Abstract
The hydrochemical characteristics of groundwater in Songnen Plain’s agricultural area were analyzed based on aquifer types and topography classification to evaluate irrigation suitability and factors influencing groundwater quality. Samples of different groundwater types and topographical conditions within the research area were collected and chemical indices, such as sodium adsorption ratio, %Na+, residual sodium carbonate, and magnesium hazard values, were calculated to assess the groundwater suitability for irrigation. The results indicated that groundwater was generally neutral, with low total dissolved solids and slightly high hardness; the dominant anion in groundwater was HCO3−, while Ca2+ was the relatively stable primary cation found in water samples from the high plain and river valley plain. The nitrate in groundwater significantly exceeded WHO drinking water standards, especially in the unconfined water of the high plain, which was due to the large-scale agricultural production activities in the eastern regions. The main reactions in the groundwater system were weathering and dissolution of carbonates and sulfates and ion-exchange reactions. Horizontal zoning in water chemical characteristics was prominent; from the high plain to river valley plain and low plain, the hydrochemistry gradually transitioned from HCO3-Ca-Na to HCO3-Na-Ca and HCO3-Na. Based on the chemical indices, the majority of samples were suitable for agricultural irrigation except for some in the western area with high salinity and sodium hazards. Treatment measures to groundwater and soil should be taken to reduce the possibility of soil salinization and promote crop growth in these latter regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
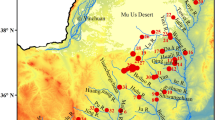
The Songnen Plain is located in the Northeastern region of China (121° 21′–128° 18′ E, 43°36′–49° 26′ N), with a total area of 18.7 × 104 km2. This region is an important base for large-scale commercial grains and animal husbandry; the fertile black soil that runs through the Eastern high plain in a North-South direction is one of the three major black soil regions in the world; and the Central plain with vast pastures that are rich in forage is an important livestock and dairy production base in China (Wang et al. 2009; Chen et al. 2013; Wang et al. 2004). In the Songnen Plain, groundwater also plays a significant function in the social and economic development (Chen et al. 2011; Bian et al. 2016). At present, 46% of the economic and social water consumption in Songnen Plain is drawn from groundwater, and in the central region, the proportion of groundwater exceeds 65%.
With social and economic development, increase in water consumption and advances in groundwater extraction technologies, problems such as decrease in the water level and the yield of groundwater as a result of long-term over-exploitation cannot be ignored (Wen et al. 2005). For example, the over-exploitation of groundwater in the city of Harbin has turned its confined groundwater into unconfined interlayer water, which also caused its groundwater level to be 10–18 m lower than the aquifer roof; as for the Daqing Oilfield, the long-term over-exploitation of groundwater has resulted in a groundwater depression cone of 5560 km2 (Zhao et al. 2009). Furthermore, water pollution has caused reduction in available resources. Increases in the amount of industrial wastewater, municipal solid wastes, domestic wastewater, and frequent agricultural activities have led to the expansion of non-point source pollution, further aggravating groundwater pollution caused by the “3-Nitrogen” (ammonia, nitrite, and nitrate); and significant increases in the total dissolved solids (TDS), total hardness, chlorides, and sulfides in the groundwater in some regions caused further reductions in available groundwater resources (Salama et al. 1999; Sun et al. 2011; Xiao et al. 2014). Additionally, under the influence of factors such as climatic conditions, groundwater burial and cycling conditions, and human irrigation activities, the Songnen Plain has become one of the three centralized distribution areas of soda saline soil in the world, with saline land area accounting for 16.8% of the Plain’s total area. The agricultural development in the area has been severely affected (Yang et al. 2011a, b).
Using mathematical statistics analysis and water chemical analysis methods, this study investigated the evolution of water chemical characteristics in the vast central and eastern region of the Songnen Plain. Because the research area is an important base for grain production, where agriculture is the primary use for water resources, large amounts of groundwater need to be exploited for agricultural irrigation; therefore, the quality of groundwater will directly affect the permeability of soil and crop yield. Therefore, we selected suitable hydrogeochemical indicators to evaluate the suitability of groundwater for irrigation. In past studies, researchers have mainly classified groundwater resources into phreatic water and confined water based on the aquifer types while evaluating water chemistry (Zhang et al. 2007; Chen et al. 2011; Yang et al. 2011a, b). However, here, because the research area is vast and there is a gradual slope from the eastern region towards the central region in topography, there are multiple hydrogeochemical processes at work. Groundwater leaching, evapo-concentration, and ion-exchange reactions are significantly influenced by changes in topography, and there are corresponding changes in water chemical types.
To reveal the interaction mechanisms between groundwater and external environment, both in the vertical and horizontal directions, this study conducted water chemical analyses based on aquifer types and topography classification, respectively, reflecting the current groundwater conditions of the study area comprehensively. The research conclusions can be used as the scientific basis for developing and utilizing groundwater, ensuring a sustainable supply of groundwater resources while preventing soil salinization.
Study area
Geographic location
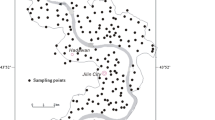
The research area comprises of 24 cities (counties) with a total area of 65,732 km2, including Harbin, Daqing City, Suihua City, and the cities and counties under their jurisdiction (Fig. 1). The topography within the area includes the high plain, the low plain, and the Songhua River valley plain. The high plain is located to the east of the research area, with an altitude of 180–400 m; the differences in topography are relatively large, with a gradual slope running from the eastern region towards the central region. The low plain is distributed to the west of the high plain, with altitudes generally between 130 and 160 m; and the topography is flat and open, with a gentle tilt running from the north to the Songhua River valley. The Songhua River valley plain is distributed in a zonal pattern along the Songhua River and its tributaries. The research area is characterized by temperate continental monsoon climate, exhibiting semi-humid and semi-arid characteristics, where rainfall decreases gradually from East to West, with multi-year average precipitation between 450 and 550 mm and an evaporation rate between 1200 and 1600 mm.
Hydrogeology
Quaternary unconfined water of the study area is not developed and has poor water quantity. Lithology of the aquifer in the high plain consists of the middle-Pleistocene sand and gravel, while in the low plain consists of the upper-Pleistocene silty sand. The aquifer consists of fluvial sand and gravel layers containing large amounts of sediment, clay, and lens. The recharge and discharge relationship among the aquifers is complex. The high plain mainly comprises of the Quaternary and Cretaceous confined aquifers, where the Quaternary confined aquifer is the main aquifer. The Quaternary aquifer lithology is characterized by sand and gravel in either middle-Pleistocene or lower-Pleistocene, with multiple layers of clay layer sandwiched in-between, and the particle size is coarse with good psephicity. In the south region, the aquifer is rich in water resources, while the thickness of aquifer decreases in the Suihua, Hailun, and Zhaodong in the North. The aquifer is surrounded by Cretaceous mudstone and argillaceous sandstone which are also buried at the bottom; therefore, the recharge condition is poor as the water resource can only be replenished via leaking recharge when precipitation seeps through the overlying clay layer.
As for the low plain, Quaternary, Tertiary, and Cretaceous multi-layer confined aquifers are distributed within the region, lithology of the Quaternary confined aquifer is mainly characterized by gray gravel of medium gravel psephicity. The lowermost Cretaceous aquifer is generally not significant in water supply, except for the Zhaodong and Daqing City, where it serves as one of the main aquifer for Daqing Oilfield, as the burial depth of the aquifer is relatively shallow, with relatively great cumulative thickness and good water-richness.
Agricultural irrigation
Agriculture, the main consumer of water resource, generally consumes more than 70% of all groundwater exploitation. In terms of the layer of exploitation in the research area, groundwater is mainly extracted from the unconfined water and Quaternary confined water; moreover, for the position of exploitation in the Songnen Plain, the region with the most intense groundwater extraction is at the Songhua River groundwater system in the Southeast of the research area, the groundwater extraction modulus is 5.31 × 104/ (km2 a), mainly due to the high water consumption by agriculture in the region (Zhao et al. 2009). Not only does irrigated water affect the salt and water absorption of crops by means of osmolality, it also affects the content of exchangeable sodium and residual sodium carbonate in soil. Therefore, the quality condition of groundwater being exploited and used in large quantities is a key factor influencing agriculture production and soil properties.
Materials and methods
Sampling and chemical analysis
Taking into account the actual distribution of aquifer and current status of topography, 94 unconfined wells and 113 confined wells were collected with 500-mL polyethylene bottles in August of 2013 (Fig. 1). After being washed by deionized water and distilled water, prior to sampling, the bottles were thoroughly rinsed for two to three times using the groundwater to be collected. Immediately following the collection of a sample, conductivity meter and pH meter were used to determine the electrical conductivity (EC) and the pH value of the sample, through these processes all pH values and 170 EC data (including 79 unconfined water and 91 confined water) were obtained; the remaining water samples were transported to laboratory immediately after being sealed and labeled, the water samples were stored at 4 °C for testing other basic parameters. Of the major cations, potassium (K+) and sodium (Na+) were analyzed using atomic absorption spectrophotometry, the total hardness (the sum of Ca2+ and Mg2+) and calcium (Ca2+) were determined using the EDTA titration method, then the magnesium (Mg2+) was determined by the difference of total hardness and calcium, and ammonium (NH4+) was analyzed by spectrophotometry. Of the anions, sulfate (SO42−) was analyzed using ionic chromatography, chloride (Cl−) was measured by AgNO3 titration method, carbonate (CO32−) and bicarbonate (HCO3−) were determined using double indicator titration, and nitrate (NO3−) and nitrite (NO2−) were analyzed by spectrophotometry. The total dissolved solids (TDS) were determined by 105°°C weight method.
Methods for water quality assessment
Ion balance can be used to determine the reliability of water analysis data. Formula is as follows:
where E is relative error and nc and na respectively represent the milligram equivalent of cation and anion.
In the experiment, assuming no errors, the total amount of anionic milligram equivalent in water is very close to that of cation. If Na+ and K+ are measured values, their relative error value E should be < 5%. If Na+ and K+ are calculated, E should be zero or near zero; if the value of E exceeds this range, the data of the water analysis is unreliable and must be re-collected and re-measured.
This paper selected key irrigation water quality parameters for conducting the suitability evaluation of groundwater for irrigation.
Sodium adsorption ratio (SAR) is calculated as
As the interaction between Na+ and soil reduces the permeability of soil, calculation Na+ percentage is also a common method for evaluating the suitability of water for agricultural irrigation (Singh et al. 2011; Raju 2011; Kshetrimayum and Bajpai 2012).
Percent sodium (%Na+) is calculated as
When the concentration of carbonate is higher than the concentration of alkaline earth metal in agricultural irrigation water, the excess carbonate would bind Na+ to form NaHCO3, and affect the structure of soil. Therefore, the contents’ relationship between carbonate and alkaline earth metal can be calculated to evaluate the suitability of agricultural irrigation water. The concentration of excessive carbonates is termed as residual sodium carbonate (RSC), which is an indicator that reflects the alkaline hazards in irrigation water (Eaton 1950).
Residual sodium carbonate (RSC) is calculated as
Szaboles and Darab have proposed a magnesium hazard to assess the suitability of agricultural irrigation water. Under normal conditions, a high level of Mg2+ is caused by exchangeable Na+ in irrigated soils; when the content of Mg2+ in irrigation water reaches a certain level, magnesium alkalizing effects may take place in soil, thereby affecting the structure of soil and producing poisonous effects on crops.
The magnesium hazard is expressed in terms of magnesium hazard (MH), which is calculated as
where all ionic concentrations are expressed in milliequivalents per liter and figures of USSLS and Wilcox were discussed to evaluate the groundwater irrigation applicability in the study area.
Results and discussions
Descriptive statistics
Statistical analysis results of the chemical parameters of the water samples collected are shown in Tables 1 and 2. The tables are based on aquifer types (94 unconfined water samples and 113 confined water samples) and topography conditions (52 samples in low plain, 102 samples in high plain and 53 samples in river valley plain), respectively. Apart from pH values, the measurement unit for the concentration of all other chemical parameters is milligrams per liter (mg/L). SD and CV are short for standard variance and coefficient of variation, respectively.
According to the ion balance, the relative error range for 94 confined water samples varied as 0.43–3.14 and the mean value is 1.24. The range in error for 113 unconfined water points is 0.34–2.11, with an average value of 0.97. In terms of topography, the maximum relative error of the valley plain is 2.44, minimum relative error is 0.61, and mean is 1.06. The error range for high plain samples is 0.29–3.3 and the mean value is 2.12. The relative error range for valley plain samples is 0.43–2.67 and the mean value is 2.04. The ion balance relative errors are within 5%, so the water quality data is reliable.
The following conclusions are drawn from the results presented in Table 1.
-
i.
Groundwater pH in the study area is generally neutral, with low TDS and slightly high hardness. A few slightly alkaline water samples were found in confined water; while the total hardness and TDS values of unconfined water were significantly higher than those of confined water, which may have been caused by more common mineral dissolution and salinization in unconfined water under the influence of topography, climate, and human activity.
-
ii.
The chemical composition of anions in groundwater was primarily HCO3− while Ca2+ was the relatively stable primary cation, with the highest average value and relatively small coefficient of variation.
-
iii.
Compared to HCO3− and Ca2+, the coefficient of variation of Mg2+ was relatively small, indicating stable concentrations. As for Na+, Cl−, and SO42−, although their average values were slightly lower, their contents in some local areas were high, and the coefficients of variation were relatively large. For example, in confined water, the average value of Na+ was close to that of Ca2+, but the maximum value was significantly greater than that of Ca2+.
These conclusions indicate that Na+, Cl−, and SO42− are ions sensitive to changes in the external environment, and were the main variables contributing to the evolution in hydrochemistry.
The analyses presented in Table 2 reveal the following.
-
i)
The mean total hardness values for groundwater in different regions were generally similar; however, the average value of TDS in groundwater samples from the low plain was significantly higher than those from the high plain and river valley plain, possibly as a result of more intense evapo-concentration effects in the low plain.
-
ii)
HCO3− remained the primary anion in groundwater across different topographies; however, compared to the high plain and river valley plain, the average HCO3− in groundwater in the low plain was significantly higher.
-
iii)
Ca2+ was the primary cation with relatively stable content in groundwater samples of the high plain and river valley plain. In contrast, in the low plain, the average value of Na+ was significantly higher than that of Ca2+, and its coefficient of variation was also lower.
These statistical results all indicate a distinct hydrogeochemical evolution process in the low plain.
The nitrogen speciation statistical results, i.e., ammonium, nitrate, and nitrite, in Table 1 indicate that the concentrations of nitrogen compounds in groundwater significantly exceed the standard; the maximum content of nitrate in unconfined and confined water samples were as high as 777.9 and 545.5 mg/L, respectively. In particular, the average concentration of NO3− in unconfined water was 96.5 mg/L, which was also significantly higher than the threshold provided in World Health Organization’s (WHO) Drinking Water Quality Standards (50 mg/L). Of the water samples tested, 32 samples exceeded the standards, accounting for 34% of all unconfined water samples. Due to non-artesian water pollution infiltration, nitrate pollution of confined water was obvious, as 17.7% of confined water samples did not meet the standards. The statistical results in Table 2 indicate that locations with high content of NO3− were more concentrated in the high plain in the eastern region, causing the average NO3− content in groundwater in the area to be as high as 96.8 mg/L, with an over-limit ratio of 38.2%. In the low plain and valley plain, the respective average concentrations of NO3− were 23.7 and 31.1 mg/L, and their over-limit ratios were 9.6 and 15.1%, respectively. In addition, two samples of confined water taken from groundwater in the low plain and valley plain, respectively, also indicated over-limit NO2− concentrations (3 mg/L). Although NO2− is a minor component, whose average content is also within the threshold value indicated in the WHO Drinking Water Quality Standards, its high environmental toxicity poses significant threats to human health, and is therefore an important nitrogen pollution indicator to be monitored closely.
In conclusion, the statistical results indicate relatively severe nitrate pollution in the research area groundwater. The nitrogen in the water is sourced mainly from the metabolism of organisms, corruption, loss of nitrogen fertilizer, and discharge of industrial wastewater and domestic sewage. The study area is a major grain-producing region, with a large range of crops and high fertilizer usage. The infiltration coefficient of rainfall is 0.3–0.7 and the permeability is high. The fertilizer nitrogen applied to the farmland is vulnerable to precipitation or water leaching into groundwater, which caused the nitrate content of groundwater to rise. Human activity is the direct cause of the groundwater nitrogen pollution. Therefore, effective management and control should be prioritized to address this problem.
Factor analysis of ion ratios
According to groundwater flow system theory and hydrogeochemical principles, groundwater chemistry field is the real “record” of groundwater flow system. To understand and illustrate hydrogeochemical processes, concentrations of different major elements and their interrelationship were studied. If the groundwater facies formed in different causes or conditions, the ratios of certain ions may have clear differences, which can be used to infer groundwater evolution (Reddy and Kumar 2010).
Dominant dissolution reactions of calcite, dolomite, and gypsum in a system will result in samples plotted in (Ca2+ + Mg2+) versus (HCO3− + SO42−) space close to the 1:1 line; in areas where Ca2+ is the main cation, those that fall below the 1:1 line would result from the effects of ion-exchange reactions. In addition, the Na-Cl relationship has often been used to identify the mechanisms for acquiring salinity in semi-arid regions. The ratio of Na+ versus Cl− will be 1 if halite dissolution is responsible, whereas if the ratio is greater than 1, it is typically interpreted as Na+ released from a silicate weathering reaction or ion-exchange reactions. Furthermore, studies of Ca2+/Mg2+ ratios in groundwater have suggested that ratios close to 1 indicate dissolution of dolomite, a higher ratio indicates greater calcite contribution, and ratios larger than 2 indicate significant the silicate mineral dissolution (Maya and Loucks 1995). Figure 2 shows the ion ratio relationships under different burial and topographical conditions. The oblique lines in Figs. 2a–d are 1:1 equilibrium lines, while the lines in Fig. 2e, f are the 1:1 and 2:1 lines, respectively.

As shown in Fig. 2a, a majority of groundwater points are distributed along or near the 1:1 line, which implies that the primary reactions in the groundwater system were weathering and dissolution of carbonates and sulfates, with additional ion-exchange reactions. In Fig. 2c, most water points lie above the 1:1 line, which indicates that the groundwater had ion-exchange reactions during runoff. Silicates, such as granite, are widely distributed in the lower strata; therefore, it is very likely that Na+ was released through the reaction expressed below:
The above reaction would lead to an increase in Na+ concentrations in the aquifer, and would have produced the kaolinite that is widely distributed as lenses in the lower strata of the research area. In addition, the rock-soil Na+ of some points would have exchanged with Ca2+ in the water, leading to a larger Na+ concentration than Cl− concentration. Statistical results in Fig. 2e indicate that the ion ratio conditions for Ca2+ (meq/L) and Mg2+ (meq/L) were complex in the unconfined water due to relatively larger differences in the nature of the subsystems. In comparison, the figure indicates that dissolution of silicates was a key reaction process in the confined water, which is consistent with the previous discussion.
The differences become more prominent based on the ion ratio analysis performed based on different topographical conditions. Figure 2b shows that groundwater samples from the high plain mainly are distributed above or near the equilibrium line, while river valley plain samples are primarily distributed near the 1:1 line, and low plain samples are primarily below the equilibrium line. Flow alternation is more active on the high plain and river valley plain, and the main reactions were weathering and dissolution of carbonates and sulfates; for samples in the high plain with ratios greater than 1, silicate dissolution would also have taken place (Azaza et al. 2011; Kumar et al. 2009). As a result, Ca2+ and Mg2+ components in the groundwater could fully exchange with Na+ in the soil during the runoff process, thereby leading to a decrease Ca2+ and Mg2+ concentrations and an increase in Na+. As also shown in Fig. 2d, due to the effects of ion exchange, the ratios of Na+ versus Cl− in most water samples in the low plain were greater than 1. In the river valley plain, precipitation has a significant impact on groundwater due to its shallow burial, and groundwater is also influenced by the discharge of high plain groundwater and dissolution of carbonates. As a result, the Na+ and Cl− concentrations in river valley plain groundwater were low. Furthermore, it can be inferred from Fig. 2f that the rock minerals undergoing weathering and dissolution in the low plain were likely dolomite and calcite, whereas groundwater in the high plain indicates some dissolution of silicates (Fig. 2b).
Groundwater is an open system. According to Tóth (Tóth 1963) proposed by that in the groundwater flow system, the groundwater was continuously carrying out material exchange with the aquifer medium and the external environment in the flow system. In the process, ion exchange, dissolution and precipitation would occur, resulting in the evolution of water chemical composition. It is an important aspect of groundwater system evolution research. According to the water chemistry characteristics of aquifer types and hydrogeological conditions, the specific hydrogeochemistry processes in local or regional systems should be studied using isotopes, microorganism, and other methods, which would help to provide direction for future research.
Groundwater types
Expressions of groundwater types are were calculated by using the Shoka Lev groundwater classification method, and the results of greater frequency results are shown in Tables 3 and 4. The Piper trilinear diagrams in Fig. 3 were constructed using AquaChem Scientific Software to illustrate the relative contents of the major ions; Fig. 3a, b represents Piper diagrams based on aquifer types and topography condition classifications, respectively.
As shown in Table 3, in terms of anionic components, the bicarbonate type dominated both confined and unconfined water, whereas sulfuric, chloride, and nitrate types were distributed locally. As for cations, calcium type, followed by sodium type dominated. HCO3-Ca was the chemical type with the highest frequency of occurrence, accounting for 18.09 and 30.97% of unconfined and confined water samples, respectively. Based on statistics, there were 35 hydrochemical types of unconfined water and 22 types of confined water in the study area. Unconfined water is comprised of complex and diverse chemical components under the direct influence of factors such as topography, climate, soil and vegetation, and forms nitric acid type water under the influence of long-term agricultural activities. In addition, in cities and towns with dense population and developed industries, human production and living activities, in particular the impacts from industrial waste discharge, has resulted in an increase in chloride ion content in unconfined water and even the formation of chloride type of water (Table 3). The confined water hydrochemical type remained dominated by HCO3-Ca, as unconfined water resources is relatively scarce in the study area, and the top layer of confined aquifer is shallow, thereby a semi-open weak reduction environment has formed (Zhao et al. 2009). However, under the influence of a deep geological environment, confined water exhibited a more prominent increase in the ratio of HCO3-Ca-Na, HCO3-Na-Ca, and HCO3-Na types compared to unconfined water.
A relatively prominent horizontal zoning phenomenon was also observed in the water chemical characteristics of groundwater in the study area. Groundwater where is from the high plain drains into groundwater in the river valley plain and low plain. During this process, hydrology alternation gradually changes from active to slow, and the main hydrochemical reactions transitioned from lixiviation to evapo-concentration and ion-exchange. Additionally, hydrochemical types also gradually changed from HCO3-Ca and HCO3-Ca-Mg types to HCO3-Ca-Na, HCO3-Na-Ca and HCO3-Na types of groundwater. Compared to groundwater in the river valley plain, groundwater in the low plain was characterized by gentle inclining topography and continuous subsidence, which created a more conducive environment for soda saline reactions in sediments and groundwater (Zhang et al. 2007). As a result, the common water chemical types in the region are HCO3-Na-Ca and HCO3-Na, which jointly accounted for 55.77% of all groundwater samples taken from the low plain.
The Piper trilinear diagram (Fig. 3) (Piper 1944) and the previous analysis indicate that the water sample points in the study area were primarily distributed in the 5th zone in the diamond-shaped area, where carbonate hardness exceeds 50%. In contrast, groundwater samples distributed in the 4th zone resulted from pollution from human activity. Generally, the groundwater chemical components in the high plain and river valley plain were alkaline earth metal > alkali metal and weak acid radicals > strong acid radicals, which are key characteristics of low-salinity water formed through lixiviation. This finding also shows that in natural environments, the concentration effects in these regions were not strong and continental salinization was at the early stages. However, with the transition from recharge and runoff areas to discharge area, the relative Ca2+ and Mg2+ concentrations decreased and Na+ increased in the low plain, and the chemical components gradually evolved into primarily alkali metal and weak acids.
Evaluation of agricultural irrigation suitability
Salinity hazard versus sodium hazard
Salinity has significant impacts on soil alkalization and normal crop growth; when groundwater with high salinity is used for irrigation, it can cause the soil to harden and inhibit crop growth. For assessing the suitability of irrigation water, salinity hazard can be expressed using the water EC values.
The measured EC values for unconfined water ranged between 362 and 3080 μS/cm in the study area; the calculated SAR values were between 0.31 and 9.71. The measured EC values in the confined water ranged between 216 and 3140 μS/cm, while the calculated SAR values were between 0.27 and 29.61. To facilitate a more intuitive assessment of irrigation water quality, groundwater points were plotted in the USSLS diagram, which provides a comprehensive reflection of salinity and sodium hazards (Fig. 4).
The USSLS diagram classifies water quality into 16 zones based on the suitability of water for irrigation; the salinity hazard is then subdivided into low-salinity (C1, < 250 μS/cm), medium-salinity (C2, 250–750 μS/cm), high-salinity (C3, 750–2250 μS/cm), and very-high-salinity (C4, > 2250 μS/cm) hazards, which are considered good, moderate, poor, and very poor water classes, respectively. Similarly, the sodium hazard can also be subdivided into low-sodium (S1, < 10 μS/cm), medium-sodium (S2, 10–18 μS/cm), high-sodium (S3, 18–26 μS/cm), and very-high-sodium (S4, > 26 μS/cm) hazards, or good, moderate, poor, and very-poor classes, respectively.
The statistical results indicate that approximately 26.6% of unconfined water samples and 57.1% of confined water samples were in the C2–S1 zone, indicating medium-salinity hazard (C2) and low-sodium hazard (S1), which can be used for irrigation on most soil types. In comparison, 60.8% of unconfined water samples and 28.6% confined water samples were in the high-salinity (C3) and low-sodium (S1) hazard zones. Groundwater samples in this zone were unsuitable for irrigation of soils with restricted drainage; soils with good drainage systems and special management for salinity control can use water from this zone if crops are selected with a certain level of salt tolerance. Most water samples from the study area fall in zones C2–S1 and C3–S1, which indicates that the hazard level for exchangeable sodium in groundwater is low. However, the saline content of groundwater in the study area should be managed carefully, especially when unconfined water is used for irrigation, some saline filtering should be performed.
Figure 4 shows that all other water samples, excluding four C1–S1 water samples in the Southwest, have relatively high irrigation hazards and should be monitored closely (Table 5). These hazardous groundwater samples were primarily from the low plain to the western study area, e.g., the Daqing, Lindian, and Anda regions. As explained in the previous analysis, these zones are located in the groundwater discharge zones, and the hydrological cycle in these regions is relatively slow while the evaporation are intense, resulting in relatively high saline-alkali concentration groundwater. When using these groundwater samples for irrigation, specific soil management measures should be put in place based on different saline contents, such as good drainage systems, saline filtering, and increasing organic matter; in addition, for regions where the saline-alkali hazard in groundwater has reached to very high level, measures should be taken in advance to prepare for likely adverse effects on crops.
Percent sodium
The sodium value ranges in unconfined and confined groundwater were 5.79~74.31% and 4.93~97.2%, respectively. Generally, when SSP > 60%, the water becomes unsuitable for agricultural irrigation. Statistical results indicate that 3.2% of unconfined water samples and 14.2% of confined water samples in the study area were unsuitable for irrigation, as shown in Tables 6 and 7.
The relationship between percent sodium (%Na+) and total salt concentration (EC) is shown in terms of Wilcox diagram for classifying groundwater irrigation quality (Wilcox 1981). Results indicate that 29.1% of unconfined water samples and 58.2% of confined water samples were in the excellent to good zone, and 50.6% of unconfined water samples and 30.8% of confined water samples were in the good to permissible zone; based on these results, the groundwater in the study area was generally suitable for agricultural irrigation. However, due to high EC values and sodium contents in some unconfined water and confined water points, four unconfined water samples and four confined water samples fell within the permissible to doubtful zone, ten unconfined water samples and four confined water samples fell within the doubtful to unsuitable zone, and the remaining four water points fell within the unsuitable zone, as shown in Fig. 5 and Tables 6 and 7.
Residual sodium carbonate
Irrigation suitability is classified based on residual sodium carbonate (RSC) calculation results. An RSC < 1.25 meq/L indicates water quality is suitable for irrigation; 1.25 meq/L < RSC < 2.5 meq/L indicates water quality is relatively suitable for irrigation; and RSC > 2.5 meq/L indicates water quality is unsuitable for irrigation. An increase in the RSC value indicates an increased Na+ absorption by soil and decreased soil permeability, which is unsuitable for normal crop growth.
The RSC values for unconfined water were between − 18.2 and 11.2 meq/L and between − 16.1 and 12.8 meq/L for confined water, showing that the groundwater study area varies significantly, and different regions are under the control of various factors, which contributed to significant differences in ionic components across different zones. Approximately 16.0% of unconfined water samples and 23.0% of confined water samples had an RSC > 2.5 meq/L, which is unsuitable for irrigation (Tables 6 and 7). Smaller percentages, 8.5% of unconfined water samples and 12.4% of confined water samples, were within the critical range (1.25–2.50 meq/L). The majority of the RSC values, 75.5% of unconfined water samples and 64.6% of confined water samples, were < 1.25 meq/L, indicating suitability for agricultural irrigation. Furthermore, 47.3% of the groundwater samples showed negative RSC values, as the carbonates were less than alkaline earth, and calcium and magnesium also bond with other ions in addition to carbonates (Jasmin and Mallikarjuna 2016).
Magnesium hazard
Szaboles and Darab proposed a magnesium hazard to assess the suitability of agricultural irrigation water. Under normal conditions, a high level of Mg2+ is caused by exchangeable Na+ in irrigated soils; when Mg2+ concentrations in irrigation water reach a certain level, magnesium alkalizing effects may take place in soil, thereby affecting the soil structure and poisoning crops.
Groundwater with MH > 50% is generally unsuitable for irrigation purposes. In the groundwater samples analyzed, the MH variation range in unconfined water was 11.07–76.08 and 6.21–62.79% in confined water; both exceeded the limit, by 11.7 and 4.4%, respectively. The unsuitable water samples were mainly distributed in cities or counties in the western study area, such as Lindian, Daqing, and Zhaozhou (Tables 6 and 7); these areas have potential for magnesium alkalization in the soil from irrigation.
Conclusions
This study has conducted water chemistry analysis based on the aquifer types and terrain characteristics, to examine the current groundwater conditions. The following conclusions were drawn.
-
(1)
The groundwater in the study area was generally neutral, with low TDS and slightly high hardness. The main anion in groundwater was HCO3−, while Ca2+ was the primary cation in the high plain and river valley plain. Na+, Cl−, and SO42− were the main variables contributing to the evolution of the hydrochemical type. Nitrate concentrations in groundwater significantly exceeded WHO standards, especially in the unconfined water of the high plain, which was due to the large-scale agricultural production activities in the eastern regions.
-
(2)
The main reactions in the groundwater system were weathering and dissolution of carbonates and sulfates, and ion-exchange reactions were prevalent throughout the system. Groundwater samples were primarily bicarbonate type in both confined and unconfined water, whereas sulfuric, chloride, and nitrate types were distributed locally. Under the influence of topography, groundwater from the high plain drains into groundwater in the river valley plain and low plain resulted in horizontal zoning in water chemical characteristics across the study area. The hydrology gradually changes from being active to slow, which resulted in the main hydrochemical reactions transitioning from lixiviation to evapo-concentration and ion-exchange.
-
(3)
HCO3-Ca was the chemical type with the highest frequency of occurrence. With the transition from recharge and runoff areas to discharge area, the relative concentrations of Ca2+ and Mg2+ decreased and Na+ concentrations increased in the low plain, and the chemical components gradually evolved to being primarily alkali metal and weak acids. Hydrochemical types gradually changed from HCO3-Ca and HCO3-Ca-Mg types to HCO3-Ca-Na, HCO3-Na-Ca, and HCO3-Na types. The regional variation in groundwater chemical type contains important information on the groundwater circulation evolution.
-
(4)
According to EC, SAR, %Na+, RSC, and MH values, the majority of samples are suitable for agricultural irrigation; however, some samples from the low plain in the western study area, e.g., Lindian, Daqing, and Zhaozhou, had levels of salinity and sodium that reached relatively high hazard classes, and there is a possibility of magnesium alkalization in the soil. Therefore, different treatment measures in groundwater and soil should be taken to reduce the possibility of soil salinization and promote crop growth.
References
Azaza, F. H., Ketata, M., Bouhlila, R., Gueddari, M., & Riberio, L. (2011). Hydrogeochemical characteristics and assessmentof drinking water quality in Zeuss–Koutine aquifer, southeastern Tunisia. Environmental Monitoring and Assessment, 174, 283–297.
Bian, J. M., Liu, C. H., Zhang, Z. Z., Wang, R., & Gao, Y. (2016). Hydro-geochemical characteristics and health risk evaluation of nitrate in groundwater. Polish Journal of Environmental Studies, 25(2), 521–527.
Chen, Z. Y., Wei, W., Liu, J., Wang, Y., & Chen, J. A. (2011). Identifying the recharge sources and age of groundwater in the Songnen Plain (Northeast China) using environmental isotopes. Hydrogeology Journal, 19, 163–176.
Chen, Y. Q., Zhang, G. X., Xu, Y. J., & Huang, Z. G. (2013). Influence of irrigation water discharge frequency on soil salt removal and rice yield in a semi-arid and saline-sodic area. Water, 5, 578–592.
Eaton, F. M. (1950). Significance of carbonates in irrigation waters. Soil Science, 69, 123–133.
Jasmin, I., & Mallikarjuna, P. (2016). Evaluation of groundwater suitability for irrigation in the Araniar River Basin, South India—a case study using Gis approach. Irrigation & Drainage, 64(5), 600–608.
Kshetrimayum, K. S., & Bajpai, V. N. (2012). Assessment of groundwater quality for irrigation use and evolution of hydrochemical facies in the Markanda River Basin, Northwestern India. Journal of the Geological Society of India, 79, 189–198.
Kumar, S. K., Rammohan, V., Sahayam, J. D., & Jeevanandam, M. (2009). Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environmental Monitoring and Assessment, 159, 341–351.
Maya, A. L., & Loucks, M. D. (1995). Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. Journal of Hydrology, 172, 31–59.
Piper, A. M. (1944). A graphical interpretation of water–analysis. Transactions American Geophysical Union, 25, 914–928.
Raju, N. J. (2011). Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environmental Geology, 52, 1067–1074.
Reddy, A. G. S., & Kumar, K. N. (2010). Identification of the hydrogeochemical processes in groundwater using major ion chemistry: a case study of Penna-Chitravathi River basins in southern India. Environmental Monitoring and Assessment, 170, 365–382.
Salama, R. B., Otto, C. J., & Fitzpatrick, R. W. (1999). Contributions of groundwater conditions to soil and water salinization. Hydrogeology Journal, 7, 46–64.
Singh, A. K., Tewary, B. K., & Sinha, A. (2011). Hydrochemistry and quality assessment of groundwater in part of Noida metropolitan city, Uttar Pradesh. Journal of the Geological Society of India, 78, 523–540.
Sun, X. Y., Zhou, Q. X., Ren, W. J., Li, X. H., & Ren, L. P. (2011). Spatial and temporal distribution of acetochlor in sediments and riparian soils of the Songhua River Basin in northeastern China. Journal of Environmental Sciences, 23, 1684–1690.
Tóth, J. (1963). A theoretical analysis of groundwater flow in small drainage basins. Journal of Geophysical Research, 68(16), 4795–4812.
Wang, H. X., Wan, Z. J., Yu, S. P., Luo, X. Z., & Sun, G. Y. (2004). Catastrophic eco-environmental change in the Songnen Plain, Northeastern China since 1900s. Chinese Geographical Science, 14(2), 179–185.
Wang, L., Seki, K., Miyazaki, T., & Ishihama, Y. (2009). The causes of soil alkalinization in the Songnen Plain of Northeast China. Paddy and Water Environment, 7(3), 259–270.
Wen, X., Wu, Y., Su, J., Zhang, Y., & Liu, F. (2005). Hydrochemical characteristics and salinity of groundwater in the Ejina Basin, Northwestern China. Environmental Geology, 48, 665–675.
Wilcox, L. V. (1981). The quality of water for irrigation use. Technical Bulletins, 113(4), 277–284.
Xiao, H. F., Zang, S. Y., Guan, Y., Liu, S. J., Gao, Y., Sun, Q. Z., Xu, H. F., Li, M., Wang, J. J., & Pei, X. Y. (2014). Assessment of potential risks associated with heavy metal contamination in sediment in Aobaopao Lake, China, determined from sediment cores. Ecotoxicology, 23, 527–537.
Yang, F., Zhang, G. X., Yin, X. R., & Liu, Z. J. (2011a). Field-scale spatial variation of saline-sodic soil and its relation with environmental factors in Western Songnen Plain. International Journal of Environmental Research and Public Health, 8(2), 374–387.
Yang, F., Zhang, G. X., Yin, X. R., Liu, Z. J., & Huang, Z. J. (2011b). Study on capillary rise from shallow groundwater and critical water table depth of a saline-sodic soil in Western Songnen Plain of China. Environmental Earth Sciences, 64, 2119–2126.
Zhang, G. X., Deng, W., Yang, Y. S., & Salama, R. B. (2007). Evolution study of a regional groundwater system using hydrochemistry and stable isotopes in Songnen Plain, northeast China. Hydrological Processes, 21(8), 1055–1065.
Zhao, H. Q., Zhao, Y. S., Yang, X. K., & Yang, S. G. (2009). Investigation and assessment of groundwater resources and their related environmental problems in the Songnen Plain. Beijing: Geological Press.
Acknowledgments
The authors appreciate the anonymous reviewers and editors for their contributions and help to this research.
Funding
This study was supported by the 111 project (No. B16020), the National Natural Science Foundation of China (No. 41072255), and Science Foundation of Jilin province (20150101116JC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bian, J., Nie, S., Wang, R. et al. Hydrochemical characteristics and quality assessment of groundwater for irrigation use in central and eastern Songnen Plain, Northeast China. Environ Monit Assess 190, 382 (2018). https://doi.org/10.1007/s10661-018-6774-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6774-4