Abstract
To assess arsenic contaminations and its possible adverse health effects, food samples were collected from Kandal, Kratie and Kampong Cham in Cambodia. The highest and the lowest concentrations were observed in fish (mean 2,832 ng g−1, ww) collected from Kandal province and cattle stomach (1.86 ± 1.10 ng g−1, ww) collected from Kratie, respectively. The daily intake of arsenic via food consumption was 604, 9.70 and 136 μg day−1 in Kandal, Kratie and Kampong Cham, respectively. The arsenic dietary intake in Kandal ranked No. 1 among all the 17 compared countries or regions. Fish consumption contributed the greatest proportion of total arsenic daily intake in Kandal (about 63.0 %) and Kampong Cham (about 69.8 %). It is revealed to be a much more important exposure pathway than drinking water for residents in Kampong Cham. The results of risk assessment suggested that the residents in Cambodia, particularly for people in Kandal province, suffer high public health risks due to consuming arsenic-contaminated food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toxic substances contamination in foodstuffs is a global important health issue (Ng et al. 2003). As the most widely distributed toxic metal in food (Llobet et al. 2003), there is no evidence that arsenic is essential in human bodies. In contrast, chronic oral consumption of arsenic could cause skin lesions, pigmentation of the skin and the development of hard patches of skin on the palm of the hands and soles of the feet (Fatmi et al. 2009). Toxicological studies show that chronic exposure to excessive arsenic would increase the risk of developing cancer of the bladder, liver, kidney and skin (Chu and Crawford-Brown 2006; Lamm et al. 2006). Individuals could be exposed to arsenic through several pathways, with food consumption and drinking water as the two most important ones. Recently, diet studies that included evaluations of total arsenic in foods have been reported for many counties such as USA, UK, Canada, Japan and Mexico (Del Razo et al. 2002; Gunderson 1991; Tsuda et al. 1995). The total dietary study performed by US Food and Drug Administration (FDA) indicated that food contributes 93 % of the total intake of arsenic and seafood contributes 90 % of that (Adams et al. 1994).
Cambodia is one of the least developed countries in the world, and arsenic contamination is one of the most important public health issues. For example, some 1 million people in Kandal province have stopped using surface water due to waterborne bacterial diseases and arsenic contamination that are partly responsible for a high infant mortality (71 deaths/1,000 live births) (Sthiannopkao et al. 2008). The arsenic concentrations in groundwater collected from Mekong River floodplain ranged from 1 to 1,340 μg L−1, with 48 % exceeding 10 μg L−1. The arsenic levels were particularly high in Kandal province, with an average concentration of 233 μg L−1 (Buschmann et al. 2007). In fact, there were many studies concerning the high arsenic concentrations in surface drinking water and groundwater collected from different regions in Cambodia, such as Kratie, Kandal, and areas south and southeast of Phnom Penh (Berg et al. 2007; Buschmann et al. 2008; Luu et al. 2009). However, there are no published data about the arsenic contamination in foodstuffs collected from these areas, although it is well known that levels of arsenic in foods generally are accumulated from the local environments.
Hence, the identification of total daily dietary intake and health risk assessment of arsenic based on market basket studies is urgent for the Cambodian population. Specifically, the objectives of the present study were (1) to measure arsenic concentrations in different food items collected from the Mekong River basin of Cambodia; (2) to evaluate human exposure to arsenic via dietary intake and the associated health risks and (3) to identify the major food sources at the three studied sites. To our knowledge, this is the first study to investigate the dietary intake and human risk assessment of arsenic based on Cambodia market basket data.
Materials and methods
Sampling sites
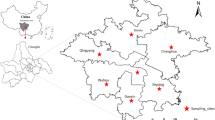
Food samples were collected from three areas in the Mekong River Basin of Cambodia (Fig. 1). According to our previous study about the arsenic contamination in groundwater (Phan et al. 2010), Kampong Kong commune (Preak Russey and Lvea Toung villages) in Kandal province was extremely contaminated, Khsarch Andaet commune (Preak Samrong I and II villages) in Kratie province was moderately contaminated and Ampil commune (Andoung Chros and Veal Sbov villages) in Kampong Cham province was considered to be uncontaminated. Kratie and Kampong Cham provinces are located along the Mekong River, upstream of Phnom Penh, whereas Kandal province is located between the Mekong and the Bassac Rivers, downstream of Phnom Penh.
Food consumption survey
A semiquantitative food frequency questionnaire was used to characterize the food consumption pattern of each participant. In total, 59 types of food items were included. Personal information data (sex, age, body weight, period of residency) were also included in the questionnaire. The detailed information about the participants in three sampling sites was reported in our previous study (Wang et al. 2011). The number of participants from Kampong Cham, Kratie and Kandal was 58, 31 and 69, respectively. Among the three study areas, cereals (46.8 %), vegetables (23.8 %), fruits (13.5 %) and fish (10.2 %) contributed to greater proportions of the total consumption, while foods of other animal origins, such as meat (3.11 %) and viscera (1.73 %), contributed lesser portion. Within Cambodia, the residents in Kandal province consumed significantly (p < 0.05) less fish than residents in the other two studies areas.
Food sample collection and arsenic analyses
The selection of food items for analyses was based on the occurrence of arsenic in these food groups, local consumption habits, as well as their availability in the local markets or grocery stores. As a result, seven major food groups, namely (1) cereals, (2) vegetable, (3) fruit, (4) fish, (5) meat, (6) viscera and (7) dairy product, comprising of 22 food items in total, were chosen for arsenic analyses. At least three samples were collected for each food category at each sampling site, with 154 samples in total for the present study. The fish sample analyzed in the present study was the mixture of common fish in Cambodia [Hypsibarbus malcolmi, family name is Cyprinidae (Minnows and Carps), 8 samples] collected from fish pond (2 sites) and river (1) in each village. Only the edible parts of food items were analyzed, with inedible parts such as bone and skin removed prior to analyses. Fish samples were analyzed with the skin since it is commonly eaten. All the edible parts of samples were separated, freeze-dried and then grounded separately.
About 0.2 g of each dried sample was used for arsenic determination using acid washed PTFE tubes by microwave oven (Li et al. 2011). Five milliliters of nitric acid and hydrogen peroxide mixture (2:1, v/v ratio) was added. All tubes were then kept for an hour at room temperature and placed in a PTFE covered container. Then, it was heated following a one-stage digestion programme (80 % of total power 900 W) for 3 min. After cooling, the extra acid was evaporated to obtain a semidried mass, 10 mL of 0.1 M HNO3 was added, all digests were filtered through a Whatman 42 filter paper, transferred into a 25 mL flask and the volume was made up to the mark with Milli-Q processed water. The arsenic concentration was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES). The limit of detection within the 95 % confidence interval was 0.5 ng/mL for arsenic. For quality control, blank extractions (without sample) and standard reference materials [wholemeal flour BCR 189 and fish (DORM-2)] were carried out throughout the complete procedure. There were no indications of contamination. The recovery rates of the arsenic of the standard reference material were within the certificate values (95 ± 7.3 %).
Data analyses
The arsenic concentrations reported in ng g−1, wet weight (ww), in all samples were not adjusted with the recovery rate due to the satisfactory recovery. The data analyses were performed using SPSS 17.0 for Windows. Normality was confirmed by the Kolmogorov–Smirnov test. Two independent t tests, Wilcoxon rank sum test, one-way ANOVA and Kruskal–Wallis test were used to compare the arsenic contaminations among different food groups and sampling sites appropriate.
Results and discussion
Arsenic levels in foodstuffs
The arsenic concentrations in the foodstuffs collected from the three study areas are listed in Table 1. These results were given in fresh weight basis, for estimating arsenic intake from food items. Among different food samples, the arsenic concentrations ranged from 4.38 to 495 ng g−1 in Kampong Cham (median 15.4 ng g−1), 1.86 to 40.6 ng g−1 in Kratie (median 5.40 ng g−1) and 10.3 to 2,832 ng g−1 in Kandal (median 74.0 ng g−1), respectively. The same food items such as cabbage, morning glory, winter melon, fish and beef collected from Kandal province contained significantly (paired t test, p < 0.05) higher arsenic concentrations than those from the other two areas. For example, the mean concentration of fish collected from Kandal (2,832 ng g−1, ww) was about 5.7 and 350 times higher than that in fish collected from Kampong Cham (mean 495 ng g−1, ww) and Kratie (mean 8.11 ng g−1, ww), respectively. This was in line with our previous study (Phan et al. 2010), and the surrounding countries such as Bangladesh (Das et al. 2004) which suggested areas that had higher amount of arsenic concentrations in groundwater also showed higher amount of arsenic in foodstuffs such as rice, fish and meat.
The highest and the lowest concentrations of arsenic were, respectively, noted in the fish (2,832 ± 1,606 ng g−1, ww) collected from Kandal province and the cattle stomach (1.86 ± 1.10 ng g−1, ww) collected from Kratie. As shown in Fig. 2, the arsenic concentrations in fish (mean 1,112 ng g−1, ww) were significantly (one-way ANOVA, p < 0.01) higher than other food items such as cereal, vegetable, meat and fruit collected from the three studied areas. Generally, the mean concentrations of total arsenic from high to low were in the order of: fish > cereal > viscera > diary > meat > vegetable > fruit. The results of the present study were in line with former studies conducted in USA (Schoof et al. 1999), UK (Al Rmalli et al. 2005), Mexico (Del Razo et al. 2002) and Bangladesh (Ohno et al. 2007) that high arsenic contents were observed in fish and seafood comparing to other foodstuffs.
In reference to other countries, the levels of arsenic concentrations in fish (mean 2,832 ng g−1, ww) collected from Kandal province were higher than those collected from Croatia (mean 498 ng g−1, ww) (Sapunar-Postruznik et al. 1996), Chile (mean 1,351 ng g−1, ww) (Munoz et al. 2005), UK (fish, 97 to 1,318 ng g−1, ww) (Al Rmalli et al. 2005), Mexico (ranged from 160 ng g−1 in freshwater fish to 2,360 ng g−1 in saltwater fish, ww) (Del Razo et al. 2002) and France (mean 1,920 ng g−1, ww) (Millour et al. 2011), and comparable with or lower than those collected from Taiwan (0.75–9.90 μg g−1, ww) (Liang et al. 2011). The fish collected from the other two areas, particularly from Kratie, the arsenic concentrations were much less than those collected from the above countries or regions. The arsenic concentrations in rice (247 ± 187 ng g−1, ww) collected from Kandal were significantly greater than that collected in the surrounding areas such as Kampong Cham, Cambodia (29.1 ± 24.0 ng g−1, ww, the present study), Bangladesh (136 ± 80 ng g−1, ww) (Das et al. 2004) and India (130 ± 60 ng g−1, ww) (Mondal and Polya 2008). Furthermore, the residual levels of arsenic exceeded the global “normal” range of 0.14 ng g−1 for arsenic concentrations in rice (WHO 2012). The arsenic concentrations in viscera (mean 122 ng g−1, ww) and meat (96.7 ng g−1, ww) collected from Kandal province were also higher than that collected from Chile (meat, 34 ng g−1, ww) (Munoz et al. 2005). The average concentrations of arsenic in vegetable (mean 66.1 ng g−1, ww) collected from Kandal were higher than those in vegetable collected from India (mean 20.9 ng g−1, ww) (Roychowdhury et al. 2003) and UK (ranged from 5–87 ng g−1, mean 24.2 ng g−1, ww) (Al Rmalli et al. 2005). Generally, the arsenic contamination in foodstuffs collected from Kandal was found to be severe when comparing other counties and regions.
Estimation of dietary intake
As there were large variations of arsenic levels detected in different types of food items even among the same food group, geometric means were used in the calculation of the mean levels of arsenic in the cereal, vegetable, meat and egg. The calculated dietary intakes were obtained by multiplying the amount and frequency of food consumed based on our food consumption survey, and the corresponding mean concentrations of arsenic detected in each food group (Xing et al. 2010). This approach has been previously applied for the investigation of dietary intake of pollutants such as organochlorine pesticides (OCPs) and lead via mixed food categories (Othman 2010; Skrbic and Predojevic 2008).
The concentrations, consumption rates and estimated daily intakes of arsenic in different food groups collected from Cambodia are listed in Table 2. The daily intake of arsenic via food consumption was 604, 9.70 and 136 μg day−1 in Kandal, Kratie and Kampong Cham, respectively. Based on the average body weight of local residents, the estimated daily intake (EDI) for the residents in Kandal, Kratie and Kampong Cham was 11.8, 0.19 and 2.52 μg kg−1 bw day−1, respectively. Former studies suggested the most two important pathways for arsenic exposure were dietary intake and drinking water (Alam et al. 2003; Steinmaus et al. 2005). For residents of these three areas, the intake of arsenic via drinking water ranged from 3.0 to 3.7, 0.05 to 0.11 and 0.004 to 0.005 μg kg−1 bw day−1 in Kandal, Kratie and Kampong Cham (Phan et al. 2010), respectively. Therefore, the dietary intake via food consumption was the dominant pathway for human exposure to arsenic for people in Cambodia, particularly for residents in Kampong Cham (accounting for more than 99 %). Our results indicated that the systematic monitoring programmes for arsenic in foodstuffs and drinking water in Cambodia should be instituted.
As shown as Fig. 3, our data indicated that daily intake of arsenic via foodstuffs in Cambodia, particularly for Kandal, was significantly higher than that of resident in Mexico, USA, Canada, UK, Germany, Japan and Australia. The arsenic daily intake in Kandal, Kratie and Kampong Cham provinces ranked No. 1, 16 and 9 among all the 17 compared counties and regions, respectively. The EDI for the residents in Kandal 11.8 μg kg−1 day−1 was much higher than the recommended provisional tolerable intake value recommend by WHO benchmark dose (BMDL0.5 = 3.0 μg kg−1 bw day−1) (JECFA 2011). The high daily intake values of arsenic in Cambodia were mainly due to the high concentrations of arsenic in food items, particularly for food items available in Kandal province. For residents of Kandal province, they are affected by the high concentrations of arsenic in groundwater (Phan et al. 2010) and heavy pesticide contamination in food (Wang et al. 2011).
The comparison of arsenic daily intake (μg day−1) in the three studied areas in Cambodia with other regions and countries. 1 Kandal, Cambodia (the present study), 2 Mexico (Del Razo et al. 2002), 3 Spain (Urieta et al. 1996), 4 Sindh, Pakistan (Arain et al. 2009), 5 Domkal blocks, India (Roychowdhury et al. 2002), 6 Jalangi, India (Roychowdhury et al. 2002), 7 Japan (Tsuda et al. 1995), 8 Bangladesh (Ohno et al. 2007), 9 Kampong Cham, Cambodia (the present study), 10 Norway (Meltzer et al. 1994), 11 USA (Gunderson 1991), 12 UK (MAFF 2000), 13 Sweden (Jorhem et al. 1998), 14 Canada (Dabeka et al. 1993), 15 Austria (Pfannhauser and Pechanek 1977), 16 Kratie, Cambodia (the present study), 17 Germany (Wilhelm et al. 2003). The red dash line represents the recommendation arsenic daily intake benchmark dose (BMDL0.5 3 μg kg−1 day−1 × 55 kg = 165 μg day−1) by WHO/FAO (JECFA 2011) (See online version for color)
We did not determine arsenic species (organic and inorganic arsenic compounds) in the food samples, although it is necessary from the toxicological point of view. Based on the previous reports on inorganic arsenic in food composites (Chowdhury et al. 2000; Dabeka et al. 1993; Heitkemper et al. 2001), inorganic arsenic may account for at least 50 % of the total arsenic in the studied foodstuffs in the present study. Therefore, the average daily dietary intakes of inorganic arsenic from foodstuffs in Kandal, Kratie and Kampong Cham were about 5.92, 0.094 and 1.26 μg kg−1 bw day−1, respectively. The value of residents in Kandal was much higher than the recommended benchmark dose (BMDL0.5 = 3.0 μg kg−1 bw day−1) (JECFA 2011).
Contribution of different food groups
The contributions of arsenic from different food items to the dietary intake are summarized in Fig. 4. Fish consumption contributed the greatest proportion of total arsenic intake in Kandal (about 63.0 %) and Kampong Cham (about 69.8 %). It might be due to that fish contained much higher concentrations of arsenic than other food items (Fig. 2). Particularly for fish collected from Kandal and Kampong Cham, the concentrations ranged from 1,609 to 4,650 ng g−1 and from 349 to 616 ng g−1, respectively. Our results were in line with previous studies showing that fish accounted 75–90 % of the total arsenic intakes via dietary source (Borak and Hosgood 2007; Das et al. 2004; Hughes 2006). For example, the seafood accounted for 90 % of daily arsenic intake in USA (Gunderson 1991), about 70 % in Canada (Dabeka et al. 1993) and about 60 % in Japan (Tsuda et al. 1995). Our previous study indicated that arsenic contamination in groundwater collected from Kratie was much higher than Kampong Cham (Phan et al. 2010). The site in Kampong Cham was selected as a reference area and less contaminated than the Kratie. However, the present results revealed that daily intakes of arsenic via foodstuffs in Kampong Cham were much higher than that in Kratie due to the high arsenic concentrations in fish collected from Kampong Cham. High concentrations of arsenic of edible fish might be due to the fact that arsenic-contaminated commercial fish produced by other areas was sold in this place. Therefore, much more attention should be paid to the daily intakes of arsenic via fish consumption although background information was available.
Cereals contributed to the second largest proportion of total arsenic intake in Kandal (27.5 %) and Kampong Cham (19.5 %). Previous studies revealed that anaerobic conditions together with excessive water will lead to the mobilization and an elevated accumulation of As in rice (Ma et al. 2008; Xu et al. 2008). Our previous study indicated that the groundwater collected from Kandal was extremely contaminated by arsenic (100 % detected As > 50 μg L−1) (Phan et al. 2010). The contribution of arsenic dietary intake via different food groups in the present study was similar with that in Bangladesh, where the highest contribution was found in seafood, followed by rice/rice cereal (Das et al. 2004).
Vegetables contributed the largest proportion of total arsenic intake in Kratie (62.4 %), due to the high consumption rates and high arsenic concentrations. Although the arsenic contents in all vegetable samples were found to below the food hygiene concentration limit of 1.0 μg g−1 in UK and Japan as described by Abedin et al. (2002), the higher consumption rate of vegetables according to local habits could also lead to higher intake of arsenic. The other food groups, such as meat, viscera and egg, contributed limited proportion of arsenic intake among all foodstuffs in the present study, which was in line with the former studies conducted in USA (Schoof et al. 1999) and Chile (Munoz et al. 2005).
In the present study, some limitations associated with the exposure can lead to uncertainty in the overall risk assessment. We did not determine the arsenic species (organic and inorganic arsenic compounds) in the studied foodstuffs. Other than the seafood (contains mainly non-toxic organic forms of arsenic and rapidly excreted through urine) (Fattorini et al. 2004), inorganic arsenic may be the major contribution of arsenic in many foods. Furthermore, the investigation in the present study only focused the selected food items and arsenic contamination. It is well known that drinking water is also an important pathway for human expose to arsenic (Kozul et al. 2009). Some other pollutants, such as OCPs, had also high concentrations in food samples collected from Cambodia (Wang et al. 2011). Considering that arsenic could accumulate in the organism and there were possibilities of synergistic effects when other pollutants are taken up simultaneously (Zhang et al. 2012), the arsenic contamination in food samples collected from Cambodia, especially from Kandal province, is an alarming public health concern. Our study, however, has provided a clear picture on the high dietary intakes of arsenic via food consumption in Cambodia, particularly in Kandal. More attention should be paid to food safety issues in this area. Furthermore, potential contamination in other provinces of Cambodia remains unclear and needs to be included in future studies.
References
Abedin, M. J., Feldmann, J., & Meharg, A. A. (2002). Uptake kinetics of arsenic species in rice plants. Plant Physiology, 128(3), 1120–1128.
Adams, M. A., Bolger, P. M., & Gunderson, E. L. (1994). Dietary intake and hazards of arsenic. In W. R. Chappell, C. O. Abernathy, & R. A. Calderon (Eds.), Arsenic: Exposure and health, science and technology letters (pp. 41–49). UK: Northwood.
Al Rmalli, S. W., Haris, P. I., Harrington, C. F., & Ayub, M. (2005). A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Science of the Total Environment, 337(1–3), 23–30.
Alam, M. G., Snow, E. T., & Tanaka, A. (2003). Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Science of the Total Environment, 308(1–3), 83–96.
Arain, M. B., Kazi, T. G., Baig, J. A., Jamali, M. K., Afridi, H. I., Shah, A. Q., et al. (2009). Determination of arsenic levels in lake water, sediment, and foodstuff from selected area of Sindh, Pakistan: Estimation of daily dietary intake. Food and Chemical Toxicology, 47(1), 242–248.
Berg, M., Stengel, C., Trang, P. T. K., Viet, P. H., Sampson, M. L., Leng, M., et al. (2007). Magnitude of arsenic pollution in the Mekong and Red River Deltas—Cambodia and Vietnam. Science of the Total Environment, 372(2–3), 413–425.
Borak, J., & Hosgood, H. D. (2007). Seafood arsenic: Implications for human risk assessment. Regulatory Toxicology and Pharmacology, 47(2), 204–212.
Buschmann, J., Berg, M., Stengel, C., & Sampson, M. L. (2007). Arsenic and manganese contamination of drinking water resources in Cambodia: Coincidence of risk areas with low relief topography. Environmental Science and Technology, 41(7), 2146–2152.
Buschmann, J., Berg, M., Stengel, C., Winkel, L., Sampson, M. L., Trang, P. T. K., et al. (2008). Contamination of drinking water resources in the Mekong delta floodplains: Arsenic and other trace metals pose serious health risks to population. Environment International, 34(6), 756–764.
Chowdhury, U. K., Biswas, B. K., Chowdhury, T. R., Samanta, G., Mandal, B. K., Basu, G. C., et al. (2000). Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environmental Health Perspectives, 108(5), 393–397.
Chu, H. A., & Crawford-Brown, D. J. (2006). Inorganic arsenic in drinking water and bladder cancer: A meta-analysis for dose-response assessment. International Journal of Environmental Research and Public Health, 3(4), 316–322.
Dabeka, R. W., McKenzie, A. D., Lacroix, G. M., Cleroux, C., Bowe, S., Graham, R. A., et al. (1993). Survey of arsenic in total diet food composites and estimation of the dietary intake of arsenic by Canadian adults and children. Journal of AOAC International, 76(1), 14–25.
Das, H. K., Mitra, A. K., Sengupta, P. K., Hossain, A., Islam, F., & Rabbani, G. H. (2004). Arsenic concentrations in rice, vegetables, and fish in Bangladesh: A preliminary study. Environment International, 30(3), 383–387.
Del Razo, L. M., Garcia-Vargas, G. G., Garcia-Salcedo, J., Sanmiguel, M. F., Rivera, M., Hernandez, M. C., et al. (2002). Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico. Food and Chemical Toxicology, 40(10), 1423–1431.
Fatmi, Z., Azam, I., Ahmed, F., Kazi, A., Gill, A. B., Kadir, M. M., et al. (2009). Health burden of skin lesions at low arsenic exposure through groundwater in Pakistan. Is river the source? Environmental Research, 109(5), 575–581.
Fattorini, D., Alonso-Hernandez, C. M., Diaz-Asencio, M., Munoz-Caravaca, A., Pannacciulli, F. G., Tangherlini, M., et al. (2004). Chemical speciation of arsenic in different marine organisms: Importance in monitoring studies. Marine Environmental Research, 58(2–5), 845–850.
Gunderson, E. L. (1991). FDA total diet study, July 1986-April Dietary intakes of pesticides, selected elements, and other chemicals. Journal of AOAC International, 78(6), 1353–1363.
Heitkemper, D. T., Vela, N. P., Stewart, K. R., & Westphal, C. S. (2001). Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. Journal of Analytical Atomic Spectrometry, 16(4), 299–306.
Hughes, M. F. (2006). Biomarkers of exposure: A case study with inorganic arsenic. Environmental Health Perspectives, 114(11), 1790–1796.
JECFA (2011). Evaluation of certain contaminants in food: Seventy-second report of the joint FAO/WHO expert committee on food additives. World Health Organization, p. 115.
Jorhem, L., Becker, W., & Slorach, S. (1998). Intake of 17 elements by Swedish women, determined by a 24-h duplicate portion study. Journal of Food Composition and Analysis, 11(1), 32–46.
Kozul, C. D., Hampton, T. H., Davey, J. C., Gosse, J. A., Nomikos, A. P., Eisenhauer, P. L., et al. (2009). Chronic exposure to arsenic in the drinking water alters the expression of immune response genes in mouse lung. Environmental Health Perspectives, 117(7), 1108–1115.
Lamm, S. H., Engel, A., Penn, C. A., Chen, R., & Feinleib, M. (2006). Arsenic cancer risk confounder in southwest Taiwan data set. Environmental Health Perspectives, 114(7), 1077–1082.
Li, H., Wu, C., Ye, Z. H., Wu, S. C., Wu, F. Y., & Wong, M. H. (2011). Uptake kinetics of different arsenic species in lowland and upland rice colonized with Glomus intraradices. Journal of Hazardous Materials, 194, 414–421.
Liang, C. P., Liu, C. W., Jang, C. S., Wang, S. W., & Lee, J. J. (2011). Assessing and managing the health risk due to ingestion of inorganic arsenic from fish and shellfish farmed in blackfoot disease areas for general Taiwanese. Journal of Hazardous Materials, 186, 622–628.
Llobet, J. M., Falco, G., Casas, C., Teixido, A., & Domingo, J. L. (2003). Concentrations of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. Journal of Agricultural and Food Chemistry, 51(3), 838–842.
Luu, T. T. G., Sthiannopkao, S., & Kim, K. W. (2009). Arsenic and other trace elements contamination in groundwater and a risk assessment study for the residents in the Kandal Province of Cambodia. Environment International, 35(3), 455–460.
Ma, J. F., Yamaji, N., Mitani, N., Xu, X. Y., Su, Y. H., McGrath, S. P., et al. (2008). Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proceedings of the National Academy of Sciences, 105(29), 9931.
MAFF (2000). Duplicate diet study of vegetarians-dietary exposures to 12 metals and other elements (sheet 193). Food surveillance information sheet. In: Ministry of Agriculture FaF (Hrsg.).
Meltzer, H. M., Mundal, H. H., Alexander, J., Bibow, K., & Ydersbond, T. A. (1994). Does dietary arsenic and mercury affect cutaneous bleeding time and blood lipids in humans? Biological Trace Element Research, 46(1–2), 135–153.
Millour, S., Noel, L., Kadar, A., Chekri, R., Vastel, C., Sirot, V., et al. (2011). Pb, Hg, Cd, As, Sb and Al levels in foodstuffs from the 2nd French total diet study. Food Chemistry, 126(4), 1787–1799.
Mondal, D., & Polya, D. A. (2008). Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: A probabilistic risk assessment. Applied Geochemistry, 23(11), 2987–2998.
Munoz, O., Bastias, J. M., Araya, M., Morales, A., Orellana, C., Rebolledo, R., et al. (2005). Estimation of the dietary intake of cadmium, lead, mercury, and arsenic by the population of Santiago (Chile) using a Total Diet Study. Food and Chemical Toxicology, 43(11), 1647–1655.
Ng, J. C., Wang, J., & Shraim, A. (2003). A global health problem caused by arsenic from natural sources. Chemosphere, 52(9), 1353–1359.
Ohno, K., Yanase, T., Matsuo, Y., Kimura, T., Rahman, M. H., Magara, Y., et al. (2007). Arsenic intake via water and food by a population living in an arsenic-affected area of Bangladesh. Science of the Total Environment, 381(1–3), 68–76.
Othman, Z. A. (2010). Lead contamination in selected foods from Riyadh city market and estimation of the daily intake. Molecules, 15(10), 7482–7497.
Pfannhauser W. & Pechanek U. (1977). Lebensm Ernaehr 30: 88.
Phan, K., Sthiannopkao, S., Kim, K. W., Wong, M. H., Sao, V., Hashim, J. H., et al. (2010). Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Research, 44(19), 5777–5788.
Roychowdhury, T., Tokunaga, H., & Ando, M. (2003). Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Science of the Total Environment, 308(1–3), 15–35.
Roychowdhury, T., Uchino, T., Tokunaga, H., & Ando, M. (2002). Survey of arsenic in food composites from an arsenic-affected area of West Bengal, India. Food and Chemical Toxicology, 40(11), 1611–1621.
Sapunar-Postruznik, J., Bazulic, D., & Kubala, H. (1996). Estimation of dietary intake of arsenic in the general population of the Republic of Croatia. Science of the Total Environment, 191(1–2), 119–123.
Schoof, R. A., Yost, L. J., Eickhoff, J., Crecelius, E. A., Cragin, D. W., Meacher, D. M., et al. (1999). A market basket survey of inorganic arsenic in food. Food and Chemical Toxicology, 37(8), 839–846.
Skrbic, B., & Predojevic, Z. (2008). Levels of organochlorine pesticides in crops and related products from Vojvodina, Serbia: Estimated dietary intake. Archives of Environmental Contamination and Toxicology, 54(4), 628–636.
Steinmaus, C., Carrigan, K., Kalman, D., Atallah, R., Yuan, Y., & Smith, A. H. (2005). Dietary intake and arsenic methylation in a U.S. population. Environmental Health Perspectives, 113(9), 1153–1159.
Sthiannopkao, S., Kim, K. W., Sotham, S., & Choup, S. (2008). Arsenic and manganese in tube well waters of Prey Veng and Kandal Provinces, Cambodia. Applied Geochemistry, 23(5), 1086–1093.
Tsuda, T., Inoue, T., Kojima, M., & Akoi, S. (1995). Market basket and duplicate portion estimation of dietary intake of cadmium, mercury, arsenic, copper, manganese, and zinc by Japanese adults. Journal of AOAC International, 78(6), 1363–1368.
Urieta, I., Jalon, M., & Eguilero, I. (1996). Food surveillance in the Basque Country (Spain). II. Estimation of the dietary intake of organochlorine pesticides, heavy metals, arsenic, aflatoxin M1, iron and zinc through the Total Diet Study, 1990/91. Food Additives & Contaminants, 13(1), 13–29.
Wang, H. S., Sthiannopkao, S., Du, J., Chen, Z. J., Kim, K. W., Mohamed Yasin, M. S., et al. (2011). Daily intake and human risk assessment of organochlorine pesticides (OCPs) based on Cambodian market basket data. Journal of Hazardous Materials, 192(3), 1441–1449.
WHO (2012). Proposed Draft Maximum Levels for Arsenic in Rice. Joint FAO/WHO Food Standards Programme Codex committee on contaminants in foods. Sixth Session Maastricht, The Netherlands, 26–30 March 2012, p. 16.
Wilhelm, M., Wittsiepe, J., Schrey, P., Lajoie-Junge, L., & Busch, V. (2003). Dietary intake of arsenic, mercury and selenium by children from a German North Sea island using duplicate portion sampling. Journal of Trace Elements in Medicine and Biology, 17(2), 123–132.
Xing, G. H., Wu, S. C., & Wong, M. H. (2010). Dietary exposure to PCBs based on food consumption survey and food basket analysis at Taizhou, China–The World’s major site for recycling transformers. Chemosphere, 81(10), 1239–1244.
Xu, X., McGrath, S., Meharg, A., & Zhao, F. (2008). Growing rice aerobically markedly decreases arsenic accumulation. Environmental Science and Technology, 42(15), 5574–5579.
Zhang, W., Zhang, M., An, S., Lin, K. F., Li, H., Cui, C. Z., et al. (2012). The combined effect of decabromodiphenyl ether (BDE-209) and copper (Cu) on soil enzyme activities and microbial community structure. Environmental Toxicology and Pharmacology, 34(2), 358–369.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 31101071), the China Postdoctoral Science Foundation (No. 2012M511868), the Research Grants Council of the University Grants Committee of Hong Kong (Collaborative Research Fund, HKBU1/CRF/08 and Special Equipment Grant, HKBU09) and the Mini-AOE (Area of Excellence) Fund from Hong Kong Baptist University. The authors thank International Environmental Research Center, Gwangju Institute of Science and Technology, Republic of Korea, United Nations University-International Institute for Global Health, Malaysia, Mr. Kongkea Phan, Dr. H. M. Leung and Mr. K. W. Chan for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, HS., Sthiannopkao, S., Chen, ZJ. et al. Arsenic concentration in rice, fish, meat and vegetables in Cambodia: a preliminary risk assessment. Environ Geochem Health 35, 745–755 (2013). https://doi.org/10.1007/s10653-013-9532-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-013-9532-0