Abstract
Heavy metals in varying quantities exist in the earth’s surface as well as natural aquatic bodies. However, beyond a certain threshold concentration, all these heavy metals pose toxicity to a multitude of life forms that come in contact with these metals or ingest substantial quantities through food. The Sundarbans mangrove ecosystem lies at a critical junction between the pristine mangroves in the south and the urbanized setup in the north. This ecosystem receives substantial industrial effluents and signatures of heavy metal accumulation have been observed in the estuarine water column, sediments, floras, and faunas. However, the lentic aquatic systems like that of ponds, which are integral to the daily life of the millions that reside in this zone, have received the least attention. This study was aimed to develop a preliminary idea on certain selected heavy metal accumulation levels in the brackish water ponds of Indian Sundarbans. Concentrations of Cd, Pb, Zn, and Fe were detected in two such ponds of the Indian Sundarbans mangrove ecosystem at two different locations namely Canning (north) and Frazergaunge (south). The observations indicate that immediate anthropogenic activities are increasing the heavy metal levels in the north of the Sundarbans ecosystem. Whereas, the Hooghly River Estuary, which has been carrying a substantial load of heavy metals for a long time has led to significant heavy metal accumulation in the brackish water ponds situated down south through tidal activities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The study was accomplished in the Indian Sundarbans delta, which is a mangrove ecosystem situated on the northeast coastal part of India. Marine water of the Bay of Bengal mixes with river water (fresh water) flowing through the Hooghly River and other tidal rivers like Matla, Vidyadhari, Raimangal, Ichhamati, along with thousands of creeks make the water network of the Indian Sundarbans. The resulting brackish water submerges many low-lying areas of the Indian Sundarbans. Farmers are using such flooded lands filled with saline water for the production of shrimp with temporary embankments. Such brackish water aquacultures are used to culture Tiger shrimp and many other mullets (i.e. Liza tade, Liza parsia, Lates calcarifer).
Several researchers noticed that heavy metal concentrations are steadily increasing in the water and sediment of the Indian Sundarbans (Mitra and Chaudhury 1993; Guhathakurta and Kaviraj 2000). However, the fate of metal concentrations from the estuaries into these low-lying areas is very rarely documented. The goal of the present study was to estimate the metal concentration in the sediment and water of the brackish water pond ecosystem of the Indian Sundarbans.
11.2 Materials and Methods
11.2.1 Study Area
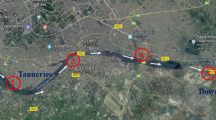
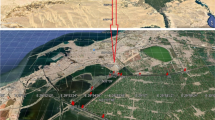
Brackish water ponds were selected at two different localities of Indian Sundarbans (Fig. 11.1). Among these two localities, Frazergaunge (B) lies closer to the Bay of Bengal than Canning (A). In these two sampling sites, tidal waters almost nourished all the ponds through narrow creeks. We have selected two brackish water ponds each from Canning (A) and Frazergaunge (B). The most important difference between these two study points is the tide timing and the salinity range of estuarine water. It is expected that the salinity range will be higher in the Frazergaunge than Canning due to the distance from the Bay of Bengal.
11.2.2 Sampling Strategy
Water and sediment samples from the brackish water pond were collected during August 2017 from at least two ponds situated in Canning and Frazergaunge, respectively. Two samples from each location were shared together to make a composite of each sample for Canning (A) and Frazergaunge (B).
The pond water samples were collected into a pre-rinsed plastic container. Water temperature and water salinity were analyzed on-site. Water samples were stored from 50 to 60 cm in depth. Pond water samples were filtered (0.45 μm filters, cellulose nitrate, Millipore) using a plastic syringe (BD Plastipak, 50 mL) for dissolved trace metals. Samples were acidified with HNO3 (0.24 M). After that samples were kept at 4 °C (in dark environment) until analysis.
For the determination of nitrate and phosphate, filtered pond water was acidified with 1 ml HCl per litre. Acidified water was stored in glass bottles made by borosilicate. Dissolved oxygen was fixed in water samples collected in 100 ml BOD bottles by using alkaline potassium iodide and manganous sulphate. To determine pH, alkalinity, conductivity, ammonia, and nitrite; water samples were stored in airtight polyethylene bottles. Samples were brought to the laboratory as soon as possible. Water samples were preserved at −4 °C before digestion for trace metal analysis.
Sediment samples were collected randomly from the same ponds of Canning and Frazergaunge using a tubular auger. 03 different sediment cores i.e. (0–10 cm), (10–20 cm), and (20–30 cm) were collected from each sampling point. Each core was packed in clean polyethylene bags (acid-soaked) and brought to the University laboratory (store in ice buckets). In the University laboratory the sediments were dried at 105 °C, then stored in clean polyethylene packets (acid-soaked) at −20 °C.
11.2.3 Analytical Protocol
The pond water salinity was measured using a Multikit (WTW 340i Set; Merck, Germany). Water temperature was measured by micro-pH meter made of Eutech Instruments, Singapore (precision—0.001). Acid washed polyethylene bottles (Tight-capped) were used for sample storing. Whatmann filter papers were used to filter the water samples. 2–3 drops of conc. HNO3 were added to water samples to attain a pH ~2 to stop the biological growth and precipitation of metals (Kramer 1994; Eaton et al. 1995). Thereafter, samples were stored at 4 °C until analysis.
Physico-chemical parameters of pond water were measured according to APHA (1995). Sediment sample was mixed with deionized water with a ratio of 1:20 for the measurement of conductivity and pH of sediment using digital conductivity meter and digital lectronic pH meter. Dichromate oxidation method is used to determine the total organic carbon in the sediment. Measureable Phosphate concentration of the sediment was measured according to Olsen et al. (1954), Dickman and Bray’s (1940) and Jackson (1967).
11.2.4 Total Heavy Metal Measurement of Pond Water
200 ml water were digested with 5 ml of acid mixture (HNO3: HClO4 = 9: 4). Then digested sample was filtered using Whatman filter (No. 42) and made up to 50 ml using double distilled water for heavy metal analysis (i.e. Cu, Pb, Cd, and Cr) (APHA 2005). Finally, atomic absorption spectrophotometer (AAS) (Perkin Elmer-4000) is used to detect metal concentration (Scientific Research Laboratory, Kolkata).
11.2.5 Total Heavy Metal Measurement of Sediment Sample (Pond)
Dried sediment were screened through a metallic sieve (63 μm). Then one g of the screened sample was extracted with 10 ml mixture of nitric acid/hydrochloric acid (3:1 v/v) at 130 °C (Otte 1991).
AAS was used to determine the metal concentration in the acid solution. Cd, Zn, Pb and Fe were measured at the wavelength of 228.8 nm, 213.9 nm, 217.0 nm, and 248.3 nm respectively. According to Nafde et al. (1998), the accuracy and precision were checked. The minimum detection limit of Cd, Zn, Pb and Fe were 0.01, 0.01, 0.02 and 2. 50 mg l−1.
11.3 Result
Cd, Zn, Pb, and Fe conc. of water and sediment samples are shortened in Table 11.1. Cd conc. in brackish pond water were very less (0.04–0.14 μg ml−1) in both locations while conc. of Fe were very high (15.4–163.7 μg ml−1) in both. Zn value ranged from not detectable to 0.40 μg ml−1. The mean value of Pb was high in the Canning pond water than in Frazergaunge. Metal conc. showed no variation among the locations (p > 0.05). Metals conc. were very high in the sediment sample except Cd. Moreover, metal conc. (sediment) varied with the locations and depth (Table 11.1, Fig. 11.2).
Background conc. (at 20–30 cm depth) of Fe in sediment was very high in both study area (Fig. 11.2) while the Zn (background) was not detectable in Canning (A) and Frazergaunge (B), on the contrary, showed a high surface conc. of Zn in the sediment of Frazergaunge (B). A significant disparity among the locations was found in Zn and Fe conc. in the sediment samples. Whereas Pb concentration showed very near values among locations and depths. Background concentration of Cd was nine times higher in Canning than Frazergaunge but the surface concentration was four times higher in the Frazergaunge than Canning. Whereas the mean value of Cd was almost similar in both stations (Table 11.1). A very important observation in this study was the almost similar concentration of Fe in all the depth of both station (Fig. 11.2).
Mean (±SD) of the physicochemical parameters of sediment and water of Canning and Frazergaunge brackish water pond are shown in Tables 11.2 and 11.3, respectively. This study found a higher DO value in the brackish pond water of Frazergaunge than Canning but alkalinity showed the opposite trend (Table 11.2). The brackish pond water salinity was two and half times higher in the Frazergaunge than Canning. Pond water temperature was almost similar value during August. Whereas mean nitrate values of pond water were nearly similar in both locations but the phosphate mean value was seven times higher in the Canning than Frazergaunge (Table 11.2). The pH of the pond sediment was similar in both the station but pond water pH was more basic in Frazergaunge than Canning pond (brackish) water (Tables 11.2 and 11.3). The sediment of the Canning pond contained higher organic carbon than Frazergaunge.
11.4 Discussion
The present study reported the metal concentrations of the pond (brackish) water and sediment of the two different kind locations of Indian Sundarbans namely Canning (A) and Frazergaunge (B). The Canning area is densely populated and the anthropogenic impact is higher than the Frazergaunge area. Overall, we have found higher metal concentration in the pond water of Canning than Frazergaunge except for Zn. Higher anthropogenic input might be the reason to observe such kinds of results. On the contrary, metal concentration in the sediment of brackish ponds has shown opposite trend i.e. metal concentration of sediment was higher in the Frazergaunge than Canning except for Zn. The above-mentioned result indicated the metal source in the Frazergaunge also. Mitra (1998) and Bastidas et al. (1999) reported that riverine water supply is the leading source of metal pollution in coastal areas. Hooghly Estuary is the principal supplier of metallic pollutants in the Indian Sundarbans (Guhathakurta and Kaviraj 2000). There are also pieces of evidence of heavy metal accumulation in different biota of Hooghly estuary (Kaviraj 1989; Mitra et al. 1995). Frazergaunge is located adjacent to the mouth of the Hooghly estuary (Fig. 11.1) and incessantly obtain industrial pollution from the adjacent cities of Howrah, Haldia and Kolkata through the Hooghly estuary. Chattopadhyay and Saha (1982) reported that a significant amount of Fe and Zn also enters into the Frazergaunge areas from the Bay of Bengal through tidal waters. Whereas the Canning area (Study point A of the present study) is situated about 22 km away from the Bay of Bengal. So there is no freshwater flow from the Hooghly River. Instead of getting fresh water from the Hooghly Estuary, Canning obtains tide induced sea waters through the Matla Estuary. Thus, the heavy metal load is moderately low in the pond sediment except for Fe. From the above-mentioned discussion, we understand that there are two theories to explain the metal concentration of pond water and sediments in the Canning and Frazergaunge area. First of all, recent higher anthropogenic pressure increases the metal concentration in the pond water of Canning area than Frazergaunge. Secondly, the Frazergaunge area has been receiving industrial run-off through Hooghly estuaries for a long time, and the metal concentration is higher in the sediments of the brackish water ponds than in Canning. The Zn conc. has not increased in the different depths of the pond sediment of Canning and Frazergaunge, because of the controlled use of tidal water in aquaculture during last few years.
The present study showed that Fe conc. (sediment) is very high in the pond ecosystem of the Indian Sundarbans. The most probable reason behind this is the increasing use of shallow tube well water in the aquaculture and agriculture of Indian Sundarbans (Mitra 1998; Guhathakurta and Kaviraj 2000) and tube-well (shallow) water contains a high value of Fe leading to higher deposition of Fe (pond sediment). Bansal (1998) also reported that constant drainage of the nearby city’s sewage may also lead to such a high level of Fe. Guhathakurta and Kaviraj (2000) also described the geological reason for such a higher value of Fe in the sediment of Indian Sundarbans. So that impact of both reasons (anthropogenic and geological) causing the variation of iron conc. in the water and sediment of the pond of Indian Sundarbans. Zn conc. (sediment) also differs between the A and B. In both the stations (A and B) Zn was identified only from the surface sediment (0–10 cm layer) of the pond representing the anthropogenic source of the metal.
From the literature review, we have found only two studies on the sediment Pb concentration of the Indian Sundarbans. Whereas Mitra et al. (1999) reported the value of 9–20 μg g−1 Pb in the sediment (brackish water ponds) in the Kulti, Indian Sundarbans. Another study conducted in the different regions of the Indian Sundarbans revealed that of 7.2–25.2 μg g−1 Pb conc. in the sediment-of the brackish water pond (Guhathakurta and Kaviraj (2000). The present study detected a little bit higher range (12–30 μg g−1) of Pb in the sediment (brackish water pond) of the Indian Sundarbans due to the increasing anthropogenic pressure.
11.5 Conclusion
Overall, this study exhibited significant Cd, Pb, Zn, and Fe levels in the two sampled ponds from the Indian Sundarbans mangrove ecosystem at two different locations namely Canning (north) and Frazergaunge (south). The analysis revealed that the current anthropogenic pollutant load discharged from domestic and industrial sectors are enhancing the degree of pollution in the Canning region. However, in the southern part, the heavy metal load in the mighty Hooghly River Estuary accumulated substantial quantities of heavy metal in the brackish water ponds through tidal activities. Compared to the number of ponds and varying geomorphic setting, the number of studies are acutely scarce to develop a holistic understanding of the heavy metal pollution scenario in these ponds. The future researchers should emphasize on this aspect as heavy metals in the long run are capable of posing severe threat by bioaccumulating in aquatic fauna like fishes and crabs, and biomagnification to higher life forms including the human beings. Human health risk assessment from heavy metal ingestion and dermal contact has already grabbed the attention of the scientific community working in the Sundarbans. However, the scenario from the ponds of Indian Sundarbans remain poorly constrained to date.
References
APHA (1995) Standard method for examination of water wastewater, 19th edn. American Public Health Association, Washington DC
APHA (2005) Standard method for examination of water wastewater, 21st edn. American Public Health Association, Washington, DC, p 3030
Bansal OP (1998) Heavy metal pollution of soil and ponds due to sewage irrigation. Indian J Environ Health 40:51–57
Bastidas C, Bone D, Garcia EM (1999) Sedimentation rates and metal content of sediments in a Venezuelan coral reef. Mar Pollut Bull 38:16–24
Chattopadhyay GN, Saha MS (1982) Distribution of diethylenetriamine pentaacetic acid extractable iron, manganese, copper and zinc in some brackish water mullet pond sediments of West Bengal, India. J Inland Fisher Soc India 12:56–62
Dickman SR, Bray RH (1940) Colorimetric determination of phosphorus. Ind Eng Chem Anal Ed 12:665–668
Eaton AD, Clesceri LS, Greenberg AE (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC
Guhathakurta H, Kaviraj A (2000) Heavy metal concentration in water, sediment, shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackish water ponds of Sunderban, India. Mar Pollut Bull 40(11):914–920
Jackson ML (1967) Sediment chemical analyses. Prentice-Hall, New Delhi
Kaviraj A (1989) Heavy metal concentration in mullet and prawn from the Hooghly estuary. Sci Cult 55:695–699
Kramer KJM (1994) Inorganic contaminants in the water column: sampling and sampling strategy. Int J Environ Anal Chem 57(3):179–188
Mitra A (1998) Status of coastal pollution in West Bengal with special reference to heavy metals. J Indian Ocn Std 5:135–138
Mitra A, Chaudhury A (1993) Heavy metal concentration in oyster Crassostrea cuculata of Sagar Island. Indian J Environ Health 35:119–123
Mitra A, Mandal T, Bhattacharya DP (1999) Concentrations of heavy metals in Penaeus spp. of brackish water wetland ecosystem of West Bengal, India. Indian J Environ Ecoplan 2:97–106
Mitra A, Trivedy S, Gupta A, Bag M, Ghosh I, Chaudhury A (1995) Balanus balanoides as an indicator of heavy metals. Indian J Environ Health 37:42–45
Nafde AS, Kondawar VK, Hasan MZ (1998) Precision and accuracy control in the determination of heavy metals in sediment and water by atomic absorption spectrophotometry. J Indian Assoc Environ Manage 25:83–91
Olsen SR, Cole CV, Watanbe FS, Dean LA (1954) Estimation of available phosphorus in sediments by extraction with sodium bi-carbonate. US Department of Agriculture, CIO, p 939
Otte ML (1991) Heavy metals and arsenic in vegetation of salt marshes and floodplains. Doctoral dissertation. Centrale Huisdrukkerij Vrije Universiteit
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Das, S. (2022). Assessment of Heavy Metal Concentration in Water and Sediment of Brackish Water Ponds of the Indian Sundarbans. In: Das, S., Chanda, A., Ghosh, T. (eds) Pond Ecosystems of the Indian Sundarbans. Water Science and Technology Library, vol 112. Springer, Cham. https://doi.org/10.1007/978-3-030-86786-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-86786-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86785-0
Online ISBN: 978-3-030-86786-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)