Abstract
Uranium mill tailings are the crushed rock residues of the uranium extraction process from ores. The tailings effluent and tailings solids from the mill are discharged as slurry to a waste retention pond, called tailing pond. Natural radionuclides’ and trace metals are present in mine tailing/soil in varying concentrations, and some of these are found in elevated concentrations in uranium waste tailings. Uranium mine tailing ponds at Jaduguda and Turamdih receive waste from ores mined at the six mine stations at Jharkhand state, India. A study was undertaken to evaluate the potential of native plant species for the phytoremediation of these site. Three sampling stations were selected at Jaduguda (TP1, TP2, TP3) and Turamdih and at the downstream of effluent treatment plant. pH, electrical conductivity, metals (12-Al, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Se, Cd, Pb) and radionuclides’ (3-Co, Sr and U) were analyzed using inductively coupled plasma mass spectrophotometry. From the analysis, four elements—Al, Mn, Fe and U—were found to be much higher in concentration in water with range (mg/kg) of 0.02–2.38, 0.30–31.67, 0.00–0.75 and 0.03–5.50, respectively, and 10 elements—of U, Mn, Al, V, Fe, Ni, Cu, Zn, Co and Se—were found to be higher in concentrations in soils with range (mg/kg) of 22–99, 1,072–8,065, 14,053–21,213, 66–139, 15,163–44,640, 149–240, 135–350, 89–191, 34–140 and 12–122, respectively. Among them, U and Mn were identified as predominant contaminants. Out of all the native plants, 21 species were screened for phytoaccumulation and transfer factor study. P. digitalis (for Al, V, Ni and Co), E. ferox (for Mn and Cu), A. indica (for Fe), B. vitisidae (for Zn), P. hydropiper (for Se) and S. spantanium (for U) were identified for hyper-accumulation, and A. indica (for Al, Co, Se and U), C. bunplandianus (for Mn, Fe, Ni and Cu), E. ferox (for V) and C. procera (for Zn) were listed for non-accumulation of respective contaminant. Besides this, taking consideration of the parameters such as shallow-rooted plant species, easy to adapt, growth, harvest and biomass production and simultaneous accumulation of multiple contaminants, following plants were found to be candidate species for phytoremediation of tailing ponds of uranium mines: For hyper-accumulation: P. vittata (can accumulate Al, V, Ni, Co, Se and U simultaneously) followed by P. digitalis, C. compressus and S. spantanium. For non-accumulation: C. bunplandianus (can non-accumulate Al, Mn, Fe, Ni, Co, Cu, Zn, Se and U simultaneously) followed by B. moneri, C. procera and A. indica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
“Nuclear energy is not only cost-effective; it is also a cleaner alternative to fossil fuels” (Manmohan Singh 2004). Uranium mines in the world usually produce low-grade ores containing 0.1–0.3 % U3O8; mines in India having still lower grades of low-grade (<0.05 % U3O8) uranium (Sethy et al. 2011; Sarangi 2003). Uranium mining residues from ore processing operations are characterized by high concentration of radionuclides and toxic elements. The form in which a metal exists strongly influences its mobility and contamination thus its severe effect on the environment (Mishra et al. 2008). After the waste rock is removed and the ore is extracted, the ore must be processed to separate the target mineral from the valueless portion. While the soil in the Jharkhand region has naturally occurring radionuclides, the uranium mining/milling process extracts and concentrates predominantly U. Thus, U is much higher in the mined ore and in the milled product, and the final product produced from the mill, commonly called “yellow cake” (magnesium diuranate or U3O8 with impurities), is packed and shipped in casks (Sarangi 2003; Shirinian-Orlando 2007). Waste from uranium mines and mills are in solid, liquid and gaseous forms. Once the minerals are processed and recovered, the remaining rock becomes another form of mining waste called tailings which is deposited in pond, i.e., tailing pond.
Uranium milling waste (tailings) containing hazardous contaminants such as radionuclides and heavy metals may be leached into the soil and enter into the environment via subsequent transport in the aquifer into surface and groundwater consequence of which is tremendous losses to the environment mainly to ecosystem which can last for decades of generations (Mishra et al. 2008, 2009). In the process of uranium mine ore, the leachate not only extracts the uranium itself, but also extracts numerous other elements such as Iron, vanadium, molybdenum, selenium, lead and arsenic, cadmium, chromium, zinc, copper, nickel, and manganese (Shirinian-Orlando 2007; Salvarredy-Aranguren et al. 2008). Besides in such residual, mineral salts, metals and uranium are priority contaminants worldwide, and therefore, a need for treatment or remediation and the approach that must be safe for soil and ground water (Cardenas et al. 2008) is important. Protection of air, soil and water from the hazardous contaminants is of at most priority in the management of uranium waste. If neglected in any sense, contaminants may leach out of mine waste and release into the environment which can result in severe destruction of ecosystems (EEB 2000). Initial and highest goal of the company in mine operation is water and soil protection. Adopting this ideal-sense ethic will be the only way we can ensure that “the golden dreams of mining do not turn into the nightmare of poisoned streams” (De Rosa 1997). In the end, radioactively contaminated and toxic elemental scrap, that is mine tailings, are produced, which have to be disposed. Uranium mill tailings are normally disposed of (dumped) as slurry or sludge in special ponds or piles called tailing pond, where they are abandoned (Shirinian-Orlando 2007). In this regard, Abandonment of uranium mines which were developed in the 1950s and 1960s are examples of major environmental insults (Bastias 1989).
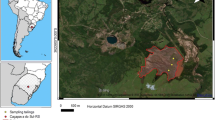
In India, Jadugoda uranium mines at Jharkhand state have the largest Uranium mining and exploration (Basu et al. 2000; Hiroaki KOIDE 2004; Fig. 1). Uranium Corporation of India Limited now operates six underground mines and one open pit mine in the state in addition to an underground mine in Andhra Pradesh (UCIL). It has been the site of extensive mining and milling of uranium for over 4 decades (Sethy et al. 2011) continuously operating since 1967 and providing the basic raw material for the nuclear power programme of the country (Sarangi 2003). Uranium mine tailing ponds at Jaduguda and Turamdih receive waste from ores mined at the six mine stations at Jaduguda, Bhatin, Turamdih, Bagjata, Narwapahar and Banduhurang (Jharkhand). Smelter only in Jadugoda processes 1,000 tons of ore per day. Since the ore is of low-grade, milling operations itself result in 99.9 % waste. Roughly 200 tonnes of uranium is produced every year, generating more than 360,000 tonnes of tailings. Thus, the uranium industry generates large quantities of waste and has increased tremendously as various new mining operations are taking place. Almost the entire mined ore comes out as a waste after recovery (Singh and Soni 2010). This tailing contains the bulk of decay products of uranium which are radioactive (Sarangi 2003; Sudhakar and Muralidhar 2008) and also high proportion of poly-metallic minerals elsevier (Mishra et al. 2009; Wislocka et al. 2006). Because tailing constitutes substantial amount of acid-generating properties of pyrite, two types of contaminants tend to leave the solid and migrate to the pore water. These are the radioactive element (U) and heavy metals (Mn, Pb, Cu, Fe, Zn, Cd, Cr, As Ni and Hg) present in mine tailing/soil in varying concentrations (Wislocka et al. 2006). Some of these are found in elevated levels in uranium waste tailings (Mishra et al. 2008; Basu et al. 2000). It shows that there is a migration of these metals into groundwater (Mishra et al. 2009). The potential environmental hazards arise when the disposal site is abandoned after decommissioning of the uranium mill. Pollution problem may rise if toxic heavy metals are mobilized into soil solution and are either taken up by plants or transported in drainage water. The metals may take up either the human food chain through the consumption of such plants.
Usually the contaminated sites are treated with traditional methods like physical, chemical and thermal processes resembling excavation and transportation. Several chemical treatments are currently available to treat uranium-contaminated sites, but these methods require expensive extraction of the soil and exposure of workers to uranium (Gavrilescu et al. 2009). Traditional physical methods involving removal of 1 m3 soil from a 1-acre contaminated site is estimated as US $0.6–2.5 million (McIntyre 2003).
The ideal solution for pollution abatement is therefore “bioremediation” (especially for uranium mine tailing ponds) and the emerging technology is ‘phytoremediation.’ Broadly, phytoremediation is the use of vegetation for in situ treatment of contaminated soils, sediments and water. Phytoremediation in its general sense means cleaning of contaminated sites with appropriate plants which offers not only greater potential to remediate contaminated sites over conventional and costly methods but also offer means to use ‘green’ sustainable process (Pulford and Watson 2003; Niu et al. 2007), which generally have a high public acceptance and can often be carried out on site to remedy contaminated soils, sediments and/or groundwater (Vidali 2001; Dietz and Schnoor 2001). Except few limiting factors, this technology has the ability to rejuvenate the contaminated environments effectively. Due to its approach, it is an environmentally friendly (eco-friendly), safe and cheap way to clean up contaminants or pollutants; aesthetic advantages; and long-term applicability (Shukla et al. 2010; Schnoor et al. 1995). It is applicable at sites containing organic, nutrient or metal pollutants that can be accessed by the roots of plants and sequestered, degraded, immobilized or metabolized in place. In the last few years, a greater understanding has been achieved about the uptake and metabolism of organic xenobiotic chemicals by plants, especially chlorinated solvents, some pesticidesand explosives compounds (Anderson et al. 1993; Schnoor et al. 1995; Newman et al. 1997; Hughes et al. 1997; Burken and Schnoor 1998; Thompson et al. 1998; Raskin and Ensley 2000; Terry and Banuelos 2000). This has particularly been proposed as a promising technique for remediation of U-contaminated soils (Huang et al. 1998; Zhu and Shaw 2000; Shahandeh et al. 2001).
Recent vegetation programmes on disturbances have begun to emphasize the use of native vegetation. Soni et al. (1992) made an attempt to revegetate rock phosphate mine with various native trees, shrubs and grasses. They reported that the mixture of natives has improved the soil fertility status and productivity capacity of the spoil material besides favoring the biological invasion of various natural invaders. Munshower (1993) emphasized that native species were less competitive and can be used in rehabilitation, and the disturbances permit the germination and development of non-seeded species. The development of ecosystem was accompanied by improvement in soil characteristics. Some species play a key role in nutrient conservation and were thus important in any rehabilitation programme.
However, Plants contribute to the circulation of heavy metals in the food chain through their active and passive absorption, accumulation in tissues as well as subsequent grazing by animals or consumption by humans (Wislocka et al. 2006). And the foreign plants may not grow or accumulate the contaminants and may have deeper root system that leads to deeper penetration into the tailing there by severe release of radon gas (IAEA 2004; Gupta et al. 2003; Smith and Weston 1999). Therefore, the research aim was to investigate the potential of wild native plant species (which are non-edible to animals and human beings), growing in the vicinity of Uranium mine tailing ponds in Jaduguda and Turamdih, which accumulates or absorbs toxic metals and remediate Uranium mine tailing ponds.
2 Materials and methods
2.1 Site description
In India, Jaduguda Uranium mine is located at east longitude of 86°20′ and north latitude of 22°40′ and Turamdih Uranium mine is located at east longitude of 86°09′ and north latitude of 22°43′ at 24 km east and 5 km south of Tatanagar railway station, in Jharkhand State, India (Jha et al. 2000). The details of mining and processing technique are being described elsewhere (Tripathi et al. 2010; IAEA 1980). In the course of mining to milling, bulk of the ore processed emerges as tailings (residues from ore processing) and are pumped into a TP. There are three valley–dam types of TPs at Jaduguda and fourth at Turamdih (Figs. 1, 2) (22°39′17.70″N and 86°19′43.48″E Google Earth; Hiroaki KOIDE 2004).
The first (JTP1) and second (JTP2) stages of the TPs are located adjacent to each other in a valley with hills on three sides and engineered embankments on downstream side of natural drainage (Mishra et al. 2008). These two TPs are filled up and now left abandoned, and second stage TP was completely buried with 30-cm-thick land soil on the top (Singh and Soni 2010). The third (JTP3) and fourth (TTP at Turamdih) stage of the TPs which are currently in use were also put up in a similar setting. These two active TPs are filled with effluent obtained after the ion exchange process of uranium removal and the fine particles obtained after the secondary filtration of barren liquor. The precipitates settle down in the TP, and the clear liquid is continuous to decant from abandoned (closed) and active uranium mine TPs through a series of decantation wells, and the decanted effluent has subsequently been manifested at various stages to treat through effluent treatment plant (ETP). The treated AMD found its way into an adjoining natural water source through Gala River and flowed toward downstream and finally mixing into Subarnarekha River (Mishra et al. 2009).
2.2 Sampling locations and sampling
Disposal of mine tailing wastes by landfill in the form of slurry is the most widely practiced method in world including India. For the present study, two open landfill (JTP & TTP) sites and the downstream of ETP were selected. Based on accessibility, sampling has been done from each sampling points in the month of March/April and September/October over a period of 3 years (2009–2010, 2010–2011 and 2011–2012) in every 4-month intervals. Samples were collected according to standard methods and analyzed for pH, electrical conductivity (EC) and chemical parameters (Roy et al. 2007; NEERI 2010). Representative replicate samples were sampled regularly by random sampling method (Mishra et al. 2008) in each different sampling point of respective sites (Fig. 2; also details in Table 1; “Appendix”). Samples away from tailing ponds served as controls. The control samples were taken only for comparison to find contaminants in study area. Therefore, sampling was done for controls only in dry season as it is not suitable to sample in rainy or Rabi season. Samples were collected according to standard methods (books by Jaiswal and Radojecic 2008; Radojevic and Bashkin 2006) and also according to EPA (Sample Collection Procedures for Radiochemical Analytes in Environmental Matrices EPA 2006).
2.3 Sample preparation
All reagents and water used here were of analytical grade. Before collecting the samples, containers were cleaned by soaking in 2 N HNO3, rinsed with pure water and then air-dried in fume hood. After collection, samples were transported to laboratory at Environment Protection Training and Research Institute (EPTRI) and stored in room and not exposed to light. Water: Samples of surface water were collected in preconditioned plastic carboys. Using whattman filter paper (No. 1 filter paper-120 mm Ø), the samples were filtered and fixed with 2 % (v/v) conc. 8 N nitric acid for metal analysis (Friel et al. 1990; Giri et al. 2007). Soil: Samples were collected in polythene bags at a depth of 25–30 cm (from surface). Soil samples were crushed, mixed thoroughly and air-dried for 5–6 days then dried in hot air oven for 24 h at 65 °C and finally ground into fine powder to pass through 2-mm sieve (EPA 2006). Plant: The collected samples were cleaned with tap water (on site) and the root and shoot parts of individual plant species were separated, weighed (fresh weight) and placed in separate paper bags. In lab, the samples were again cleaned with distilled water, air-dried and kept at 110 °C for 2 days in hot air oven and dry weight were taken (Jha et al. 2007). After taking dry weight, the samples were ground into fine powder with Wiley mill followed by coffee grinder (Kenstar mixer grinder MG 0411) to pass through a 2-mm sieve (Jha et al. 2007; Wislocka et al. 2006).
2.4 Instrumentation and analytical procedure
Water: pH and EC were measured in water before acidification for metal fixation (Following APHA 4500H and B and 2510B protocols) and kept for elemental analysis. Soil: pH was measured by adding 50 ml of distilled water to 20-g of air-dried soil and allowed to equilibrate for 30 min (AOAC SW 846 9040 protocol), and EC was measured by adding 40 ml of distilled water to 20-g of air-dried soil and allowed to equilibrate for 30 min (SW 846 9050 protocols). For elemental analysis, 50-mg of soil was taken in Poly Tetra Fluoro Ethylene (PTFE) Teflon beakers and moistened with few drops of ultra pure water. Then, 10 ml of an acid mixture (7:3:1 HF–HNO3–HClO4) was added and swirled thoroughly, covered with lids and kept overnight for digestion. Next day, the samples were heated at 200 °C for about an hour on a hot plate, the lids were then removed and the contents were evaporated to incipient dryness until a crystalline paste was obtained. The remaining residues were then dissolved using 10 ml of 1:1 HNO3:H2O and kept on hot plate for 10 min at 70 °C to dissolve all suspended particles. One milliliter (5 μg/ml) of Rhodium (Rh) solution was added to act as an internal standard, and then, the volume was made up to 250 ml with purified water (18 MΩ) and stored in polyethylene bottles (Roy et al. 2007). Plant: Approximately 0.5 g of the plant samples were accurately weighed and transferred to a Teflon container. Five milliliters of 65 % HNO3 and 1 ml 30 % H2O2 were added. After microwave/hot plate digestion cycle, digested samples were made up to 25 ml with de ionized water (Sahan et al. 2007). Radionuclide’s (3-Cobalt-Co, Strontium-Sr and Uranium-U) and metals (12-Aluminum-Al, Vanadium-V, Chromium-Cr, Manganese-Mn, Iron-Fe, Nickel-Ni, Copper-Cu, Zinc-Zn, Arsenic-As, Selenium-Se, Cadmium-Cd, Lead-Pb) in water, soil and plant samples were analyzed by Inductively Coupled Plasma Mass Spectrometry (ICPMS make PerkinElmer Sciex ELAN DRC II)) at the Central Research Facility available at National Geophysical Research Institute (NGRI), Hyderabad. The international geostandard certified values of SO-1 for soil (Govindaraju 1994) and NIST for water and plant sample were used for standard references. Subsequently results were corrected using blanks.
2.5 Determination of transfer factor (TF)
Soil-to-plant transfer factor is one of the important parameters used to estimate the concentrations of radionuclides in plants according to a transfer model. The uptake of radionuclides or elements by plants from the soil is normally described as transfer factor (TF), i.e., the ratio of concentration of radionuclides or element in plant tissue and soil (in Bq kg−1 or mg kg−1; Hegde et al. 2004; Dobrin et al. 2006; Abu-Khadra et al. 2008; Alharbi and El-Taher 2013), and it is represented as below.
The transfer factors vary significantly according to plant properties and soil type (Abu-Khadra et al. 2008).
2.6 TL (threshold limits)
2.6.1 Definition of threshold limits (TL) for identifying the contaminants
Limits are most usefully defined in terms of the point or range of conditions beyond which the benefits derived from a natural resource system are judged unacceptable or insufficient (Haines-Young et al. 2006). There were definite reference standards (CPCB 1986; WHO 2004, 2006; CPHEEO 2009; BIS 1993; USSR 2006; EPA 2009) already available for water. However, to identify the actual contaminants in the samples, the largest value of control along with available stands needs to be considered here as threshold limits. For soil, there were no definite reference standards found. Hence, the greatest value of control and UCC (2011) was considered here as threshold limit (Table 2).
3 Results and discussion
3.1 Physico-chemical characteristics of water and soil samples
In each sampling intervals, in individual sampling points, the sampling and analysis have been done following standard methods discussed above. The analysis indicated that water, soils and plants for all study sites differed significantly with respect to the contents of the examined elements. The mean concentrations of elements at sampling locations are presented in “Appendix”.
3.1.1 pH and EC characteristics of water and soil samples
pH: All water samples have shown pH ranging between 5.62 and 7.24. The JTP1 and JTP3 water samples were found to be slightly acidic in nature (pH 5.98 and 5.62). This may be due to direct discharge of acidic effluent in TP3, and in the course of time by natural process, the acidity of the pond may decrease and turn it into alkaline. However, the pH of the soil collected from all the sampling points were more than the pH of the control (Fig. 3a). EC: All the samples (soil and water) were found to have higher levels of EC and are more than the permissible limit [(Water: 2,250 µmohs/cm (CPCB); soil: 292.29] (Fig. 3b). The greater their concentration of ions or elements, the greater the conductivity, and for acidic solution, the lower the pH, the greater the conductivity. Therefore, it is confirm that the samples have high concentration of elements or metals. In the present investigation, since the area is low in organic carbon with pH range of 5.98–7.24 in water and pH range of 6.45–6.93 in soil, mobility of elements is likely to be greater (Mishra et al. 2009).
3.1.2 Metals characteristics of water and soil samples
3.1.2.1 Water metals characteristics
The water chemical analysis has been done following standard methods. The concentration with decreasing order of elements according to the control samples in water was identified as follows.
Average metal concentration of water from tailing ponds and its downstream areas has shown significant higher concentration levels of four elements—Al, Mn, Sr and U. The concentrations of other metals are significantly in low. The actual values after subsequent deduction of TL values were shown to significantly exceeded concentration levels of AL, Mn and U. The concentration of Al in TP3 was in average 2.8 mg/kg, followed by pipe (average 0.12 mg/kg) and TTP (average 0.07 mg/kg). Mn in all location (range 0.11 mg/kg in ETP to saturation in TP3 and ETP) and U in all location (range 0.05 mg/kg in pipe to 4.42 mg/kg in TTP with average of 1.38 mg/kg) showed exceeding concentrations with respect to the control water and also to the CPCB permissible limits. Mn is showing elevated levels of concentration in all tailing ponds, i.e., up to the saturation This effect was due to the excess addition of manganese oxide (MnO2) powder as an oxidizer uranium process (Mishra et al. 2009; Seidel 1980). The other metals were having very low concentration than the TL (Fig. 4).
The decreasing order of concentration of hazardous elements in sampling locations with reference to the TL is as follows.
-
TP1 (mg/kg): Mn (5.20) > U (1.48) > Al (0.00) > V (0.0) > Cr (0.0) > Fe (0.0) > Ni (0.0) > Co >
-
TP3 (mg/kg): Mn (Sturation) > Al (2.80) > U (0.70) > V (0.0) > Cr (0.0) > Fe (0.0) > Ni (0.0) > Co
-
TTP (mg/kg): U (4.42) > Mn (1.70) > Al (0.07) > V (0.0) > Cr (0.0) > Fe (0.0) > Ni (0.0) > Co >
-
ETP (mg/kg): U (0.24) > Mn (0.11) > Al (0.00) > V (0.0) > Cr (0.0) > Fe (0.0) > Ni (0.0) > Co >
-
Pipe (mg/kg): Mn (Sturation) > Al (0.12) > U (0.05) > V (0.0) > Cr (0.0) > Fe (0.0) > Ni (0.0) > Co
3.1.2.2 Soil metals characteristics
After appropriate preparation, the soil physicochemical analysis has been done following standard protocol. The concentration with decreasing order of elements according to the control samples in soil was identified as follows.
Average metal concentration in tailing ponds and its affected areas shows much higher concentration levels of seven elements—Al, Mn, Fe, Ni, Cu, Zn and U. The other metals show significantly in low concentration. The actual values after subsequent deduction of TL showed significantly higher concentration levels of certain elements (Fig. 5). The elements whose concentrations were found to be beyond TL were Mn, Ni and Co (average 7,555, 151 mg/kg and 117 mg/kg, respectively) in TP1; Al, Cr and Se (average 8,685, 19 and 116 mg/kg, respectively) in TP2 samples; V, Zn and Pb (average 79 mg/kg 98 mg/kg, and 6 mg/kg, respectively) in TP3 samples; Fe and Cu (average 38,225 mg/kg and 281 mg/kg, respectively) in TTP samples, and the concentration of U (average 95 mg/kg) was found higher in ETP samples. In average in concentrations of Al, Mn, Fe and U in all locations prominent higher levels were found. The decreasing order of concentration (in mg/kg) of hazardous elements in sampling locations with reference to the TL is as follows.
-
TP1 (mg/kg): Fe (32,216) > Mn (7,555) > Al (3,542) > Ni (151) > Co (117) > U (87) > Cu (72) > Zn
-
TP2 (mg/kg): Fe (8,749) > Al (8,685) > Mn (562) > Se (116) > Cu (84) > Ni (75) > V (34) > Cr (19)
-
TP3 (mg/kg): Fe (33,327) > Al (7,102) > Mn (2,483) > Cu (140) > Ni (128) > Zn (98) > V (79) > U
-
TTP (mg/kg): Fe (38,225) > Al (3,767) > Mn (2,206) > Cu (281) > Ni (80) > Se (73) > Zn (48) > U
-
ETP (mg/kg): Fe (27,200) > Mn (3,415) > Al (1,525) > U (95) > Zn (92) > V (75) > Cu (66) > Ni (60
3.2 Contaminants identified
By comparing the results with control values as well as permissible limits of reference standards simultaneously discussed above, in the above analysis it must be borne in mind that elements that exceed their concentrations beyond the threshold limits subsequently were identifies as contaminants in each sampling location. For the same, we can suspect or confirm the presence of pollution when the concentrations are higher than the typical values for soils and water found in literature and exceed the levels present in the nearby areas. In this study, in order to define the presence of contamination, for soil, the concentrations of elements were compared with the normal ranges in control soils (Alloway 1990) and Earth’s crust of upper continent crust (UCC; Turekian and Wedepohl 1961), and for water, the maximum acceptable levels according to the CPCB, WHO, CPHEEO, BIS, USSR and EPA which were universal standard should be followed for contaminants identification and also for the reclamation of contaminated sites.
The elements of mainly geochemical origin, such as V, Cr, Fe, Ni, Co, Cu, Zn, As, Se, Sr, Cd and Pb are instead present at concentrations lower than the typical values in water and same was seen for Cr, As, Sr, Cd and Pb in soil samples. This may be due to a “dilution effect” of the sludge carried by the floods in this area on the native lithogenic element content (Malandrino et al. 2011). The elemental contamination is high in the soil samples collected in the core of the site, and it seems to decrease the same in the soil samples collected at the border of site, where the spontaneous vegetation is present (Prach et al. 2009).
3.2.1 Water contaminants
Identified based on standards (TL-values) results indicated that mainly three elements—U, Mn and Al—were identified as major water contaminants in the selected sites (Fig. 6). Al in TP2 (3.2 mg/kg), Mn in TP1 (7.2 mg/kg), TP2 (10.0 mg/kg) and ETP (10.0 mg/kg) and U in TP3 (4.4 mg/kg) showed the higher concentration, and it was found that the pattern of Mn and U were comparable with measured concentrations (Mishra et al. 2009).
3.2.2 Soil contaminants
Identified based on the control (threshold) values, ten elements—Fe, Al, U, Mn, V, Ni, Cu, Zn, Co and Se have been identified as major contaminants (Fig. 7). Fe (15,163–44,640 mg/kg), Ni (149–240 mg/kg), Cu (135–350 mg/kg) and U (22–99 mg/kg) in all sites; Al in TP2 (21,213 mg/kg) and TP3 (19,631 mg/kg); Mn in ETP (3,915 mg/kg); V in TP3 (139 mg/kg) and ETP (135 mg/kg); Zn in TP3 (191 mg/kg) and ETP (184 mg/kg); Co in TP1 (140 mg/kg); Se in TP2 (122 mg/kg) and TTP (80 mg/kg) showed the higher concentration and the pattern of Mn, Pb, Cu, Fe, Zn and U which was comparable with measured concentrations was found (Mishra et al. 2008).
Samples with high Fe, Al, V, Ni, Cu, Zn, Co and Se concentration may be due to geochemical origin, aquatic process such as neutralization, precipitation, flocculation as well as adsorption occurred in the receiving water (Wang 1999). For the samples with high Mn and U concentrations, due to technical limitations all of the uranium present in the ore cannot be extracted. Therefore, the sludge also contains 5–10 % of the uranium initially present in the ore (Shirinian-Orlando 2007); hence, the index of the contamination related to uranium mine is uranium (Hiroaki KOIDE 2004). The samples with high Mn concentration may due to addition of manganese dioxide or KMnO4 used as oxidant in acid leaching uranium circuit and also common contaminant in mining process (Mandal and Sengupta 2005; Mishra et al. 2009; Landa 2003). Therefore, out of ten contaminants identified above, only two elements U and Mn were considered here as major contaminants in the selected sites that need to be remedied.
3.3 Plants metal characteristics
From the water and soil analysis, it is found that out of all only 10 elements—Al, Mn, Fe, V, Ni, Cu, Zn, Co, Se and U—have been identified as contaminants. Therefore, these elements were selected for plant samples analysis. A total of 21 plant species of about same age (~1–2 years) were screened for phytoremediation studies. Concentrations of Al, Mn, Fe, V, Ni, Cu, Zn, Co, Se and U in all the plant species varied considerably across the seven sampling intervals in same species and between sites. Plant elemental concentration was mostly affected by concentration of the same in the soil. However, concentration of Al, Mn, Fe and Zn were significantly similar apparent in all the plant species, i.e., comparatively higher concentration. The average concentrations of identified contaminants in plant samples collected from tailing ponds and its downstream areas were detailed as follows (Table 3).
3.3.1 Identification of plant species for bio-concentrators of identified contaminants
In order to find plant species which concentrate the selected contaminants, all the collected plant tissue samples initially were analyzed for their elemental concentrations, and based on the concentration, the plants were screened accordingly. All the selected species were shorted according their decreasing concentration. Two species with highest concentrations were identified as bio-concentrator, and two plant species with lowest concentrations were identified as non-concentrator of the respective contaminant, and the same is presented below in Table 4.
Of the above, the six plant species namely P. vittata (concentrated with Al, V, Mn, Fe, Ni, Co and U), V. negundo (Fe, Cu and Zn), C. procera (Al and Se), B. vitisidae (Fe, Cu and Zn), D. annulatum (Ni and Se) and L. Camera (Zn) from tailing pond and one plant species namely P. digitalis (Al, V, Mn, Ni, Co and Se) from ETP external channel have been Identified for multi-elemental concentrators. Among the all plant samples collected, P. vittata and P. digitalis has shown significant higher concentration of multiple elements (Table 5).
3.3.2 Screening of plants for hyper- and non-accumulation of contaminants
To identify plant species for hyper- or non-accumulator, transfer factor (TF), i.e., the translocation of contaminants from soil to plant needs to be calculated.
3.4 Determination of transfer factor (TF)
The transfer factor (TF) of contaminants from soil to plants of collected samples was determined based on dry weight (Hegde et al. 2004; Dobrin et al. 2006). The average metal TF of plant sample collected are placed in Fig. 8.
It is obvious that the TF of contaminants is nearly the same for each plant species at different sampling location. On the other hand, the TF of element of the plant increased as the concentration increased.
A. conyzoides and paspaladium spp., T. Latifolia and V. negundo showed comparatively the similar TF character for all the contaminants. The other plant species showed varied TF character, i.e., for example A. indica showed the lowest TF: 0.000 for U to much higher TF: 0.463 for Zn. By comparing with the other species, H. Suaeulance, P. digitalis, P. vittata and P. hydropiper plants showed relatively high magnitude of TF and B. Moneri, C. Bunplandianum and C. Esculentam plants showed significantly low magnitude of TF. However, in this study, no clear correlation was found in TF value between any two plant species.
3.4.1 Identification of plant species for hyper- and non-accumulation of contaminants
For a plant to be considered a hyper-accumulator, the plant should typically contain, at least, a few times more of a metal than that occurs in same plants from non-polluted environments or other plants grown in the same soil (Zu et al. 2005). Plant species with high and low TF values for individual metals were screened. The plant species with higher TF value were considered here as hyper-accumulators, and the plant species with lower TF value were considered as non-accumulators. In Table 5, it is apparently seen that minimum 22 times accumulation of Cu was seen in E. ferox and maximum 2,295 times accumulation of U was seen in S. spantanium samples than that in the plant samples of the studied area.
3.4.2 Identification of plant species for multi-accumulation and non-accumulation of contaminants
By seeing simultaneous accumulations of multiple elements, only P. vittata has shown highest multi-contaminants accumulation, i.e., up to six (hexa grade) elements—AL, V, Ni, Co, Se and U. Five plant species namely T. latifolia, D. annulatum, S. celearis, Paspalidium spp. and S. spantanium showed least or single elemental accumulation, i.e., V, Ni, Co, Se and U, respectively. While seeing in plant species with simultaneous non-accumulation of multiple elements, only C. bunplandianus has shown highest multi-contaminant non-accumulation, i.e., up to nine (nona grade) elements—Al, Mn, Fe, Ni, Co, Cu, Zn, Se and U. The same is presented in Table 6.
3.5 Plant species recommended for remediation of uranium tailing ponds
Analysis of results of metals and radionuclides in in situ studies of different plant species has shown different bio-concentration and accumulation patterns of different identified contaminants. Besides the above phytoaccumulation studies, TF of identified contaminants above and also taking into consideration the limits (such as shallow-rooted plant species, easy to adapt, growth, harvest and biomass production), and simultaneous multiple contaminant accumulation, following plant species were found to be the candidate species each in accumulation and non-accumulation and recommended for phytoremediation of tailing ponds of uranium mines (Singh and Soni 2009, 2010).
Such as plant species with shallow rooted, adapt, growth and biomass production whether
-
For hyper-accumulation of contaminants: Among various plants, P. vittata was found to be the best accumulator of various contaminants mainly the U and Mn along with Al, V, Ni, Co and Se. This plant showed the maximum accumulation of U (3.89 mg/kg; TF: 0.087) and Mn (197.62 mg/kg; TF: 0.041) followed by P. digitalis (Al, V, Ni and Co with the grade of 36, 183, 34 and 465 times more accumulation, respectively), C. compressus (penta grade accumulation of Al, Mn, Ni, Co and Se) and S. spantanium (greatest time accumulator of U, i.e., 2,595 times comparatively).
-
For non-accumulation of contaminants: Among various plants, C. bunplandianus was found to be the best non-accumulator of contaminants mainly U and Mn along with Al, Fe, Ni, Co, Cu, Zn and Se. This plant showed the lowest accumulation of U (0.023 mg/kg; TF: 0.0002) and Mn (4 mg/kg; 0.002) followed by B. moneri (penta grade non-accumulation of Al, V, Fe, Co and U), C. procera (even accumulate Al and Fe, it shows the best in tetra grade non-accumulation of Mn, Cu, Zn and Se) and A. indica (with the penta grade least accumulation of Al, Ni, Co, Se and U).
3.6 Quality checking of inter-laboratory data
The reproducibility of these procedures was compared to the results of an inter-laboratory study by digesting and analyzing the reference material (Lucid Laboratories Private Limited, Hyderabad, India) for quality assurance using the more sensitive technique of ICP-OES Varian Liberty, and the results presented in Fig. 9 show a comparison of the results. Values were found to be within 97 ± 4 %. It is also compared with the previous works of the study area and all the results presented here are more or less following to the published works.
4 Conclusion
Trace element concentrations in water and soil or tailing varied widely within and between sites, and mainly three elements—U, Mn and Al—were found common in water and soil and other seven elements—Fe, V, Ni, Cu, Zn, Co and Se—in soil exceeded the control (threshold) values therefore were identified as major contaminants. Samples with high Fe, Al, V, Ni, Cu, Zn, Co and Se concentrations may due to aquatic or natural process such as neutralization, precipitation, flocculation as well as adsorption and the samples with high Mn and U concentration may due to technical limitations in ore processing that all the uranium present in the ore cannot be extracted and MnO4 exclusively used in the process of leaching the UO3. Therefore, of the above six elements, only two elements U and Mn were identified as major contaminants in the selected sites that need to be remedied.
In-situ study was performed to know bio-concentration and accumulation (TF) of metals and radionuclides in native plant species in uranium-mine-tailing ponds. Variability in elemental concentration and TF has been observed within the same plant species growing under different physicochemical environment and also different plant species growing under the same environment. Bio-concentration and accumulation of elements were found to be more apparent in some plants species; therefore, hyper-accumulator and non-accumulator were identified. Highest accumulation of Al, V, Ni and Co was seen in P. digitalis, and Mn and Cu accumulation favored in E. ferox. The maximum accumulation of Fe, Zn, Se and U was favored in A. indica, B. vitisidae, P. hydropiper and S. spantanium, respectively. It is also identified that plant species with multiple elemental accumulators, i.e., P. vittata can accumulate six elements—Al, V, Ni, Co, Se and U—followed by C. compressus that can accumulate five elements—Al, Mn, Ni, Co and Se—simultaneously, and so on. Plant species with non-accumulation of contaminants Al, Co, Se and U was more apparent in A. indica, Mn, Fe, Ni and Cu non-accumulation favoured in C. bunplandianus and the non-accumulation of V and Zn was seen in E. ferox and C. procera, respectively. It is also observed that some plants with multi-elemental non-accumulation, i.e., C. bunplandianus can non-accumulate of nine elements—Al, Mn, Fe, Ni, Co, Cu, Zn, Se and U followed by A. indica and B. moneri each can non-accumulate of 5 elements and so on.
However, taking in consideration such as plant species with shallow rooted, adapt, growth and biomass production and of the entire above criterion, following plant species were identified each in accumulation and non-accumulation of the identified contaminants and recommended for phytoremediation of tailing ponds of uranium mines.
-
For accumulation of contaminants: P. vittata followed by P. digitalis C. compressus and S. spantanium
-
For non accumulation of contaminants: C. bunplandianus followed by B. moneri, C. procera and A. indica
Soil characteristics and environmental conditions will affect the metal transfer processes. However, there is lack of TF on radionuclides and heavy metals data, and sources reporting on such adequate TF data are very few. The knowledge of the soil–plant relationships in contaminated sites is an important issue, but a striking observation was that the majority of soil-to-plant TF data and phytoremediation studies were reported without information on bioavailability properties of contaminants. Therefore, further comprehensive bioavailability studies are required to compare those contaminants on the availability for root uptake and its accumulation by plants, and also greenhouse experiments are needed to confirm whether the species are accumulators or excluders of the contaminants, only then the conclusion will be made on bio- or phytoremediation. In this scenario, it is essential to invade a simple and cost-effective phytoremediation technique that allows the reduction in metal assimilation from contaminated soils by non-edible indigenous plants.
References
Abu-Khadra, S. A., Abdel-Sabour, M. F., Abdel-Fattah, A. T., & Eissa, H. S. (2008). Transfer factor of radioactive Cs and Sr from Egyptian soils to roots and leafs of wheat plant. IX radiation physics & protection conference, 15–19 November 2008, Nasr City—Cairo, Egypt.
Alharbi, A., & El-Taher, A. (2013). A study on transfer factors of radionuclides from soil to plant. Life Science Journal, 10(2), 532–539.
Alloway, B. J. (1990). Heavy metals in soils. Blackie. Glasgow, London, New York: Wiley.
APHA 4500H + B and 2510B.
Anderson, T. A., Guthrie, E. A., & Walton, B. T. (1993). Bioremediation in the rhizosphere: Plant roots and associated microbes clean contaminated soil. Environmental Science and Technology, 27, 2630–2636.
Bastias, J. G. (1989). Retreatment of radioactive gold bearing tailings and rehabilitation of mill and tailings dump sites at Rockhole and Moline, Northern Territory (a personal view). Australian Mining Industry Council Environment Workshop Papers (Adelaide, 21–25 September 1987), Australian Mining Industry Council, Canberra. Proceedings of Conference ‘Reclamation, A Global Perspective’, Calgary, Alberta, 27–31 August 1989, pp. 319–346.
Basu, S. K., Jha, V. N., & Khan, A. H. (2000). Uptake of radionuclides and trace metals by plants growing on or near uranium tailings, Ninth Natinal Symposium on Environment, Bangalore University, India, June, June 5–7, 2000, pp. 138–141.
BIS (Bureau of Indian Standards). (1993). Specification for drinking water. Indian Standards (IS: 10500). New Delhi.
Burken, J. G., & Schnoor, J. L. (1998). Predictive relationships for uptake of organic contaminants by hybrid poplar trees. Environmental Science and Technology, 32, 3379–3385.
Cardenas, E., Wu, W., Leigh, M., Carley, J., Carroll, S., Gentry, T., et al. (2008). Microbial communities in contaminated sediments, associated with bioremediation of uranium to submicromolar levels. Applied and Environment Microbiology, 74, 3718–3729. doi:10.1128/AEM.02308-07.
CPCB. (1986). General standards for discharge of environmental pollutants part-A: Effluents. The Environment (Protection) Rules, 1986.
CPHEEO. (2009). Recommended guidelines for physical and chemical parameters of drinking water. Volume I: Surveillance, ministry of urban development, New Delhi.
De Rosa, C., Lyon, J., Golden Dreams, & Poisoned Streams. (1997). Mineral Policy Center, Washington DC.
Dietz, A. C., & Schnoor, J. L. (2001). Advances in phytoremediation. Environmental Health Perspectives, 109(Suppl 1), 163–168.
Dobrin, R. I., Dulama, C. N., & Toma, A. (2006). Soil–plant experimental radionuclide transfer factors. Romanian Journal of Physics, 51(1–2), 73–76.
EEB. (2000). The environmental performance of the mining industry and the action necessary to strengthen European legislation in the wake of the Tisza–Danube pollution. European Environmental Bureau. Document No. 2000/016. 32 pp.
EPA U.S. (2006). Sample collection procedures for radiochemical analytes in environmental matrices. December 2006 EPA/600/S-07/001.
EPA. (2009). National recommended water quality criteria. United States Environmental Protection Agency. Office of Water, Office of Science and Technology (4304T). View this document online at http://water.epa.gov/scitech/swguidance/standards/current/.
Friel, J. K., Skinner, C. S., Jackson, S. E., & Longerich, H. P. (1990). Analysis of biological reference materials, prepared by microwave dissolution, using inductively coupled plasma mass spectrometry. Analyst, 115, 269–273.
Gavrilescu, M., Pavel, L., & Cretescu, I. (2009). Characterization and remediation of soils contaminated with uranium. Journal of Hazardous Materials, 163, 475–510. doi:10.1016/j.jhazmat.2008.07.103.
Govindaraju, K. (1994). Compilation of working values and sample description for 383 geostandards. Geostandards Newsletter, 18(Special Issue), 1–158. doi:10.1046/j.1365-2494.1998.53202081.x-i1.
Gupta, R., Kundu, A. C., & Sarangi, A. K. (2003). Uranium mining, milling and tailings disposal—best practices. Presented and published in the proceedings of the National Seminar on “Environmental and sociological Implications of Mining (Coal, Limestone and Uranium) and exploitation of Oil and natural gas in North – East India”, North—East India Council for social Science, Shillong; Jun. 5–6, 2003.
Haines-Young, R., Potschin, M., & Cheshire, D. (2006). Defining and identifying environmental limits for sustainable development. A scoping study. Final Full Technical Report to Defra, 103 pp + appendix 77 pp, Project Code NR0102.
Hegde, A. G., Desai, M. V. M., & Hemalatha, P. (2004). Transfer factors of radionuclides and elements in the terrestrial and fresh water environment of India. Bhabha Atomic Research Center Report.
Huang, J. W., Blaylock, M. J., Kapulnik, Y., & Ensley, B. D. (1998). Phytoremediation of uranium-contaminated soils: Role of organic acids in triggering uranium hyperaccumulation in plants. Environmental Science and Technology, 32, 2004–2008.
Hughes, J. B., Shanks, J., Vanderford, M., Lauritzen, J., & Bhadra, R. (1997). Transformation of TNT by aquatic plants and plant tissue cultures. Environmental Science and Technology, 31, 266–271.
IAEA. (1980). Production of yellow-cake and uranium fluorides. In Proceedings of an advisory group meeting—Paris, 5–8 June 1979.
IAEA-Tecdoc-1403. (2004). The long term stabilization of uranium mill tailings. Iaea, Vienna, 2004. ISBN 92–0–108904–X. ISSN 1011–4289.
Jaiswal, P. C., & Radojecic, M. (2008). Soil, plant and water analysis. Book by Kalyani Publishers/Lyall Bk Depot. ISBN 10: 8127232262.
Jha, S., Khan, A. H., & Mishra, U. C. (2000). Environmental Rn levels around an Indan U complex. Journal of Environmental Radioactivity, 48, 223–234.
Jha, V. N., Shukla, A. K., Tripathi, R. M., Sethy, N. K., et al. (2007). Radionuclide uptake by native vegetation Growing around upcoming U-mining project at Banduhurang, Jharkhand. 15th National Symposium on Environment, Bharathiar University, Coimbatore, June 5–7.
Kerrich, R., & Wyman, D. A. (2007). Review of developments in trace-element fingerprinting of geodynamic settings and their implications for mineral exploration. Australian Journal of Earth Sciences, 44(4). doi:10.1080/08120099708728327.
Koide, H. (2004). Radioactive contamination around Jadugoda uranium mine in India. Research Reactor Institute, Kyoto University April 27, 2004.
Landa, E. R. (2003). Mobilization of radionuclides from uranium mill tailings and related waste materials in anaerobic environments. Journal of Radioanalytical and Nuclear Chemistry, 255, 559–563.
Mandal, A., & Sengupta, D. (2005). Radionuclide and trace element contamination around Kolaghat Thermal Power Station, West Bengal—environmental implications. Current Science, 88(4).
Prach, K., Bartha, S., Joyce, C. B., Pyšek, P., van Diggelen, R., & Wiegleb, G. (2009). The role of spontaneous vegetation succession in ecosystem restoration: A perspective. Applied Vegetation Science, 4(1), 111–114. doi:10.1111/j.1654-109X.2001.tb00241.x.
Raskin, I., & Ensley, B. D. (2000). Phytoremediation of toxic metals: Using plants to clean up the environment. New York: Wiley. ISBN 978-0-471-19254-1.
Shirinian-Orlando, A. (2007). Hazards of uranium mining and milling. Armenian News Network/Groong, 2/5/2007.
Shukla, K. P., Singh, N. K., & Sharma, S. (2010). Bioremediation: Developments, current practices and perspectives. Genetic Engineering and Biotechnology Journal, GEBJ-3.
Singh, L., & Soni, P. (2009). Species selection for revegetation and consolidation of uranium tailings at Jadugud (Written by Administrator 08 Nov 2009 - Last Updated 09 Nov 2009).
Singh, L., & Soni, P. (2010). Concentration of radionuclides in uranium tailings and its uptake by plants at Jaduguda, Jharkhand, India. Current Science, 98(1), 37–49.
Smith, G. M., Weston, R. F., Inc. (1999). Evolution of disposal cell cover design used for uranium mill tailings long-term containment. Wm’99 Conference, February 28—March 4, 1999.
AOAC SW 846 9040 and SW 846 9050. Environment Protection Training and Research Centre, Hyderabad, India. Dated on 1. June, 2009.
APPCB General standards, general standards for discharge of environmental pollutants. http://www.appcb.ap.nic.in/Env-Standards/cat_in_unitop/lga33.htm.
Manmohan Singh. (2004). P.M.’s address at the Golden Jubilee Commemoration functions of the Department of Atomic Energy and foundation stone laying of the Fast Breeder programme. Press Information Bureau Government of India Prime Minister’s Office. 23-October-2004 13:27 IST.
Giri, S., Jha, V. N., Sethy, N. K., Shukla, A. K., Singh, G., & Tripathi, R. M. (2007). Evaluation of radionuclides in terrestrial ecosystem around proposed U-mining sites at Bagjata, Jharkhand. 15th national symposium on environment, Bharathiar University, Coimbatore, June 5–7, 2007.
Malandrino, M., Abollino, O., Buoso, S., Giacomino, A., La Gioia, C., & Mentasti, E. (2011). Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere, 82(2011), 169–178. doi:10.1016/j.chemosphere.2010.10.028.
McIntyre, T. (2003). Phytoremediation of heavy metals from soils. Advance Biochemical Engineering Biotechnology, 78, 97–123.
Mishra, S., Bhalke, S., Manikandan, S. T., Sunny, F., Nair, R. N., Pandit, G. G., et al. (2009). Migration of metals into groundwater from Uranium tailing pond. BARC Newsletter, 309, 309–330.
Mishra, S., Bhalke, S., Suseela, B., Pandit, G. G., & Puranik, V. D. (2008). Speciation of metals in uranium mill tailing using sequential extraction technique. BARC Newsletter, Founder’s day special issue, issue no. 297, October 2008, pp. 285–291.
Munshower, F. F. (1993). Practical handbook of disturbed land revegetation. Boca Raton: Lewis publishers. London, Tokyo: CRC Press (November 9, 1993). ISBN-10:1566700264.
NEERI (National Environmental Engineering Research Institute). (2010). Assessment and remediation of hazardous waste contaminated areas in and around M/s Union Carbide India Ltd., Bhopal. Final Report, June 2010.
Newman, L. A., Strand, S. E., Choe, N., Duffy, J., Ekuan, G., Ruszaj, M., et al. (1997). Uptake and biotransformation of trichloroethylene by hybrid poplars. Environmental Science and Technology, 31, 1062–1067.
Niu, Z., Sun, L., Sun, T., Li, Y., & Wang, H. (2007). Evaluation of phytoextracting cadmium and lead by sunflower, ricinus, alfalfa and mustard in hydrophonic culture. Journal of Environmental Science, 19, 961–967.
Pulford, L. D., & Watson, C. (2003). Phytoremediation of heavy metals on contaminated land by trees—a review. Environment International, 29, 529–540.
Radojevic, M., & Bashkin, V. N. (2006). Practical environmental analysis. Book ISBN: 978-0-85404-679-9.
Roy, P., Balaram, V., Kumar, A., Satyanarayanan, M., & Gnaneshwar Rao, T. (2007). New REE and trace element data on two kimberlitic reference materials by ICP-MS. Geostandards and Research Geoanalytical, 31(3), 261–273. doi:10.1111/j.1751-908X.2007.00836.x.
Şahan, Y., Basoglu, F., & Gücer, S. (2007). ICP-MS analysis of a series of metals (namely: Mg, Cr Co, Ni, Fe, Cu, Zn, Sn, Cd and Pb) in black and green olive samples from Bursa, Turkey. Food Chemistry, 105, 395–399.
Salvarredy-Aranguren, M. M., Probst, A., Roulet, M., & Isaure, M.-P. (2008). Contamination of surface waters by mining wastes in the Milluni Valley (Cordillera Real, Bolivia): Mineralogical and hydrological influences. Elsevier Ltd. All rights reserved.
Sarangi, A. K. (2003). Grade control in Jaduguda uranium mine, Jharkhand. Published in the Transactions of the Mining, Geological and Metallurgical Institute of India (MGMI), Vol. 99, nos. 1–2.
Schnoor, J. L., Licht, L. A., McCutcheon, S. C., Wolfe, N. L., & Carriera, L. H. (1995). Phytoremediation of organic and nutrient contaminants. Environmental Science and Technology, 29, 318A–323A.
Sethy, N. K., Jha, V. N., Shukla, A. K., Sahoo, S. K., Tripathi, R. M., & Puranik, V. D. (2011). Natural radionuclide (U and 226Ra) in water, sediment, fish and plant species in the aquatic environment around uranium mining and ore processing site at Jaduguda, India. Journal of Ecosystem Ecograph, 1, 1. Doi:10.4172/2157-7625.1000103.
Shahandeh, H., Lee, J. H., Hossner, L. R., & Loeppert, R. H. (2001). Bioavailability of uranium and plutonium to plants in soil water systems and the potential of phytoremediation. In G. R. Gobran, W. W. Wenzel, & E. Lombi (Eds.), Trace elements in the rhizosphere (pp. 93–124). London: CRC Press LLC.
Soni, P., Vasistha, H. B., & Kumar, O. (1992). Surface mined lands: Problems and prospectus. Report on Eco-restoration of rock phosphate mined area. ICFRE publication-18, 30 p.
Sudhakar, G., & Muralidhar, R. C. (2008). Landscaping of tailing ponds. Homi Bhabha Centenary DAE-BRNS national symposium on landscaping for sustainable environment, November 20–21, 2008 Proceedings.
Taylor, S. R., & McLennan, S. M. (1986). The chemical composition of the Archaean crust. Geological Society, London, Special Publications, Vol. 24, pp. 173–178. doi:10.1144/GSL.SP.1986.024.01.16.
Terry, N., & Banuelos, G. (Eds.). (2000). Phytoremediation of contaminated soil and water. Boca Raton: Lewis Publishers.
Thompson, P. L., Ramer, L. A., & Schoor, J. L. (1998). Uptake and transformation of TNT by hybrid poplar trees. Environmental Science and Technology, 32, 975–980.
Tripathi, R. M., Sahoo, S. K., Jha, V. N., Kumar, R., Shukla, A. K., Puranik, V. D., et al. (2010). Radiation dose to members of public residing around uranium mining complex, Jaduguda, Jharkhand, India. Oxford Journals Mathematics & Physical Sciences Radiation Protection Dosimetry, 147(4), 565–572.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth’s Crust. Geological Society of America Bulletin, 72, 175–192.
Vidali, M. (2001). Bioremediation: An overview. Pure Applied Chemistry, 73, 1163–1172.
Wang, Z. J. (1999). Major environmental and ecotoxicological process of heavy metal in lean river polluted by discharges from mining activities. Journal for Environmental Sciences, 11(3), 322–327.
WHO (World Health Organization). (2004). Guideline for drinking water quality, 3rd ed., Vol. 1. Recommendations, Geneva, Switzerland.
WHO (World Health Organization). (2006). Guidelines for drinking-water quality [electronic resource]: Incorporating first addendum. Vol. 1, Recommendations. 3rd ed. ISBN 92 4 154696 4.
Wisłocka, M., Krawczyk, J., Klink, A., & Morrison, L. (2006). Bioaccumulation of heavy metals by selected plant species from uranium mining dumps in the Sudety Mts., Poland. Polish Journal of Environmental Studies, 15(5), 811–818.
Seidel, D. C. (1980). Extracting uranium from its ores. Member of the agency’s nuclear materials and fuel cycle section, Division of Nuclear Fuel Cycle. IAEA BULLETIN, Vol. 23, no. 2.
UCIL (Uranium Corporation of India Ltd.) official website: http://www.ucil.gov.in/web/index.asp.
USSR. (2006). Calculation of concentration of hazardous substances contained in industrial emissions. OND-86. Gidrometeoizdat. USSR.
UCC, & Yanagi, T. (2011). Chemical composition of continental crust and the primitive mantle. Arc Volcano of Japan, Lecture Notes in Earth Sciences 136. Berlin, Heidelber: Springer. doi:10.1007/978-4-431-53996-4_2.
Zu, Y. Q., Yuan, L., Jianjun, C., Haiyan, C., Li, Q., & Schvartz, C. (2005). Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environment International, 31, 755–762.
Zhu, Y.-G., & Shaw, G. (2000). Soil contamination with radionuclides and potential remediation. Chemosphere, 41, 121–128.
Acknowledgments
We gratefully acknowledge the financial support from the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), and Govt. of India. The authors acknowledges for keen interest given by Shri. Indrajit Pal, Director General, EPTRI and staff of the Institute for encouragement in this work and also the kind permission given to publish it. Thanks are also due to Assistance received from scientific group of Health Physics Unit, Jaduguda and the Uranium Corporation of India Limited, for facilities and assistance provided during this study are acknowledged. The authors greatly appreciate numerous inputs from review by the committee members of technical programme discussion meeting (TPDM) are also acknowledged for their helpful discussions that helped to improve the quality and presentation of this work. Many members of the EPTRI, AMD and BARC past and present, contributed to both the research presented here and my development as Research Scholar.
Author information
Authors and Affiliations
Corresponding author
Appendix: Mean concentrations (mg/kg) of elements at sampling locations (matrix: water, soil, pant)
Appendix: Mean concentrations (mg/kg) of elements at sampling locations (matrix: water, soil, pant)
Location | Matrix | Al | V | Cr | Mn | Fe | Ni | Co | Cu | Zn | As | Se | Sr | Cd | Pb | U | pH | Ec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
C | Water | 0.37 | 0.02 | 0.29 | 0.56 | 2.08 | 0.04 | 0.12 | 0.00 | 0.14 | 0.04 | 0.12 | 0.29 | 0.00 | 0.13 | 0.00 | 8.37 | 1,057 |
PL | Water | 0.20 | 0.20 | 2.00 | 2.00 | 3.00 | 3.00 | 1.00 | 3.00 | 5.00 | 0.20 | 0.05 | 4.00 | 2.00 | 0.10 | 0.02 | 7.25 | 2,250 |
TL | Water | 0.37 | 0.20 | 2.00 | 2.00 | 3.00 | 3.00 | 1.00 | 3.00 | 5.00 | 0.20 | 0.12 | 4.00 | 2.00 | 0.13 | 0.02 | 7.25 | 2,250 |
Pipe | Water | 0.49 | 0.00 | 0.01 | S | 0.48 | 0.15 | 0.02 | 0.01 | 0.02 | 0.00 | 0.00 | 0.46 | 0.00 | 0.00 | 0.07 | 6.60 | 6,000 |
TP1 | Water | 0.02 | 0.00 | 0.01 | 7.20 | 0.42 | 0.04 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.56 | 0.00 | 0.00 | 1.50 | 6.17 | 5,575 |
Soil | 16,071 | 93 | 183 | 8,065 | 38,630 | 240 | 140 | 141 | 156 | 8 | 22 | 72 | 1 | 22 | 91 | 7 | 1,182 | |
B. moneri | 7.19 | 0.04 | – | 42.65 | 8.93 | 0.43 | 0.17 | 0.71 | 1.11 | – | 0.12 | – | – | – | 0.30 | – | – | |
C. compressus | 43.39 | 0.36 | – | 83.67 | 92.51 | 1.23 | 0.26 | 1.83 | 2.99 | – | 0.03 | – | – | – | 0.64 | – | – | |
I. carnea | 6.70 | 0.06 | – | 37.43 | 57.85 | 0.28 | 0.04 | 3.73 | 17.13 | – | 0.04 | – | – | – | 0.37 | – | – | |
L. camera | 12.21 | 0.07 | – | 41.16 | 74.21 | 0.14 | 0.17 | 6.45 | 32.71 | – | 0.10 | – | – | – | 1.13 | – | – | |
Pteris vittata | 70.61 | 0.68 | – | 197.62 | 108.58 | 1.86 | 0.64 | 1.09 | 1.51 | – | 0.06 | – | – | – | 3.89 | – | – | |
T. latifolia | 16.69 | 0.13 | – | 94.26 | 69.13 | 0.63 | 0.15 | 5.67 | 28.59 | – | 0.03 | – | – | – | 1.82 | – | – | |
R. communis | 18.79 | 0.08 | – | 32.49 | 112.81 | 0.34 | 0.08 | 8.44 | 35.56 | – | 0.00 | – | – | – | 0.47 | – | – | |
TP2 | Soil | 21,213 | 94 | 190 | 1,072 | 15,163 | 164 | 34 | 154 | 89 | 11 | 122 | 36 | 1 | 21 | 22 | 7 | 348 |
C. procera | 52.46 | 0.33 | – | 3.13 | 77.11 | 0.58 | 0.24 | 0.79 | 0.86 | – | 0.27 | – | – | – | 0.21 | – | – | |
V. negundo | 4.80 | 0.03 | – | 56.18 | 94.38 | 0.07 | 0.03 | 9.13 | 51.53 | – | 0.02 | – | – | – | 0.06 | – | – | |
TP3 | Water | 3.17 | 0.01 | 0.01 | S | 0.47 | 0.65 | 0.08 | 0.04 | 0.07 | 0.01 | 0.05 | 0.71 | 0.00 | 0.03 | 0.72 | 5.86 | 5,706 |
Soil | 19,631 | 139 | 161 | 2,993 | 39,741 | 217 | 55 | 209 | 191 | 11 | 12 | 45 | 1 | 27 | 70 | 7 | 1,648 | |
A. conynzoides | 18.50 | 0.13 | – | 43.94 | 38.10 | 0.59 | 0.27 | 4.10 | 6.08 | – | 0.02 | – | – | – | 0.58 | – | – | |
B. vitisidae | 14.98 | 0.13 | – | 74.76 | 103.95 | 0.29 | 0.23 | 17.29 | 57.74 | – | 0.07 | – | – | – | 0.55 | – | – | |
C. esculenta | 11.71 | 0.10 | – | 29.69 | 20.65 | 0.29 | 0.14 | 1.38 | 4.33 | – | 0.13 | – | – | – | 0.16 | – | – | |
D. annulatum | 41.97 | 0.32 | – | 91.20 | 77.60 | 2.15 | 0.41 | 1.87 | 1.33 | – | 0.17 | – | – | – | 0.19 | – | – | |
H. suaeulance | 18.04 | 0.12 | – | 40.17 | 26.06 | 0.51 | 0.35 | 1.27 | 1.76 | – | 0.03 | – | – | – | 2.61 | – | – | |
S. spontanium | 28.24 | 0.16 | – | 29.68 | 60.26 | 0.42 | 0.09 | 3.97 | 16.66 | – | 0.07 | – | – | – | 7.67 | – | – | |
A. indica | 3.15 | 0.06 | – | 61.47 | 84.58 | 0.08 | 0.06 | 10.45 | 25.05 | – | 0.07 | – | – | – | 0.02 | – | – | |
TTP | Water | 0.44 | 0.00 | 0.01 | 3.70 | 0.54 | 0.09 | 0.02 | 0.19 | 0.04 | 0.00 | 0.00 | 0.56 | 0.00 | 0.00 | 4.44 | 6.71 | 4,833 |
Soil | 16,296 | 66 | 108 | 2,716 | 44,640 | 169 | 36 | 350 | 141 | 5 | 80 | 34 | 1 | 19 | 29 | 7 | 1,192 | |
C. bunplandianus | 8.92 | 0.07 | – | 4.00 | 11.07 | 0.16 | 0.03 | 1.07 | 1.57 | – | 0.06 | – | – | – | 0.02 | – | – | |
Euryle ferox | 32.78 | 0.23 | – | 28.71 | 87.80 | 0.24 | 0.62 | 1.50 | 1.24 | – | 0.10 | – | – | – | 0.06 | – | – | |
Paspalidium spp | 17.39 | 0.12 | – | 35.17 | 22.79 | 0.53 | 0.11 | 1.40 | 1.66 | – | 0.03 | – | – | – | 0.22 | – | – | |
S. celearis | 30.64 | 0.15 | – | 51.77 | 42.57 | 0.79 | 0.16 | 3.64 | 2.55 | – | 0.04 | – | – | – | 0.60 | – | – | |
ETP | Water | 0.18 | 0.00 | 0.01 | 2.11 | 0.30 | 0.02 | 0.02 | 0.01 | 0.03 | 0.01 | 0.02 | 0.31 | 0.00 | 0.02 | 0.26 | 7.17 | 3,412 |
Soil | 14,053 | 135 | 152 | 3,925 | 33,614 | 149 | 38 | 135 | 184 | 6 | 20 | 86 | 0 | 20 | 99 | 7 | 890 | |
P. degitalis | 48.35 | 0.38 | – | 94.74 | 75.70 | 2.66 | 0.55 | 2.17 | 3.20 | – | 0.14 | – | – | – | 1.02 | – | – | |
P. hydropiper | 22.64 | 0.26 | – | 49.01 | 19.66 | 0.72 | 0.14 | 0.97 | 2.17 | – | 0.04 | – | – | – | 1.02 | – | – | |
C | Soil | 12,529 | 10 | 170 | 510 | 6,414 | 89 | 23 | 69 | 92 | 13 | 6 | 69 | 0 | 20 | 4 | 6 | 292 |
UCC | Soil | 15.20 | 60.00 | 35.00 | 2.20 | 4.50 | 20.00 | 10.00 | 25.00 | 71.00 | 1.50 | – | 350 | 0.10 | 20.00 | 2.80 | – | – |
TL | Soil | 12,529 | 60 | 170 | 510 | 6,414 | 89 | 23 | 69 | 92 | 13 | 6 | 350 | 0 | 20 | 4 | 6 | 292 |
Rights and permissions
About this article
Cite this article
Laxman Singh, K., Sudhakar, G., Swaminathan, S.K. et al. Identification of elite native plants species for phytoaccumulation and remediation of major contaminants in uranium tailing ponds and its affected area. Environ Dev Sustain 17, 57–81 (2015). https://doi.org/10.1007/s10668-014-9536-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-014-9536-7