2004). Therefore, much more attention should be paid to the daily intakes of arsenic via fish consumption although background information was available. The other food groups, such as fruits, meat, milk and egg contributed limited proportion of arsenic intake among all foodstuffs in the present study, which was in line with the former studies conducted in Cambodia (Wang et al. 2013) and Chile (Munoz et al. 2005).
3.3 Health risk assessment
Table 3 shows the target hazard quotients (THQ) and target carcinogenic risk factors (TR) for arsenic in different foodstuffs commonly consumed by the Bangladeshi population in their daily diet. The result shows the highest THQ of iAs in cereals and vegetables for both the rural and urban inhabitants which exceed the safe limit (>1) indicating that cereals and vegetables are the main food items contributing to the potential health risk (Table 3). Therefore, health risks of iAs from exposure to daily consumable foods are of some concern.
For carcinogens, risks were estimated as the incremental probability of an individual to develop cancer over lifetime, as a result of exposure to that potential carcinogen (i.e., incremental or excess individual lifetime cancer risk; US-EPA 1989). Results are listed in Table 3; indicated that total TR values for rural residents were (2.9E − 03 and 5.0E − 03 for adults and child, respectively) and for urban residents were (2.5E − 03 and 4.6E − 03 for adults and child, respectively). Among the food items, higher cancer risk of inorganic arsenic (iAs) was obtained due to cereals and vegetables consumption. In general, the excess cancer risk lower than 10−6 are considered to be negligible, cancer risk above 10−4 are considered unacceptable and risks lying between 10−6 and 10−4 are generally considered an acceptable range (USE-PA 1989, 2010). The carcinogenic risk of iAs was higher than the unacceptable value (10−4). Therefore, the potential health risk of the inhabitants due to iAs exposure through food consumption should not be ignored at the study area. Based on the assumptions in Table 3, the residents of the study area may be exposed to high dietary iAs concentrations through consumption of cereals and vegetables that could direct to cause cancer.
The arsenic toxicity mainly depends on the species and chemical form. The exposure time to develop arsenicosis varies from case to case reflecting its dependence on arsenic level in drinking water and food, nutritional status, genetic variant of human being and compounding factors (Banerjee et al. 2011). According to WHO, 1.0 mg of inorganic arsenic per day may give rise to skin effects within a few years (WHO 1981). The U.S. Environmental Protection Agency (EPA) estimated that drinking 1 L per day of arsenic-contaminated water (50 g/L) could be as high as 13 per 1000 persons at life risk from arsenicosis (Santra et al. 2013). However, most households still use arsenic-contaminated water for drinking, cooking and other domestic purposes, and based on their total dietary intake of arsenic from food, the Bangladeshi population are at high risk of developing arsenical skin lesions and cancer.
In the present study, some limitations associated with the exposure can lead to uncertainty in the overall risk assessment. We did not determine the arsenic species (organic and inorganic arsenic compounds) in the studied foodstuffs but we calculated iAs in foods using the reference values. While for many foods, the majority of total arsenic is made up of inorganic species (e.g., rice), there is great variability, and using total arsenic as a surrogate for inorganic arsenic will likely overestimate risk (Lynch et al. 2014). Furthermore, the investigation in the present study only focused the selected food items and arsenic contamination in drinking water. In most cases, food was subjected to boiling prior to consumption. People generally use the same water for cooking purposes as they usually drink. As a result, a higher amount of dietary arsenic especially iAs would be included in their final diet which we did not consider in this study. Considering that arsenic could accumulate in the organism and there were possibilities of synergistic effects when other pollutants are taken up simultaneously (Zhang et al. 2011), the high exposure by food is an alarming public health concern. Our study has provided a clear picture on the high dietary intakes of arsenic via food consumption in Bangladesh. The results obtained are an essential component for future more in-depth epidemiological studies which will include exposure of the population to the different arsenic species.
4 Conclusion
The concentrations of arsenic widely varied among the samples. Among the food items cereals and vegetables contributes the highest intake of arsenic. Total intake of inorganic arsenic for the exposed people was three times higher than that recommended as the MTDI, indicated that people would experience significant risks. From the human health point of view, the total THQ values for iAs were higher than 1, suggesting that people would experience significant health risks by ingesting arsenic through consuming the studied foodstuffs. Concerning the carcinogenic risk, the total TR values of iAs were clearly higher than the US-EPA threshold level (10−6). Hence, health risks associated with food consumption are not negligible and the sources of arsenic pollution should be controlled to achieve the safe food stuffs. A detailed monitoring programme, mitigation measures; rising awareness and management of arsenic problem in Bangladesh are urgently needed to overcome this burning problem. Furthermore, it is important to continue to monitor the food chain because continued use of arsenic-contaminated water and soil is likely to increase the probability and magnitude of dietary arsenic intake.
References
Adams MA, Bolger PM, Gunderson EL (1994) Dietary intake and hazards of arsenic. In: Chappell WR, Abernathy CO, Calderon RA (eds) Arsenic: exposure and health, science and technology letters. Northwood, London, pp 41–49
Ahmed MK, Shaheen N, Islam MS, Islam S, Islam MM, Kundu GK, Bhattacharjee L (2016) A comprehensive assessment of arsenic in commonly consumed foodstuffs to evaluate the potential health risk in Bangladesh. Sci Total Environ 544:125–133
Al Rmalli SW, Haris PI, Harrington CF, Ayub M (2005) A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Sci Total Environ 337(1–3):23–30
Alam MG, Snow ET, Tanaka A (2003) Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ 308(1–3):83–96
Banerjee N, Nandy S, Kearns JK, Bandyopadhyay AK, Das JK, Majumder P, Basu S, Banerjee S, Sau TJ, States JC, Giri AK (2011) Polymorphisms in the TNF-a and IL10 gene promoters and risk of arsenic-induced skin lesions and other nondermatological health effects. Toxicol Sci 121(1):132–139
Bundschuh J, Nath B, Bhattacharya P, Liu CW, Armienta MA, López MVM, Lopez DL, Jean JS, Cornejo L, Macedo LFL, Filho AT (2012) Arsenic in the human food chain: the Latin American perspective. Sci Total Environ 429:92–106
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentrations in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30(3):383–387
EFSA (European Food Safety Authority) (2014) Dietary exposure to inorganic arsenic in the European population European Food Safety Authority (EFSA), Parma, Italy. EFSA J 12(3):3597. doi:10.2903/j.efsa.2014.3597
FAO (Food and Agriculture Organization) (2006) Arsenic contamination of irrigation water, soil and crops in Bangladesh: risk implications for sustainable agriculture and food safety in Asia. Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific, Bangkok
HIES (household income and expenditure survey) (2011) Preliminary report on household income and expenditure survey-2010. Bangladesh Bureau of Statistics, Statistics Division, Ministry of Planning, Dhaka
IARC (International Agency for Research on Cancer) (2012) Monographs on the evaluation of carcinogenic risks to humans. A review of human carcinogens: arsenic, metals, fibres, and dusts, vol 100C. International Agency for Research on Cancer, Lyon, pp 41–93
Islam MS, Hoque MF (2014) Concentrations of heavy metals in vegetables around the industrial area of Dhaka city, Bangladesh and health risk assessment. Int Food Res J 21(6):2121–2126
Islam MS, Ahmed MK, Al-mamun MH, Masunaga S (2014a) Trace metals in soil and vegetables and associated health risk assessment. Environ Monit Assess 186:8727–8739
Islam MS, Ahmed MK, Al-Mamun MH, Islam K, Ibrahim M, Masunaga S (2014b) Determination of arsenic and lead in daily consumable foods and their potential risks to human in Bangladesh. Food Add Contam Part A 31:1982–1992
Islam MS, Ahmed MK, Al-Mamun MH, Masunaga S (2015a) Assessment of trace metals in foodstuffs grown around the vicinity of industries in Bangladesh. Food Comp Anal 42:8–15
Islam MS, Ahmed MK, Al-Mamun MH, Masunaga S (2015b) Assessment of trace metals in fish species of urban rivers in Bangladesh and health implications. Environ Toxicol Pharmacol 39:347–357
JECFA (2011) Safety evaluation of certain contaminants in food. Seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO food additives series: 63; FAO JECFA monographs: 8. FAO, Rome
Jiang JQ, Ashekuzzaman SM, Jiang A, Sharifuzzaman SM, Chowdhury SR (2013) Arsenic contaminated groundwater and its treatment options in Bangladesh. Int J Environ Res Public Health 10(1):18–46
Kelley K, Clark B, Brown V, Sitzia J (2003) Good practice in the conduct and reporting of survey research. Int J Qual Health Care 15(3):261–266
Li X, Gao J, Wang Y, Chen J (2006) 2000 Chinese Total Dietary Study—the dietary arsenic intakes. J Hyg Res 35(1):63–66
Li G, Sun GX, Williams PN, Nunes L, Zhu YG (2011) Inorganic arsenic in Chinese food and its cancer risk. Environ Int 37:1219–1225
Lindberg AL, Ekström EC, Nerwell B, Rahman M, Lönnerdal B, Persson LÅ, Vahter M (2008) Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res 106:110–120
Lynch HN, Greenberg GI, Pollock MC, Lewis AS (2014) A comprehensive evaluation of inorganic arsenic in food and considerations for dietary intake analyses. Sci Total Environ 496:299–313
Munoz O, Bastias JM, Araya M, Morales A, Orellana C, Rebolledo R, Velez D (2005) Estimation of the dietary intake of cadmium, lead, mercury, and arsenic by the population of Santiago (Chile) using a Total Diet Study. Food Chem Toxicol 43(11):1647–1655
Piyawat S, Suthep R, Bundit T, Nongluck R (2012) Total and inorganic arsenic in freshwater fish and prawn in Thailand. J Food Protect 10:1728–1902
Santos EE, Lauri DC, Silveira PCL (2004) Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci Tot Environ 327:69–79
Santra SC, Samal AC, Bhattacharya P, Banerjee S, Biswas A, Majumdar J (2013) Arsenic in food chain and community health risk: a study in Gangetic West Bengal. Proc Environ Sci 18:2–13
Shaheen N, Irfan MN, Khan IN, Islam S, Islam MS, Ahmed MK (2016) Presence of heavy metals in fruits and vegetables: health risk implications in Bangladesh. Chemosphere 152:431–438
Shen S, Li XF, Cullen WR, Weinfeld M, Le XC (2013) Arsenic binding to proteins. Chem Rev 113(10):7769–7792
Smith NM, Lee R, Heitkemper DT, Cafferky KD, Haque A, Henderson AK (2006) Inorganic arsenic in cooked rice and vegetables from Bangladeshi households. Sci Total Environ 370:294–301
Sun GX, Williams PN, Carey AM, Zhu YG, Deacon C, Raab A, Feldmann J, Islam MR, Meharg AA (2008) Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ Sci Technol 42:7542–7546
US-EPA (1989) Assessing human health risks from chemically contaminated fish and shellfish: a guidance manual. EPA-503/8-89-002. U.S. Environmental Protection Agency Washington, DC
US-EPA (1991) Technical support document for water quality-based toxics control (EPA/505/2-90-001). Washington, DC
US-EPA (2010) Risk-based concentration table. http://www.epa.gov/reg3hwmd/risk/human/index.htm
Wang G, Su M, Chen Y, Lin FF, Luo D, Gao SF (2006) Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ Pollut 144:127–135
Wang HS, Sthiannopkao S, Chen ZJ, Man YB, Du J, Xing GH, Kim KW, Yasin MSM, Hashim JH, Wong MH (2013) Arsenic concentration in rice, fish, meat and vegetables in Cambodia: a preliminary risk assessment. Environ Geochem Health 35:745–755
WHO (1981) Arsenic: environmental health criteria 18. World Health Organization, Geneva
WHO (2001) IPCS environmental health criteria 224. Arsenic and arsenic compounds. International Programme on Chemical Safety, World Health Organization, Geneva
WHO (2011a) Guidelines for drinking-water quality, 4th ed. http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf
WHO (2011b) Evaluation of certain contaminants in food. Seventy-second report of the joint FOA/WHO expert committee on food additives, WHO technical report series No. 959. World Health Organization (WHO), Geneva
Xu X, McGrath S, Meharg A, Zhao F (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42(15):5574–5579
Zhang W, Huang L, Wang WX (2011) Arsenic bioaccumulation in a marine juvenile fish Teraponjarbua. Aquatic Toxicol 105:582–588
Zheng N, Wang Q, Zheng D (2007) Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao Zinc Plant in China via consumption of vegetables. Sci Total Environ 383:81–89
Acknowledgements
The authors thank the authority of Patuakhali Science and Technology University (PSTU), Bangladesh and Yokohama National University, Japan for providing laboratory facilities. The authors also delighted to express their gratefulness and sincerest thanks to Professor Dr. Md Shams-Ud-Din (Vice Chancellor, PSTU), for his valuable suggestions and cooperation to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, M.S., Ahmed, M.K., Habibullah-Al-Mamun, M. et al. Arsenic in the food chain and assessment of population health risks in Bangladesh. Environ Syst Decis 37, 344–352 (2017). https://doi.org/10.1007/s10669-017-9635-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10669-017-9635-8