Abstract
The presence of highly poisonous arsenic (As) elements in food concerns humans and animals. In Bangladesh, arsenic-contaminated groundwater is frequently utilized for agricultural irrigation. This is a significant source of arsenic pollution in the human food chain. For the first time, we investigated the presence of total arsenic in various foodstuffs obtained from 30 distinct agricultural eco-zones of Bangladesh to understand human exposure to arsenic through the food chain in Bangladesh. The greatest and lowest As concentrations were reported in fish among the examined dietary items (0.55 mg/kg, fw) and fruit (0.0068 mg/kg, fw), respectively. The results show that arsenic consumption from daily diet and food with drinking water was estimated to be 0.0352 mg/day for rural residents and 0.2002 mg/day for urban residents, respectively. The highest target hazard quotients (THQ) of arsenic in the fish samples surpassed the allowable limit (> 1), proving that fish are the primary dietary items influencing the possible danger to health. However, the target cancer risk (TR) from nutritional arsenic consumption was likewise higher than tolerable. A value of 10−4 indicates that Bangladeshi people are continuously exposed to arsenic, which has carcinogenic and non-carcinogenic dangers. Overall, our results highlight that people in Bangladesh are exposed to hazardous levels of arsenic throughout the food chain, which should be addressed to ensure the country’s food safety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a well-known hazardous contaminant that threatens water resources in developing countries (Islam et al. 2019). Because of its pervasiveness, it has the potential to modify soil, water, and plants, as well as other ecosystem components, and is detrimental to human health (Bundschuh et al. 2012; Proshad et al. 2017; Moulick et al. 2021). Arsenic enrichment in Bangladeshi groundwater is regarded as one of the most concerning environmental disasters (Reza et al. 2010). Groundwater As pollution with concentrations over the Bangladesh regulatory limit (0.05 mgL−1) has afflicted 59 of Bangladesh’s 64 administrative districts, causing arsenicosis sickness in the local population (Islam et al. 2021a). Terrestrial and aquatic biota can uptake arsenic from As-contaminated water and soil and enters the human body through foodstuffs (Bundschuh et al. 2012; Bhattacharya et al. 2009, 2013; Santra et al. 2013a, b; Islam et al. 2017a). Therefore, human exposure to arsenic found in food must be monitored because humans are one of the top consumers in the food chain.
Although arsenic is the most commonly found hazardous element in food, there is no indication that it is required for human health (Ahmed et al. 2016). Arsenic, both inorganic and organic, is found in foods, water, and other environmental components, and humans are exposed to it. In Bangladesh, foods such as rice, wheat, lentils, eggplant, carrot, potato, tomato, banana, beans, chili pepper, mango, onion, jackfruit, and different fish and meats are frequently used up by the people habitually (Islam et al. 2014a, 2015a, b). Inhalation can have a substantial impact in severely arsenic-contaminated areas, but for most individuals, food consumption is the predominant cradle of arsenic exposure (Kabir et al. 2021a, b). Arsenic’s entrance into the human body via food chain routes is widely documented worldwide (Zhang et al. 2011). The binding of numerous biological ligands to inactivate an enzyme system is the utmost collective practice of arsenic poisoning in humans (Shen et al. 2013). In contrast, chronic arsenic exposure causes cancers, diabetes mellitus, hyperkeratosis, peripheral vascular disease, restrictive lung disease, melanosis, hypertension, ischemic heart disease, and gangrene (Argos et al. 2010; Ahsan et al. 2006; Samal et al. 2013, 2021). Several of these health conditions are now affecting people in South and Southeast Asia, most notably in Indonesia, Pakistan, and Taiwan. Arsenic is a dangerous substance linked to skin and lung cancer in humans. (WHO 2001). Furthermore, eating arsenic-contaminated food can deplete the body’s critical nutrients and create significant health difficulties (Samal et al. 2010; Islam et al. 2014b). It should be emphasized that, while arsenic may alter chemical forms, it cannot be decomposed or eliminated.
The human repercussions of arsenic contamination of groundwater in Bangladesh are substantially inferior to those in any other country, as evidenced by exposure to drinking water and food. At its highest, about 26 million individuals drank water with a concentration of more than 50 μgL−1, while at its lowest, around 49 million people drank water with > 10 μgL−1 (Hasan et al. 2019). In Bangladesh, people (97%) have dug hundreds of wells to collect fresh groundwater for drinking, irrigation, fish culture in hatcheries, and domestic use (Ahmed et al. 2004, 2021; Bhattacharya et al. 2020). As a result, natural processes in the groundwater and excessive groundwater withdrawal due to overpopulation, the individuals who consume this contaminated water face a major detrimental threat. Approximately 27% of Bangladesh’s wells are seriously contaminated with elevated amounts of As (> 0.05 mgL−1) (Anawar et al. 2002). In Bangladesh, arsenic-enriched groundwater is the focal point of As amassing in the food chain through the water-soil-crop-food system (Das et al. 2004; Rahman et al. 2012, 2013; Islam et al. 2017b).
Inadequate surveillance of input routes, lack of knowledge of hazard measures, limited knowledge of regulatory frameworks, and poor administration of manufacturing effluents are the leading causes of hazardous trace element pollution of the environment and food in Bangladesh (Rahman et al. 2012, 2013; Islam et al. 2017a). However, legislative flaws relating to arsenic contamination are not a top concern in Bangladeshi research. The evaluation of daily dietary arsenic exposure and health hazards based on daily food ingesting research is critical for Bangladesh (FAO 2006; Williams et al. 2006; Islam et al. 2017a, b, 2019). Although a few publications have explored the presence and toxicity profile of arsenic in Bangladesh’s food chain, soil, water, and other environmental compartments (Ahmed et al. 2019; Islam et al. 2018, 2021a,b), the primary concerns in terms of risk factors, policy, and research gaps have received little attention. Arsenic pollution and associated biogeochemistry are an extensive study topic regarding exposure and health consequences worldwide. Furthermore, arsenic poisoning, exposure lethality, gaps in updated research and coordination among the government and public, and factors responsible for increasing food security in Bangladesh are seldom investigated, and toxicity data of arsenic from foods is inadequate to estimate health hazards in Bangladesh. Hence, the purposes of this study were to (i) quantify the concentration of arsenic in foodstuffs consumed by both rural and urban people of Bangladesh living in 30 agro-ecological zones of the country; (ii) estimate the highest target hazard quotients (THQ) of arsenic in the food items; and (iii) estimate target cancer risks (TR) from ingesting dietary arsenic. Our study, for the first time, facilitated well-being and excellent valuations of the local food chain for predicting the potential fitness risk concerning dietary consumption, non-carcinogenic, and target cancer risk for the urban and rural adult inhabitants of Bangladesh.
Materials and methods
Study area
Bangladesh forms the world’s largest delta at 88o10′ to 92o41′ East and 20o34′ to 26o38′ North. The great delta is flat from the Himalayas in the north to the Bay of Bengal in the south. The Meghna, Padma, Jamuna, Karnafuli, and tributaries wash the plain. Monsoon rains soak the land and rivers. Summer rains overflow their banks, flooding low, and outlying areas annually. Two elevated tracts, Modhupur and Barind, and rows of hilly forests relieve the monotony of flatness inland. The great plain is nearly sea level along the southern coast and rises gradually northward. Rangamati Hill district’s Keocradang Hill is 4034 feet above sea level. Topography can be divided into five classes.
Bangladesh’s agro-ecological zones are based on physiography, soils, floods, and agro-climatology. Bangladesh's agro-ecological zones total 30. These 30 zones are organized into 88 agro-ecological sub-regions and 535 units (Banglapedia 2014). Physiography is the mix of soil geology and landscape. It defines Bangladesh’s agro-ecological regions. The agroecological zone’s water resource system is determined by its relationship to the Ganges–Brahmaputra-Meghna (GBM) basin and the Bay of Bengal. These zones have a humid subtropical climate. The average maximum and lowest temperatures are 43 °C and 3.6 °C, with an average annual rainfall of 2400 mm (Das and Islam 2021). The GBM delta’s Pleistocene and Holocene alluvial deposits constitute the world’s most prolific aquifer. Annual monsoons and floods refresh much of the delta system.
Sample collection and preparation
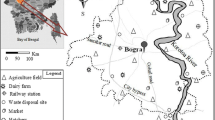
A total of 325 samples of rice and wheat grains; nine vegetables such as lentil, eggplant, carrot, bean, potato, tomato, onion, and chili pepper; three different fruits like banana, mango, and jackfruit; three different fish species like rui, pangas, and tilapia; and chicken eggs, cow milk, and chicken meat were gathered from the country and urban markets throughout Bangladesh’s 30 agro-ecological zones (AEZ) (Fig. 1). The sample took place between January and December of 2017. Collected samples were labeled properly and wrapped in polythene bags and brought to the University of Dhaka’s Institute of Nutrition and Food Science (INFS). Each food item was thoroughly washed in the laboratory using deionized water. The moisture contents in plant components were calculated by recording the fresh and dried weights. In a food processor, a composite sample for each food item was prepared and homogenized, and 50 g test samples were collected and dried at 65–75 °C to achieve a consistent weight. The pre-processed samples were then sent to Yokohama National University in Japan, where they were kept in an airtight, clean zip-lock bag in the freezer until chemical analysis.
Sample extraction procedure
Ultrapure water and analytic reagent-grade compounds were used to make all of the solutions. A microwave (Bergh of Microwave MWS-2, Germany) was used for sample digestion. 0.2 g of dried powdered material was processed with 6 mL of 69% HNO3 and 2 mL of 30% H2O2 for extraction (Wako Chemical Co, Japan) in a Teflon vessel (DAP-60 K) of microwave digestion system (Islam et al. 2022a). Three-step digestion procedures were used: step 1, 180 °C temperature and 85% power were held constant for 15 min; step 2, 200 °C temperature with 90% power for 15 min; and step 3, 100 °C temperature and 40% power for 10 min to lower the temperature. After digestion, Teflon containers were dipped in cold water to alleviate any leftover pressure within the vessel. The samples were moved to a Teflon beaker, and the total volume was increased to 25 mL using MilliQ water (Elix UV5 and MilliQ, Millipore, USA). After filtering (with a DISMIC®—25HP PTTF syringe filter with a pore size of 0.45 mm), the digest solution was maintained in a plastic crew cap tube (Islam et al. 2022b,c,d).
Instrumental analysis and quality control
An inductively coupled plasma mass spectrometer was then used to test the samples for arsenic (ICP-MS, Agilent 7700, USA). Spex Certi Prep® USA supplied standard solutions covering 10 g/L of internal standards such as indium, yttrium, beryllium, cobalt, and titanium were used to construct the standardization curve. For calculating concentration, calibration curves with an R2 > 0.999 were allowed. Working standards were generated and utilized daily in 5% (v/v) HNO3 at 69% ultrapure grade. If all test batches satisfied the stated internal quality controls, they were examined and verified using an internal quality methodology (IQCs). To check the accuracy of the instrument analysis and standard reference material of INCT-CF-3, corn flour was analyzed for arsenic concentration, and data for certified value (0.01 mg/kg) and measured value (0.0098 ± 0.001 mg/kg) was checked. The recovery percentage of the arsenic estimation was 98%, indicating that the instrumentation procedure was accurate for estimating arsenic from the samples.
Data calculations
Estimated daily intakes of arsenic
Estimated daily intakes (EDI) for arsenic were calculated by the following formula:
where FIR denotes the food intake rate (g/person/day), C denotes the arsenic amount in samples (mg/kg, fresh weight (fw), and BW is the body weight considered to be 60 kg for adult occupants in the current study (FAO 2006). On a fresh weight basis, the adult population’s daily food intake rate was determined (HIES 2017).
Non-carcinogenic risk
The non-carcinogenic risk {(target hazard quotient (THQ)} of arsenic was calculated using the USEPA region III risk-based concentration table (USEPA 2010) as follows:
where EFr is the frequency of exposure (365 days per year); ED is the length of exposure (70 years), which corresponds to the average human life time (USEPA 1991); and RfD is the oral reference dosage (mg/kg/day); BW is the average body weight (adult, 60 kg); and AT is the averaging time for non-carcinogens (365 days per year number of exposure years, assuming 70 years). Arsenic’s oral reference dosage was 0.0003 mg/kg/day (USEPA 2010). If the THQ is less than one, arsenic exposure is unlikely to influence the exposed population’s health negatively. The THQ value is one or above that indicates a possible health concern (Wang et al. 2005), and treatments and preventative measures to reduce arsenic exposure in meals would be implemented.
Carcinogenic risk
The carcinogenic risk of arsenic was used to assess the incremental likelihood of an adult developing cancer as a result of lifetime exposure to that likely carcinogen (i.e., incremental or excess individual lifetime cancer risk; USEPA 1989). The following formula (USEPA 1989) was adopted to calculate the target carcinogenic risk:
According to the USEPA (2010), the TR for arsenic was 1.5 (mg/kg/day)−1, where TR denotes the objective carcinogenic risk or lifelong cancer risk and CSFo denotes the oral carcinogenic slope factor.
Statistical analysis
Microsoft Excel 2016 computed arsenic levels’ averages and standard deviations in food samples. Arsenic datasets are tested for normality using the Kolmogorov–Smirnov test.
Results and discussion
Arsenic in foods
The study’s major focus is on Bangladesh’s rural and urban inhabitants’ total exposure to arsenic from food and consumption of water, as well as their health effects from consuming arsenic-contaminated foods. Cumulative arsenic levels (mg/kg fw) in foods widely consumed by Bangladeshi people are evaluated in this study, and the results are shown in Table 1. On a fresh bulk basis, the mean arsenic contents in the dietary groups were 0.0133 (cereals), 0.0072 (vegetables), 0.0068 (fruits), 0.557 (fish), 0.012 (egg), 0.0489 (milk), and 0.0426 (meat). Rahman et al. (2014) observed that the total arsenic concentration in different varieties of Australian rice was 0.270 and Bangladeshi rice was 0.073 mg/kg dw, which was identical to the present study (Table 1). Total arsenic concentration in Brazilian rice was 0.212 mg/kg dw (Batista et al. 2011; Ciminelli et al. 2017); Indian rice was 0.094 mg/kg dw; Chinese rice was 0.128 mg/kg dw (Chen et al. 2018); and Pakistan was 0.083 mg/kg dw and other countries (Table 1) which was in line with the current study. In this study, arsenic concentration in wheat was 0.055 mg/kg which was comparable with the other studies such as in China 0.050 mg/kg (Adomako et al. 2011); India 0.027 mg/kg (Kumar et al. 2016); Spain 0.086 mg/kg (Matos-Reyes et al. 2010); Scotland, UK 0.030 mg/kg (Williams et al. 2007); and Cornwall, UK 0.070 mg/kg (Williams et al. 2007) (Table 1). In the current study, As concentration was observed in wheat as 0.055 mg/kg, which was slightly higher than the previous study conducted by Adomako et al. (2011) in China (0.050 mg/kg); Kumar et al. (2016) in India (0.027 mg/kg); Cubadda et al. (2016) in Italy (0.009 mg/kg); Williams et al. (2007) in Scotland, UK (0.030 mg/kg) and lower than the study by Islam et al. (2017a, b) in Bangladesh (0.26 mg/kg), Bhattacharya et al. (2010) in India (0.129 mg/kg), and Matos-Reyes et al. (2010) in Spain (0.086 mg/kg) (Table 1).
The fish (0.557 mg/kg, fw) and fruits (0.0068 mg/kg, fw) had the highest and lowest levels of arsenic, respectively. Food intake could be a crucial pathway for arsenic bioaccumulation in fish (Zhang et al. 2011). The overall arsenic quantities in seafood, such as fish, are slightly higher in Cambodian meals than in other cuisines (Wang et al. 2013), the UK (Al Rmalli et al. 2005), China (Li et al. 2011a, b), and other studies in Bangladesh (Ohno et al. 2007; Das et al. 2004). As concentration in fish in the present study was comparable to the previous studies conducted by Ciminelli et al. (2017) in Brazilian fish at 0.233 mg/kg dw, in China at 0.038 mg/kg dw) (Wu et al. 2014), and in Bangladesh at 2.18 mg/kg dw (Ali et al. 2022) (Table 1).
In general, the mean arsenic concentrations of the dietary items studied were in the following order: fish > milk > meat > cereal > egg > vegetable > fruit. Arsenic concentration varied among the analyzed foodstuffs. This might be owing to variances in arsenic absorption and accumulation capacities, and changes in the development periods and rates of the dietary items (Islam et al. 2014b), and climatic variations for the vast areas of food production in Bangladesh (Santos et al. 2004). All cereals, vegetables, fruits, fish, eggs, milk, and meat samples tested had mean arsenic amounts below the acceptable limit (0.5 mg/kg) (Codex 2001; Table 1). On a dry weight basis, previous investigations have revealed arsenic concentration in eggplant of 0.2 mg/kg and potato of 0.01 mg/kg (Alam et al. 2003). Another study in Bangladesh depicted the arsenic concentration as 0.0545 mg/kg in some vegetable species (Al Rmalli et al. 2005). In Bangladesh, two investigations found mean arsenic concentrations in vegetables of 0.2 (0.009–7.9 mg/kg dw) (Islam et al. 2015a) and 0.05 (0.01–0.2 mg/kg dw) (Rahman et al. 2013). In previous studies, arsenic concentrations were reported on as dry weight basis, which showed slightly higher than the present study. If those studies could convert the concentration of arsenic in foods, then it would be identical to the present study.
Human health implications
Estimated daily intake of arsenic
The nutritional occurrence approach for arsenic from popular meals is a trustworthy instrument for examining a public meal in terms of nutrients, bioactive chemicals, and pollutant ingestion, supplying vital information on potential nutritional shortages or sufficient (exposure) to dietary contaminants such as arsenic (WHO 1985). This study assesses dietary arsenic consumption through commonly consumed daily products in the Bangladeshi population’s daily diet, including rural and urban residents. Table 2 displays arsenic content, rates of food consumption, and anticipated arsenic intakes for the studied food items. The amount of arsenic consumed through food was 0.0352 mg/day in rural and 0.0427 mg/day in urban populations, respectively (Alam et al. 2003; Steinmaus et al. 2005). The total arsenic consumption estimates were based on the sum of arsenic absorbed from a range of meals and drinking water consumed by an adult resident (average body mass of 60 kg) during 24 h. Assuming a daily water consumption of 3 L and an arsenic concentration of 0.055 mg/L (DPHE 2001), the total daily consumption of arsenic would be 0.2002 and 0.2077 mg/day for the rural and urban residents, respectively (Table 2), by a factor of two over the FAO limit, suggesting a considerable risk. Our research found that urban residents’ daily arsenic exposure to foods was substantially higher than that of rural residents. The EDI for urban residents at 0.2077 mg/day/person was much greater than the WHO benchmark dose (BMDL0.5 = 0.118 mg/day/person), suggesting an interim tolerated intake value (JECFA 2011). According to this analysis, high daily arsenic consumption in urban dwellers was primarily attributable to the increased consumption of fish, meat, milk, and fruits, all of which contain significant levels of arsenic. The estimated daily intake of arsenic from foods of the current study is compared with some other studies in Bangladesh and other countries in the world as presented in Table 3. The results showed that EDI of arsenic of the present study was slightly higher than the studies in Bangladesh (Islam et al. 2017a), Mexico (Del Razo et al. 2002), Japan (Tsuji et al. 2007), and Ghana (Adomako et al. 2011), whereas slightly higher than other studies in the world, i.e., Bangladesh (Rahmana et al. 2008), India (Samal et al. 2011, 2021), Iraq (Mustafa 2023), China (Fu et al. 2007), and Spain (Martí-Cid et al. 2008) (Table 3).
Non-carcinogenic and carcinogenic risks
Table 4 displays the non-carcinogenic risk (THQ) and carcinogenic risk (TR) of arsenic in the examined food items in Bangladesh. Based on comprehensive data on dietary arsenic in the daily diet, the predicted THQ and TR standards effectively indicate the health hazard below actual sites. The result showed that the maximum THQ of As in fish samples for both rural and urban residents was above the safe level (> 1) by a factor of two (2), indicating that fish is the principal dietary item causal to the possible health risk. Considering all examined food items, THQ standards of arsenic for rural and urban inhabitants were 1.95 and 2.37 (> 1.0), showing a possible non-carcinogenic danger to individuals in Bangladesh. As a result, the health concerns posed by inorganic arsenic exposure in everyday consumable foods are a source of anxiety in Bangladesh.
The target carcinogenic risk factors (TR) ranged from 8.3 per 10,000 to 9.5 per one million people (Table 4). The highest safe criterion for cancer risk is one per 10,000, while the lowest is one per one million. All of the expected target cancer hazards from eating arsenic have been confirmed exposure exceeded 10−6, and urban persons had a greater risk than rural residents. According to Table 4, inhabitants of metropolitan areas may be exposed to high levels of dietary arsenic through fish eating, resulting in lifetime risk of cancer. Human consumption of arsenic-contaminated farmed fish poses health hazards, according to Kar et al. (2011). Li et al. (2011a, b) stated the lowermost cancer risk of 123 per 100,000 persons and the uppermost cancer risk of 201 per 100,000 persons due to arsenic exposure from low land and upland rice.
The toxic properties of arsenic depend on the species and chemical form. Chronic arsenic poisoning indications appear gradually and rely on the dose quantity and the exposure length (Banerjee et al. 2011; Sarkar 2009). Numerous studies have indicated clinical signs such as nausea, diarrhea, anorexia, and abdominal pain (Smith et al. 2000). Cumulative arsenic poisoning has been related to several pathways that induce DNA damage. According to a study conducted in West Bengal, India, defects in DNA repair ability, alteration of methylation of the promoter regions of the p53 and p16 genes, and genomic methylation change may all play a role in arsenic-induced disease manifestation in humans. Arsenic-induced keratosis has been linked to an increased prevalence of arsenic-induced keratosis. Among the numerous genes involved in the control of arsenic metabolism, purine nucleoside phosphorylase single-nucleotide polymorphisms were associated with an increased frequency of arsenicosis (Mazumder and Dasgupta 2011). Arsenic acts as a cancer promoter rather than a starter, causing cancer, and cancer risk in the human body is dose-dependent. Bowen’s disease, basal cell carcinoma, and squamous cell carcinoma are all arsenical skin cancers induced by arsenic exposure (Santra et al. 2013a, b). The great bulk of Bangladeshi individuals continues to use water with high arsenic content for household activities, putting them at risk of developing arsenic skin lesions and cancer due to their overall dietary consumption of arsenic through food.
Contribution of arsenic exposure from food items.
Figure 2 highlights the dietary contributions of arsenic from various food sources consumed. In both rural (72%) and urban (78%) areas, fish consumption accounted for the highest share of total arsenic ingestion. This may be because fish contain substantially higher levels of arsenic than other foods (Tables 1 and 2). Our findings were consistent with prior research, which found that fish contributed 75–90% of cumulative arsenic consumption from dietary sources (Das et al. 2004; Hughes 2006; Borak and Hosgood 2007). Cereals accounted for the second greatest amount of complete arsenic consumption in both rural (18%) and urban (12%) areas (Fig. 2). A previous study has reported that higher arsenic levels in rice (Ma et al. 2008; Xu et al. 2008) may be linked to high arsenic levels in agricultural field soil and substantial use of arsenic-contaminated irrigation water for rice agriculture.
Das et al. (2004) found 16.676.61 mg/kg of arsenic in Bangladesh soil, which was greater than the global average of 10 mg/kg. They also discovered a good association between arsenic in polluted water and arsenic on soils (r = 0.74, p = 0.01). In the current study, the proportion of arsenic food consumption across several food groupings was equivalent to that of Cambodia, where the significant amount originated in fish, trailed in cereal (Wang et al. 2013). In the current study, vegetables, fruits, meat, milk, and eggs supplied a minor amount of arsenic during food ingestion, consistent with previous investigations by Wang et al. (2013) in Cambodia and Munoz et al. (2005) in Chile.
According to the ecosystem approach, the Bangladeshi population lives in complicated food chain pathways that are, of course, interconnected. Figure 3 displays the many food chain channels that expose Bangladeshis to arsenic poisoning, from source to food. Humans are one of the principal consumers of arsenic from rice, vegetables, fish, milk, and meat, and arsenic buildup in humans may occur via other sources of the environment such as soil–plant-human’s and/or plant-animal-human’s and/or soil–water-animal’s food chain pathways (Fig. 3).
Limitations and environmental implication
The authors of this study believe that there are several limitations associated with arsenic exposure through food, which may add to the risk assessment ambiguity. We could not distinguish between different types of arsenic in the foods included in our investigation. Apart from seafood (essentially non-toxic organic arsenic forms readily removed by urine) (Fattorini et al. 2004), inorganic arsenic may be the principal source of arsenic exposure from various foods. Furthermore, the current study only looked at a few of the most widely eaten foods and beverages and drinking water. Foods were usually boiled before being consumed in Bangladeshi families. Cooking and drinking water are generally done with the same water. As a result, more dietary arsenic would be present in their final diet, which we did not account for in our study. Despite certain limitations, we believe the findings of this study will be significant in conducting future in-depth epidemiological research that will cover population contact with numerous arsenic species and organic and other inorganic contaminants.
Conclusions
This study determined the concentrations of As in the commonly used foodstuffs covering 30 AEZ in Bangladesh. It estimated the highest (THQ) and target cancer risks (TR) from ingesting dietary arsenic. Our work showed that an elevated concentration of arsenic in the fish meal exceeded the maximum permitted limit in food. The mean arsenic concentrations of the dietary items studied were in the following order: fish > milk > meat > cereal > egg > vegetable > fruit. The total daily intake of arsenic from some of the studied food items was more than the (MTDI), indicating a significant risk to human health. The total THQ and TR values for arsenic from all food items surpassed the threshold values (THQs > 1, TR > 10−4), suggesting that people in Bangladesh have both non-carcinogenic and carcinogenic health hazards due to dietary arsenic exposure. A comprehensive management strategy for the entire arsenic contamination scenario is required, which includes surface water conservation and custom, rainfall collection, and the prudent use of pure groundwater for farming, aquaculture, and home needs. Because of the prolonged use of arsenic-contaminated groundwater for crop production, the soil is expected to increase the quantity of arsenic in foods through bioconcentration. To fix the unexpected problem of arsenic contamination in Bangladesh, there needs to be a regular monitoring system for the whole food chain.
Data availability
Not applicable.
Change history
24 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11356-022-24389-4
References
Adomako EE, Williams PN, Deacon C, Meharg AA (2011) Inorganic arsenic and trace elements in Ghanaian grain staples. Environ Pollut 159:2435–2442
Ahmed FT, Khan AHAN, Khan R, Saha SK, Alam MF, Dafader NC, Sultana S, Elius IB, Mamum SA (2021) Characterization of arsenic contaminated groundwater from central Bangladesh: irrigation feasibility and preliminary health risks assessment. Environ Nanotechnol Monit Manag 15:100433. https://doi.org/10.1016/j.enmm.2021.100433
Ahmed MK, Bhattacharya P, Hasan MA, Akhter SH, Alam SMM, Bhuyian MAH, Imam MB, Khan AA, Sracek O (2004) Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Appl Geochem 19:181–200. https://doi.org/10.1016/j.apgeochem.2003.09.006
Ahmed MK, Shaheen N, Islam MS, Islam S, Islam MM, Kundu GK, Bhattacharjee L (2016) A comprehensive assessment of arsenic in commonly consumed foodstuffs to evaluate the potential health risk in Bangladesh. Sci Total Environ 544:125–133
Ahmed MK, Shaheen N, Islam MS, Al-mamun MH, Islam S, Mohiduzzaman M, Bhattacharjee L (2015) Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128:284–292
Ahmed N, Bodrud-Doza M, Islam ARMT, Hossain S, Moniruzzaman M, Dev N, Bhuiyan MAQ (2019) Appraising spatial variations of As, Fe, Mn and NO3 contaminations associated health risks of drinking water from Surma basin, Bangladesh. Chemosphere 218:726–740. https://doi.org/10.1016/j.chemosphere.2018.11.104
Ahsan H, Chen Y, Parvez F, Zablotska L, Argos M, Hussain I, Momotaj H, Levy D, Cheng Z, Slavkovich V, van Geen A, Howe GR, Graziano JH (2006) Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the health effects of arsenic longitudinal study. Am J Epidemiol 163(12):1138–1148
Ali MM, Ali ML, Bhuyan MS, Islam MS, Rahman MZ, Alam MW, Das M, Mustary S, Islam MN (2022) Spatiotemporal variation and toxicity of trace metals in commercially important fish of the tidal Pasur River in Bangladesh. Environmental science and pollution research https://doi.org/10.1007/s11356-022-18821-y
Al Rmalli SW, Haris PI, Harrington CF, Ayub M (2005) A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Sci Total Environ 337(1–3):23–30
Alam MG, Snow ET, Tanaka A (2003) Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ 308(1–3):83–96
Anawar HM, Akai J, Mostofa KM, Safiullah S, Tareq SM (2002) Arsenic poisoning in groundwater: health risk and geochemical sources in Bangladesh. Environ Int 27:597–604
Argos M, Kalra T, Rathouz P, Chem Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zama M, Hasan R, Sarwar G, Slavkovich V, van Geen A, Graziano J, Ahsan H (2010) Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376:252–258
Argos M, Parvez F, Chen Y, Hussain AZ, Momotaj H, Howe GR (2007) Socioeconomic status and risk for arsenic-related skin lesions in Bangladesh. Am J Public Health 97:825–831
Banerjee N, Nandy S, Kearns JK, Bandyopadhyay AK, Das JK, Majumder P, Basu S, Banerjee S, Sau TJ, States JC, Giri AK (2011) Polymorphisms in the TNF-a and IL10 gene promoters and risk of arsenic-induced skin lesions and other nondermatological health effects. Toxicol Sci 121(1):132–139
Batista BL, Souza JMO, Souza SS, Barbosa J (2011) Speciation of As in rice and estimation of daily intake of different As species by Brazilians through rice consumption. J Hazard Mater 191:342–348
Banglapedia (2014) Agroecological Zone. Banglapedia, National Encyclopedia of Bangladesh. Asiatic Society of Bangladesh, Dhaka
Bhattacharya P, Adhikari S, Samal AC, Das R, Dey D, Deb A, Ahmed S, Hussein J, De A, Das A, Joardar M, Panigrahi AK, Roychowdhury T, Santra SC (2020) Health risk assessment of co-occurrence of toxic fluoride and arsenic in groundwater of Dharmanagar region, North Tripura (India). Groundwater for Sustainable Development https://doi.org/10.1016/j.gsd.2020.100430
Bhattacharya P, Samal AC, Majumdar J, Santra SC (2010) Arsenic contamination in rice, wheat, pulses, and vegetables: a study in an As affected area of West Bengal, India. Water Air Soil Pollut 213:3–13
Bhattacharya P, Samal AC, Majumdar J, Santra SC (2009) Transfer of arsenic from groundwater and paddy soil to rice plant (Oryza sativa L.): a micro level study in West Bengal India. World J Agri Sci 5(4):425–431
Bhattacharya P, Samal AC, Majumdar J, Banerjee S, Santra SC (2013) In vitro assessment on the impact of soil arsenic in the eight rice varieties of West Bengal, India. J Hazard Mater 262:1091–1097
Borak J, Hosgood HD (2007) Seafood arsenic: implications for human risk assessment. Regul Toxicol Pharmacol 47(2):204–212
Bundschuh J, Nath B, Bhattacharya P, Liu CW, Armienta MA, López MVM, Lopez DL, Jean JS, Cornejo L, Macedo LFL, Filho AT (2012) Arsenic in the human food chain: the Latin American perspective. Sci Total Environ 429:92–106
Chen H, Tang Z, Wang P, Zhao FJ (2018) Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ Pollut 238:482–490
Ciminelli VST, Gasparon M, Ng JC, Silva GC, Caldeira CL (2017) Dietary As exposure in Brazil: The contribution of rice and beans. Chemosphere 168:996–1003
Codex Alimentarius Commission [FAO/WHO] (2001) Food additives and contaminants. Joint FAO/WHO Food Standards Program. ALINORM 01/12A, pp. 1e289. ftp://ftp.fao.org/Codex/Reports/Alinorm01/al01_27e.pdf
Cubadda F, D’Amato M, Aureli F, Raggi A, Mantovani A (2016) Dietary exposure of the Italian population to inorganic arsenic: the 2012–2014 total diet study. Food Chem Toxicol 98:148–158
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentrations in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30(3):383–387
Das S, Islam ARMT (2021) Assessment of mapping of annual average rainfall in a tropical country like Bangladesh: remotely sensed output vs. kriging estimate. Theory Appl Climatol 146:111–123. https://doi.org/10.1007/s00704-021-03729-3
Del Razo LM, Garcia-Vargas GG, Garcia-Salcedo J, Sanmiguel MF, Rivera M, Hernandez MC, Cebrian ME (2002) Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico. Food Chem Toxicol 40:1423–1431
FAO (Food and Agriculture Organization) (2006) Arsenic contamination of irrigation water, soil and crops in Bangladesh: risk implications for sustainable agriculture and food safety in Asia. Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific, Bangkok, Thailand
FAO/WHO (2011) Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods, Food CF/5 INF/1. Fifth Session. The Hague, The Netherlands. ftp://ftp.fao.org/codex/meetings/CCCF/cccf5/cf05_INF.pdf
Fattorini D, Alonso-Hernandez CM, Diaz-Asencio M, Munoz-Caravaca A, Pannacciulli FG, Tangherlini M, Regoli F (2004) Chemical speciation of arsenic in different marine organisms: importance in monitoring studies. Mar Environ Res 58(2–5):845–850
Fu J, Zhou Q, Liu J, Liu W, Wang T, Zhang Q, Jiang G (2007) High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere 71:1269–1275
Hasan MK, Shahriar A, Jim KU (2019) Water pollution in Bangladesh and its impact on public health. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e02145
HIES (2017) Preliminary report on household income and expenditure survey 2016. Bangladesh Bureau of Statistics, Ministry of Planning, Govt. of the People’s Republic of Bangladesh, Dhaka-1000, Bangladesh
Hughes MF (2006) Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect 114(11):1790–1796
Islam MM, Karim MR, Zheng X, Li X (2018) Heavy metal and metalloid pollution of soil, water and foods in Bangladesh: a critical review. International Journal of Environmental Research and Public Health 15(12). https://doi.org/10.3390/ijerph15122825
Islam MS, Ahmed MK, Al-Mamun MH (2014a) Heavy metals in cereals and pulses: health implications in Bangladesh. J Agric Food Chem 62:10828–10835
Islam MS, Idris AM, Islam ARMT, Ali MM, Rakib MRJ (2021a) Hydrological distribution of physicochemical parameters and heavy metals in surface water and their ecotoxicological implications in the Bay of Bengal coast of Bangladesh. Environmental Science and Pollution Research https://doi.org/10.1007/s11356-021-15353-9
Islam ARMT, Bodrud-Doza M, Rahman MS, Amin SB, Chu R, Mamun HA (2019) Sources of trace elements identification in drinking water of Rangpur district, Bangladesh and their potential health risk following multivariate techniques and Monte-Carlo simulation. Groundw Sustain Dev 9:100275
Islam MS, Ahmed MK, Al-Mamun MH (2015a) Determination of heavy metals in fish and vegetables and health implications in Bangladesh. Hum Ecol Risk Assess Int J 21:986–1006
Islam MS, Ahmed MK, Al-Mamun MH, Eaton DW (2017a) Arsenic in the food chain and assessment of population health risks in Bangladesh. Environ Syst Decision 37(3):344–352
Islam ARMT, Shen S, Bodrud-Doza M (2017b) Assessment of arsenic health risk and source apportionment of groundwater pollutants using multivariate statistical techniques in Chapai-Nawabganj district, Bangladesh. J Geol Soc India 90(2):239–248. https://doi.org/10.1007/s12594-017-0705-9
Islam MS, Ahmed MK, Al-Mamun MH, Islam KN, Ibrahim M, Masunaga S (2014b) Arsenic and lead in foods: a potential threat to human health in Bangladesh. Food Addit Contam Part A 31(12):1982–1992
Islam MS, Ahmed MK, Al-Mamun MH, Raknuzzaman M (2015b) The concentration, source and potential human health risk of heavy metals in the commonly consumed foods in Bangladesh. Ecotoxicol Environ Saf 122:462–469
Islam MS, Kormoker T, Mazumder M, Anika SE, Islam MT, Hemy DH, Mimi US, Proshad R, Idris AM, Kabir MH (2021b) Trace elements concentration in soil and plant within the vicinity of abandoned tanning sites in Bangladesh: an integrated chemometric approach for health risk assessment. Toxin Reviews https://doi.org/10.1080/15569543.2021b.1925919
Islam MS, Phoungthong K, Ali MM, Ibrahim KA, Idris AM (2022a) Assessing risk to human health for potentially toxic elements in farmed and wild giant tiger prawn (Paeneas monodon) in the coastal area of Bangladesh. International Journal of Environmental Analytical Chemistry https://doi.org/10.1080/03067319.2022a.2106136
Islam MS, Islam ARMT, Phoungthong K, Ustaoğlu F, Tokatli C, Ahmed R, Ibrahim KA, Idris AM (2022b) Potentially toxic elements in vegetable and rice species in Bangladesh and their exposure assessment. Journal of Food Composition and Analysis https://doi.org/10.1016/j.jfca.2021.104350
Islam MS, Ismail Z, Jamal MH, Ibrahim Z, Jumain M, Islam ARMT, Kabir MH, Islam SMA, Ahmed S, Phoungthong K, Ibrahim KA, Idris AM (2022c) Heavy metals from different land use soil in the capital of ancient Pundranagar, Bangladesh: a preliminary study for ecological risk assessment. Chem Ecol https://doi.org/10.1080/02757540.2022c.2100360
JECFA (2011) Evaluation of certain contaminants in food: seventy-second report of the joint FAO/WHO expert committee on food additives. World Health Organization. pp. 115
Julshamn K, Lundebye AK, Heggstad K, Berntssen MHG, Boe B (2004) Norwegian monitoring programme on the inorganic and organic contaminants in fish caught in the Barents Sea, Norwegian Sea and North Sea, 1994–2001. Food Addit Contam 21:365–376
Kabir MH, Kormoker T, Shammi RS, Tusher TR, Islam MS, Khan R, Omorf MZU, Sarker ME, Yeasmin M, Idris AM (2021b) A comprehensive assessment of heavy metal contamination in road dusts along a hectic national highway of Bangladesh: spatial distribution, sources of contamination, ecological and human health risks. Toxin Rev https://doi.org/10.1080/15569543.2021a.1952436
Kabir HM, Kormoker T, Islam MS, Khan R, Shammi RS, Tusher TR, Proshad R, Islam MS, Idris AM (2021a) Potentially toxic elements in street-dust from an urban city of a developing country: ecological and probabilistic health risks assessment. Environmental Science and Pollution Research https://doi.org/10.1007/s11356-021-14581-3
Kar S, Maity JP, Jean JS, Liu C, Liu C, Bundschuh J, Lu H (2011) Health risks for human intake of aquacultural fish: arsenic bioaccumulation and contamination. J Environ Sci Health Part A Toxic/hazard Subst Environ Eng 46(11):1266–1273
Kumar M, Rahman MM, Ramanathan AL, Naidu R (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134
Li G, Sun GX, Williams PN, Nunes L, Zhu YG (2011a) Inorganic arsenic in Chinese food and its cancer risk. Environ Int 37:1219–1225
Li H, Wu C, Ye ZH, Wu SC, Wu FY, Wong MH (2011b) Uptake kinetics of different arsenic species in lowland and upland rice colonized with Glomus intraradices. J Hazard Mater 194:414–421
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105(29):9931–9935
Martí-Cid R, Llobet JM, Castell V, Domingo JL (2008) Dietary intake of arsenic, cadmium, mercury, and lead by the population of Catalonia, Spain. Biol Trace Elem Res 125:120–132
Matos-Reyes MN, Cervera ML, Campos RC, de la Guardia M (2010) Total content of As, Sb, Se, Te and Bi in Spanish vegetables, cereals and pulses and estimation of the contribution of these foods to the Mediterranean daily intake of trace elements. Food Chem 122:188–194
Mazumder DG, Dasgupta UB (2011) Chronic arsenic toxicity: studies in West Bengal, India. Kaohsiung J Med Sci 27:360–370
Mondal D, Banerjee M, Kundu M, Banerjee N, Bhattacharya U, Giri AK, Ganguli B, Roy SS, Polya DA (2010) Comparison of drinking water, raw rice and cooking of rice as arsenic exposure routes in three contrasting areas of West Bengal India. Environ Geochem Health 32(6):463–477
Moulick D, Samanta S, Sarkar S, Mukherjee A, Pattnaik BK, Saha S, Awasthi, JP Bhowmick S, Ghosh D, Samal AC, Mahanta S, Mazumder MK, Choudhury S, Bramhachari, K, Biswas, JK, Santra SC (2021) Arsenic contamination, impact and mitigation strategies in rice agro-environment: an inclusive insight. Science of the Total Environment https://doi.org/10.1016/j.scitotenv.2021.149477
Munoz O, Bastias JM, Araya M, Morales A, Orellana C, Rebolledo R, Velez D (2005) Estimation of the dietary intake of cadmium, lead, mercury, and arsenic by the population of Santiago (Chile) using a total diet study. Food Chem Toxicol 43(11):1647–1655
Mustafa RA (2023) Islam MS (2022) Preliminary assessment of trace elements in canned fish with the appraisal of health risks. Foods Raw Mater 11:1
Naito S, Matsumoto E, Shindoh K, Nishimura T (2015) Effects of polishing, cooking, and storing on total As and As species concentrations in rice cultivated in Japan. Food Chem 168:294–301
Nookabkaew S, Rangkadilok N, Mahidol C, Promsuk G, Satayavivad J (2013) Determination of As species in rice from Thailand and other Asian countries using simple extraction and HPLC-ICP-MS analysis. J Agri Food Chem 61:6991–6998
Ohno K, Yanase T, Matsuo Y, Kimura T, Rahman MH, Magara Y, Matsui Y (2007) Arsenic intake via water and food by a population living in an arsenic-affected area of Bangladesh. Sci Total Environ 381(1–3):68–76
Proshad R, Kormoker T, Islam MS, Saha BC, Hossain MR, Prince MH, Khan MM (2017) An apportionment of arsenic and iron contamination of tube-well groundwater with possible health risk in Bangladesh. J Environ Pollut Hum Health 5:117–123
Rahmana MA, Hasegawa H, Rahman MM, Miah MAM, Tasmin A (2008) Arsenic accumulation in rice (Oryza sativa L.): Human exposure through food chain. Ecotoxicol Environ Saf 69:317–324
Rahman MM, Asaduzzaman M, Naidu R (2013) Consumption of arsenic and other elements from vegetables and drinking waterfrom an arsenic-contaminated area of Bangladesh. J Hazard Mater 262:1056–1063
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem 134:1847–1854
Rahman MA, Rahman MM, Reichman SM, Lim RP, Naidu R (2014) Arsenic speciation in Australian-grown and imported rice on sale in Australia: implications for human health risk. J Agri Food Chem 62:6016–6024
Reza AHMS, Jean JS, Lee MK, Yang HJ, Liu CC (2010) Arsenic enrichment and mobilization in the Holocene alluvial aquifers of the Chapai-Nawabganj district, Bangladesh: a geochemical and statistical study. Appl Geochem 25:1280–1289
Roychowdhury T, Tokunaga H, Ando M (2003) Survey of As and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci Total Environ 308:15–35
Samal AC, Kar S, Bhattacharya P, Santra SC (2011) Human exposure to arsenic through foodstuffs cultivated using arsenic contaminated groundwater in areas of West Bengal, India. J Environ Sci Health - Part A Toxic/hazardous Subs Environ Eng 46(11):1259–1265
Samal AC, Kar S, Maity JP, Santra SC (2013) Arsenicosis and its relationship with nutritional status in two arsenic affected areas of West Bengal, India. J Asian Earth Sci 77:303–310
Samal AC, Kar S, Bhattacharya P, Santra SC (2010) Assessment of potential health risk through arsenic flow in food chain—a study in Gangetic Delta of West Bengal. Management and Sustainable Development of Coastal Zone Environments 259–269 https://doi.org/10.1007/978-90-481-3068-9_17
Samal AC, Bhattacharya P, Biswas P, PrakashMaity J, Bundschuh J, Santra SC (2021) Variety-specific arsenic accumulation in 44 different rice cultivars (O. sativa L.) and human health risks due to co-exposure of arsenic-contaminated rice and drinking water. Journal of Hazardous Materials https://doi.org/10.1016/j.jhazmat.2020.124804
Santra SC, Samal AC, Bhattacharya P, Banerjee S, Biswas A, Majumdar J (2013a) Arsenic in food chain and community health risk: a study in Gangetic West Bengal. Procedia Environ Sci 18:2–13
Santos EE, Lauri DC, Silveira PCL (2004) Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci Total Environ 327:69–79
Santra SC, Samal AC, Bhattacharya P, Banerjee S, Biswas A, Majumdar J (2013b) Arsenic in food chain and community health risk: a study in Gangetic West Bengal. Procedia Environ Sci Eng Manag 18:2–13
Sarkar A (2009) Role of inequality and inequity in the occurrence and consequences of chronic arsenicosis in India and policy implications. Environ Justice 2(3):147–152
Shen S, Li XF, Cullen WR, Weinfeld M, Le XC (2013) Arsenic Binding to Proteins. Chem Rev 113(10):7769–7792
Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 78(9):1093–1103
Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH (2005) Dietary intake and arsenic methylation in a U.S. population. Environmental Health Perspectives, 113(9):1153–1159
Tenni D, Martin M, Barberis E, Beone GM, Miniotti E, Sodano M (2017) Total As and As speciation in Italian rice as related to producing areas and paddy soil properties. J Agric Food Chem 65:3443–3452
Tsuji JS, Yost LJ, Barraj LM, Scrafford CG, Mink PJ (2007) Use of background inorganic arsenic exposures to provide perspective on risk assessment results. Regul Toxicol Pharmacol 48:59–68
USEPA (1989) Guidance manual for assessing human health risks from chemically contaminated, fish and shellfish. Washington, D.C.7 U.S. Environmental Protection Agency; EPA-503/8–89–002
USEPA (2010) Risk-based concentration table Available from: <http://www.epa.gov/reg3hwmd/risk/human/index.htm
USEPA (1991) Human health evaluation manual, supplemental guidance: “standard default exposure factors”. OSWER Directive 9285.6–03
Wang HS, Sthiannopkao S, Chen ZJ, Man YB, Du J, Xing GH, Kim KW, Yasin MSM, Hashim JH, Wong MH (2013) Arsenic concentration in rice, fish, meat and vegetables in Cambodia: a preliminary risk assessment. Environ Geochem Health 35:745–755
Wang X, Sato T, Xing B, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
WHO (1985) Guidelines for the study of dietary intakes of chemical contaminants. WHO Offset Publication No. 87. World Health Organization, Geneva, pp. 1–100
WHO (2001) IPCS Environmental Health Criteria 224. Arsenic and arsenic compounds. International Programme on Chemical Safety, World Health Organization, Geneva
Williams PN, Islam MR, Adomako EE (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in ground waters. Environ Sci Technol 40:4903–4908
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ (2007) Greatly enhanced As shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41:6854–6859
Wong WWK, Chung SWC, Chan BTP, Ho YY, Xiao Y (2013) Dietary exposure to inorganic arsenic of the Hong Kong population: results of the first Hong Kong total diet study. Food Chem Toxicol 51:379–385
Wu X, Gao M, Wang L, Luo Y, Bi R, Li L (2014) The As content in marketed seafood and associated health risks for the residents of Shandong, China. Ecotoxicol Environ Saf 102:168–173
Xu X, McGrath S, Meharg A, Zhao F (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42(15):5574–5579
Zhang W, Huang L, Wang WX (2011) Arsenic bioaccumulation in a marine juvenile fish Terapon jarbua. Aquat Toxicol 105:582–588
Acknowledgements
The authors want to thank Patuakhali Science and Technology University (PSTU), Dumki, Patuakhali-8602, and Dhaka University in Bangladesh for sample processing, as well as Yokohama National University in Japan for arsenic analysis. The authors express their gratitude to Research Center of Advanced Materials, King Khalid University, Saudi Arabia, for support (award number KKU/RCAMS/22).
Author information
Authors and Affiliations
Contributions
Md. Saiful Islam, Ramal Ahmed Mustafa, and Abu Reza Md Towfiqul Islam collected the water samples from the study area. Khamphe Phoungthong, Abu Reza Md Towfiqul Islam, Md. Saiful Islam, and Tofazzal Islam designed the total experiment. Tasrina Rabia Choudhury, Md. Saiful Islam, Md. Humayun Kabir, Mir M. Ali, and Abubakr M. Idris analyzed the data and wrote, revised, and improved the manuscript. All authors reviewed and approved this manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Islam, M.S., Mustafa, R.A., Phoungthong, K. et al. Arsenic in the foodstuffs: potential health appraisals in a developing country, Bangladesh. Environ Sci Pollut Res 30, 26938–26951 (2023). https://doi.org/10.1007/s11356-022-24119-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24119-w