Abstract
Groundwater is the most important water resources in modern water supply, and its quality is the determining factor, especially for the groundwater supply under extreme events. Groundwater quality survey has been carried out in Jilin City, which is a famous industrial city in northeast China. The results show that (1) the distribution of major ions in the groundwater is as follows: Ca2+ > Mg2+ > Na+ > K+ and HCO3− > SO4 2− > Cl− > NO3−; the Piper’s, Gibbs’s, and Langelier–Ludwig’s diagrams suggest that groundwater was evolved due to the water–rock interaction; (2) geochemical relations (Ca/Mg ratio, Ca + Mg versus HCO3 + SO4, Na versus Cl) suggest that the dissolution of carbonate minerals gypsum and halite is the significant mechanism dominating the groundwater chemistry, the relations also suggest that the ion exchange is the secondary processes for higher concentrations of Ca2+ and Mg2+ ions; (3) the influences of agricultural fertilizers and irrigation practice are the other reasons for increasing NO3 − ions in the groundwater; according to the drinking water quality standards, the groundwater is not suitable for drinking with reference to the concentrations of total hardness, Ca2+, Mg2+, NO3 −, F−, and Fe in many locations; (4) based on the parameters mentioned (sodium adsorption ratio, Kelly’s ratio, sodium percent, permeability index, and magnesium hazard), the groundwater quality is assessed and the overall irrigation qualities of wells are demarcated as suitable for irrigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the most important water resources in modern society, groundwater is playing more and more influences for the sustainable development. Groundwater quality investigation and assessment are the fundamental works in the regional groundwater management (Subramani et al. 2005; Nagarajan et al. 2010; Ako et al. 2011; Wang 2013; Agca 2014; Al-Khashman et al. 2014). As the surface water bodies are polluted seriously around the world, groundwater quality becomes controlling factors in modern water management decisions (Jang et al. 2012; Re et al. 2014; Erdlenbruch et al. 2014; El-Fadel et al. 2014). Especially for the hydrogeochemistry characteristics and its responses for the changing climate and human activities, groundwater quality assessment has more and more important influence to the changing world (Gorelick and Zheng, 2015). Groundwater has become the major source of water supply for domestic, industrial, and agricultural sectors of many countries (Du et al. 2013; Su et al. 2014a, 2014b; Fu et al. 2014).
Although main cities are located along big rivers and the water supply is always mainly based on the surface water resources, the urbanization is making an increased water demand, groundwater resources are playing important sources of water supply, especially in arid and semi-arid areas (Zhang et al. 2010; Zhai et al. 2015), groundwater even becoming sole source in some city or under critical events (Ghislain et al. 2012; Nazzal et al. 2014). In this study, Jilin City has been selected as the study area, although the groundwater resources only occupied about 2.72 % daily water supply; however, groundwater could be the sole water resource (Huan et al. 2012). There is a famous petrochemical plant that exploded in Jilin Province, China, on November 13, 2005, and approximately, 100 tons of toxic substances including benzene, aniline, and nitrobenzene spilled into the Songhua River (Zhang et al. 2010), groundwater becoming the emergent water supply source. The groundwater resources monitoring and assessment would be very useful for the social sustainable development and safety under extreme events. Hence, groundwater suitability for drinking and irrigation should be investigated, the assessment result could be important guidance for local groundwater management under an emergent event.
Study area
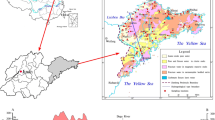
Jilin City has been selected as the study area, the location could be seen in Fig. 1; from the figures, it could be seen that the study area is along the Songhua River, which is a big river in northeastern China, and the area is about 104.5 km2.
The precipitation in this area is about 645.5 mm/a, and evapotranspiration is about 1506 mm/a. Quaternary Holocene alluvial is distributed in the floodplain, and the First terrace and quaternary upper Pleistocene alluvial is distributed in the Second terrace. Lower Pleistocene Series is continuously overlaid by Holocene alluvial and upper Pleistocene alluvial. The types of sediments in the vadose zone mainly consist of loamy sand, sandy loam, sandy clay, loam, silt clay, and silt (Huan et al. 2012, 2015).
In the study area, the phreatic aquifer is composed of Quaternary unconsolidated deposits, including sands, gravels, and pebbles. Rainfall and irrigation infiltration recharge the groundwater from May to September. The groundwater flows mainly from southeast to northwest. The groundwater discharges typically towards rivers except during the flood season. Evaporation only occurs in the area where the groundwater depth is less than 4.95 m, between late November and the following April. Abstraction of groundwater constitutes a significant resource output, especially in the groundwater source areas such as Jiuzhan, Hadawan, and Songyuanhada (Huan et al. 2015).
According to recent research, the groundwater has been polluted by human activities in Jilin City area, especially for nitrogen contamination; the process to derive a NO3 − N concentration at the pumping well is presented, based on determining the maximum nitrate loading limit to satisfy China’s drinking-water quality standards (Huan et al. 2015). Hydrogeochemistry characteristic analysis is the foundation for local groundwater resource evaluation and management.
Sampling and methodology
A regional geological survey of the study area was carried out during May 2002 and May 2004, and 262 groundwater samples were collected from private, municipal, and observation wells, which were distributed over the whole region (Fig. 1).
Samples were collected in polyethylene bottles with capacity of 1 L. Prior to their filling with sampled water, these bottles were rinsed to minimize the chance of any contamination. The sample preservation and the used analytical techniques were in accordance with the standard methods from the American Public Health Association (American Public Health Association, APHA, 1995). Unstable parameters such as hydrogen ion concentration (pH) and total dissolved solids (TDS) were determined at the sampling sites with the help of a pH meter and a TDS meter. The calcium (Ca2+), magnesium (Mg2+), sodium (Na+), and potassium (K+) ions were determined by atomic absorption spectrophotometer (Shimadzu AA-6300CF). Total iron and total manganese were analyzed by spectrophotometer. Bicarbonate (HCO3 −) and carbonate (CO3 2−) were analyzed by titrimetric methods. Total alkalinity as CaCO3 and total hardness as CaCO3 were analyzed by titration with EDTA and titration with HCl, respectively. Chloride (Cl−), sulfate (SO4 2−), nitrate (NO3 −), and fluoride (F−) were measured by ionic chromatography. Then, ionic balance was calculated by cations minus anions over total ion concentration, and samples above 5 % error were discarded (Appelo and Postma 2005).
Carbonate hardness, non-carbonate hardness, and excess alkalinity were computed. The values, which were the lowest among total alkalinity and total hardness, were placed under carbon hardness; when the value of total alkalinity was greater than total hardness, the difference value of total alkalinity was considered as excess alkalinity, and when total hardness was more than total alkalinity, the excess value of total hardness was taken as non-carbonate hardness.
Hydrogeochemical classification and groundwater evolution have been discussed using bivariate plots, Piper’s plot, Gibb’s plots, chloro-alkaline indices, and meteoric genesis indices. For drinking-water quality assessment, the results were compared with the World Health Organization (WHO, 2011) standards. The suitability for agriculture use was assessed using sodium adsorption ratio, sodium percentage, Kelley’s ratio, residual Mg/Ca ratio, permeability index, and magnesium hazard.
Results and discussions
General chemistry
According to the groundwater sample test results (Table 1), it could be seen that the groundwater samples are slightly acidic to neutral, the pH values range from 6.2 to 7.3. The total dissolved solids (TDS) range from 91.4 to 1140 mg/L with an average value of 410 mg/L, only two groundwater samples are brackish water with TDS values more than 1000 mg/L, and the other groundwater samples are fresh water with TDS values less than 1000 mg/L (Fetter 2014).
The total alkalinity is in between 35.5 and 558 mg/L (Table 1). This is due to the HCO3 − ions, as the pH varies from 6.2 to 7.3 which results in no presence of CO3 2−.
The total hardness of groundwater samples in Jilin City could be seen in Table 2; from the table, it could be seen that, the total hardness ranges from 131 to 1169 mg/L, which mean that there is about 57 % of the groundwater samples that fall into the classification of hard (150–300 mg/L) and 43 % of the groundwater samples fall into the classification of very hard (>300 mg/L).
The relationship between total alkalinity and total hardness could be used to calculate the carbonate hardness and non-carbonate hardness, the result could be seen in Table 1. From the results, it could be seen that while carbonate hardness could be removed easily by water boiling due to a presence of weak acids, the non-carbonate hardness cannot be removed due to strong acids.
The distribution orders of major ions in the groundwater samples are Ca2+ > Mg2+ > Na+ > K+ and HCO3 − > SO4 2− > Cl− > NO3 −. The concentration of Ca2+ in the study area ranges from 13.5 to 295 mg/L, 37 % of the groundwater samples exceed the limit of 75 mg/L. The concentrations of Mg2+ range from 11.7 to 134 mg/L with an average value of 35.7 mg/L. Most of the Mg2+ concentrations (52 %) are within the desirable limit of 30 mg/L. The concentrations of Na+ and K+ ions vary from 3 to 80 and 0.3 to 33 mg/L, respectively (Table 1). The major source of Ca2+ in the groundwater is due to ion exchange of minerals from rocks of this area. Further, this may also be due to the presence of CaCO3 and CaSO4 minerals present in the soil horizon, gypsum anhydrides, dolomite, etc.
The HCO3 − concentration ranges from 43 to 680 mg/L with an average of 168 mg/L. The SO4 2− concentrations range from 10 to 922 mg/L, with an average value of 107 mg/L. Only one sample has SO4 2− concentration greater than the permissible limit of 250 mg/L. The Cl− concentration ranges from 2 to 359 mg/L, averaging 53.2 mg/L. Two samples have Cl− concentration more than the permissible limit of 250 mg/L. The NO3 − concentration ranges from 0 to 193 mg/L with an average of 37 mg/L. As much as 30 % sample has more than the permissible NO3 − concentration (45 mg/L).
Hydrochemical classification
The classification of groundwater facies was done using Piper’s diagram (Piper 1944). The plots of desirable data on the diamond-shaped field classify the groundwater types into two groups (Fig. 2).
The diagram of Langelier and Ludwig can also reflect these two groundwater types. Langelier–Ludwig diagram (1942) shows four parts, and each part is divided by 50 % of the total ions (Fig. 3). They are related to Ca2+ + Mg2+ + HCO3 −, Na+ + HCO3 −, Na+ + Cl−, and Ca2+ + SO4 2− compositions. The computed percentages of Ca2+ + Mg2+, Na+ + K+, HCO3 −, and Cl− + SO4 2− vary from 68 to 95, 5 to 32, 6 to 87, and 13 to 94, respectively. Twenty-five percent of the groundwater sampling points fall in the area of Ca2+ + Mg2+ + HCO3 − composition (Fig. 3). Ca2+ + Mg2+ + HCO3 − water types represent meteoric signatures or fresh recharged water. The residual samples (75 %) fall in Ca2+ + Mg2+ + SO4 2− which show evolved groundwater type where unique chemical masking is achieved through rock–water interaction, ion exchange, and reverse ion exchange; reactions within unsaturated zones; increased resident time; and anthropogenic influences.
Hydrochemical evolution
Gibbs’s diagram
In order to know the hydrogeochemical processes during groundwater recharge, runoff, and discharge, two Gibbs’s diagrams have been proposed as Fig. 4, they are related to the weight ratios of cations (Na+:Na+ + Ca2+) and anions (Cl−:Cl− + HCO3 −) against TDS.
From Fig. 4, it could be seen that the cation ratios (Na+:Na+ + Ca2+) vary from 0.06 to 0.49 and the anion ratios (Cl−:Cl− + HCO3 −) vary from 0.01 to 0.81, which mean that the main source of dissolved ions is rock–water. The rock–water interaction generally includes the chemical weathering of rocks, dissolution–precipitation of secondary carbonates, and ion exchange between water and clay minerals. The moving of groundwater sampling points from the rock-domain towards the evaporation-domain in Fig. 4b suggests an increase of Cl− ions, and consequent higher TDS due to water contamination caused by the influences of irrigation practice; as water–rock interaction was the dominant process governing the groundwater chemistry, it is reasonable to identify the origin of different ions (Kumar et al. 2014).
Hydrogeochemical relations
Hydrogeochemical characteristics and their relationships could be used to find the groundwater species original and the controlling hydrogeochemical process during the groundwater recharge, runoff, and discharge.
Ca2+ and Mg2+ were the dominant cations, and HCO3 − was the dominant anion in wide areas. The abundance of Ca2+ and Mg2+ in the groundwater could be related to the presence of carbonate rock in the basin, while weathering of carbonate and silicates may contribute Ca2+ and Mg2+ in the groundwater. The dissolution of calcite and dolomite can be shown by the groundwater’s Ca2+/Mg2+ molar ratio. A Ca2+/Mg2+ molar ratio that is equal to one indicates dissolution of dolomite rocks (Mayo and Loucks 1995), while a greater ratio may represent a more dominant calcite contribution from the rocks. A Ca2+/Mg2+ ratio, greater than 2, may represent the dissolution of silicate minerals into the groundwater (Katz et al. 1997). While 30 % of the groundwater samples had a lower Ca2+/Mg2+ ratio than 1, which indicated that the dissolution of dolomite, 60 % of the samples had a Ca2+/Mg2+ ratio between 1 and 2, which indicated the dissolution of calcite that contributes Ca2+ and Mg2+ to the groundwater. Only a few samples (around 10 %) were indicative of the effect of silicate minerals with Ca2+/Mg2+ ratio > 2 (Fig. 5a). The dissolution of carbonate minerals could be represented in the following reactions ((1) to (4)) in natural systems (Mukherjee et al., 2009; Kura et al. 2013):
Dissolution is a simple and common weathering reaction in carbonate rocks (Drever 1997). It is specified by the 1:2 ratio of Ca2+/HCO3 − and 1:1 equivalent ratio of Ca2+ + Mg2+ / HCO3 −, in the groundwater. The lower value of Ca2+ + Mg2+ / HCO3 − indicates other sources of HCO3 − such as silicate weathering, and the higher ratio of Ca2+ + Mg2+ to HCO3 − represents that the excess of Ca2+ + Mg2+ has been balanced by Cl− and SO4 2− in the study area. The plot of Ca2+ + Mg2+ versus HCO3 − + SO4 2− will be near to 1:1 line if Ca2+, Mg2+, SO4 2−, and HCO3 − are derived from the dissolution of carbonate, such as the minerals of calcite, dolomite, and gypsum. The data points tend to shift to the right due to excess of HCO3 − + SO4 2− when ion exchange is the dominant process, the ion exchange mechanism could be described by following reaction:
If the points were above the median line, a reverse ion exchange was the active reaction for the excess of Ca2+ + Mg2+ over SO4 2− + HCO3 −, which could be represented by the following reaction:
Based on the plot, the majority of samples were scattered close to the 1:1 line, indicating that Ca2+, Mg2+, SO4 2−, and HCO3 − in the groundwater may be due to the dissolution of calcite, dolomite, and gypsum. Moreover, a considerable number of samples fall above the median line, indicating that reverse ion exchange tended to be the dominant reaction over ion exchange, which was responsible for the higher Ca2+ and Mg2+ concentration in the groundwater (Fig. 5b).
Halite is a dominant source of Na+ and Cl− in the groundwater; the molar ratio varies spatially as a result of the cation exchange (Wayland et al. 2003). The ratio of Na+/Cl− being equal to one indicated that halite dissolution could be responsible for the sodium and chloride concentration in the water samples. In most groundwater samples, the chemical data in a plot of Na+ versus Cl− fall around the uniline (Fig. 5c). This infers Na+ and Cl− are mainly from the dissolution of halite. Chloride is a conservative component in the groundwater; therefore, a higher concentration of Cl− versus Na+ indicates the decency of Na+ and the truth that groundwater samples with higher chloride concentration versus Na+ also shows higher Ca + Mg versus HCO3 + SO4. Thus, the reverse ion exchange was testified.
Quality criteria for drinking water supply and irrigation
Drinking water
In order to assess the suitability of groundwater for drinking water supply, the species chemistry concentration in groundwater samples could be compared with drinking-water quality standard (Ministry of Health of the People's Republic of China 2006).
The pH threshold limit ranges from 6.5 to 8.5 for drinking water (Fig. 6a), the water would affect the mucous membrane when the pH is beyond this limit (Rao et al. 2012). The pH values of majority groundwater samples in Jilin City range from 7.6 to 8.5, which are not beyond the recommended pH limit, the groundwater samples are potable for drinking. The TDS concentration in all groundwater samples in Jilin City ranges from 91 to 1140 mg/L, which is lower than that of threshold limit in drinking water (1000 mg/L) (Fig. 6b), the groundwater samples are potable for drinking. The threshold limit of total hardness for drinking water is 450 mg/L (Ministry of Health of the People's Republic of China 2006). Accordingly, the groundwater in 16 % of the total water samples is not suitable for drinking (Fig. 6c). The higher concentration of total hardness in potable water causes to develop gall bladders, urinary stones, and arthritis (Garg et al. 2009).
The prescribed limit of Ca2+ for drinking water is 75 mg/L, and about 37 % groundwater samples from the study area exceed the safe limit with reference to Ca2+ (Fig. 7a). The Mg2+ (12–134 mg/L), in excess of the permissible limit of 30 mg/L allowed for drinking water, has led groundwater sample to become unsuitable in 48 % of the total groundwater samples (Fig. 7b). None of the groundwater samples exceeds this limit so that the water is potable (Fig. 7c). Potassium is essential to maintain the fluid in balance stage in the body. Generally, it is less than 10 mg/L in water. In the study area, it is more than 10 mg/L in approximately 11 % of the groundwater samples (Fig. 7d).
The chemical data of the study area show that the concentration of HCO3 − (43–680 mg/L) exceeds the desirable limit of 300 mg/L in 11 % of the groundwater samples (Fig. 8a). The allowed limit of Cl− for drinking water is 250 mg/L (Fig. 8b). The groundwater samples observed from the study area show Cl− from 2 to 359 mg/L, and only two samples encountered a higher chloride concentration than the safe limit. The groundwater samples of the study area show SO4 2− from 10 to 922 mg/L, and the majority are below the recommended limit of SO4 2− for drinking water except two samples (Fig. 8c).
The data show that 30 % samples have a higher concentration than the desirable limit, which means NO3 − in the groundwater may cause a severe health threat to the local residents (Fig. 9a). The safe limit of F− from 0.5 to 1.0 mg/L is essential for normal growth of bones, in potable water. Less than 0.6 mg/L of F− causes dental caries, while more than 1.0 of F− results in fluorosis. The groundwater in the study area has the concentration of F− ranging from 0.1 to 1.6 mg/L. About 74 % of the samples have a lower fluoride concentration than 0.5 mg/L, and the rest are within the desirable limit. The deficiency of fluoride may be the result from the formation of F−-bearing minerals due to the presence of rich Ca2+ in the groundwater. Fe is an important element for human’s health, but if it exceeds the limit in groundwater, some fatal diseases would occur (WHO 2011). The safe limit of iron is 0.3 mg/L, 43 % samples have a higher iron concentration than the safe limit. Thus, the residents in the study area face the threat of excess iron in drinking water.
Groundwater quality for irrigation purposes
Groundwater is the main irrigation water sources in Jilin City, and the groundwater quality assessment for irrigation could be useful in the future regional water resource management. Different parameters have been used to assess the suitability of groundwater for irrigation, such as sodium adsorption ratio (SAR), Kelly’s ratio (KR), sodium percent (Na%), permeability index (PI), and magnesium hazard (MH).
SAR has been used to assess the suitability of groundwater for irrigation (Rao 2006; Rao et al. 2012). The calculated SAR values have been shown in Table 3. From the table, it could be seen that the values of SAR in the study area range from about 0.1 to 1.8, and there is no well with SAR value that exceeds 26, which indicate that the irrigation with these groundwater could not induce high sodium hazard. According to the calculation results of SAR, it could be seen that the groundwater in Jilin City could be used for regional irrigation.
The KR values also could be seen in Table 3; from the table, it could be seen that the KR values of groundwater samples in Jilin City range from 0.04 to 0.44, which indicate that none of the groundwater samples is unsuitable for irrigation, the results match with that of SAR.
The Na% calculated results could be seen in Table 3; from the table, it could be seen that the Na% ranges from 5 to 32 %, all the results are less than 60 % (threshold limit), which mean that all groundwater samples are suitable for irrigation in Jilin City. According to the calculated MH values, 93 % sample classifies to be in safe category and the remaining samples show groundwater moderate suitability for irrigation.
Conclusion
From the discussion, we can see that the distribution of major ions in the groundwater is as follows: Ca2+ > Mg2+ > Na+ > K+ and HCO3 − > SO4 2− > Cl− > NO3 −; the Piper’s, Gibbs’s, and Langelier–Ludwig’s diagrams suggest that groundwater was evolved due to the water–rock interaction; geochemical relations (Ca/Mg ratio, Ca + Mg versus HCO3 + SO4, Na versus Cl) suggest that the dissolution of carbonate minerals gypsum and halite is the significant mechanism dominating the groundwater chemistry, the relations also suggest that the ion exchange is the secondary processes for higher concentrations of Ca2+ and Mg2+ ions; according to the drinking-water quality standards, the groundwater is not suitable for drinking with reference to the concentrations of total hardness, Ca2+, Mg2+, NO3 −, F− and Fe in many locations; based on the parameters mentioned (sodium adsorption ratio, Kelly’s ratio, sodium percent, permeability index, and magnesium hazard), all the groundwater samples fall into the classification of suitable irrigation.
References
Agca N (2014) Spatial variability of groundwater quality and its suitability for drinking and irrigation in the Amik Plain (South Turkey). Environ Earth Sci 72:4115–4130
Ako AA, Shimada J, Hosono T, Ichiyanagi K, Nkeng GE, Fantong WY, Eyong GET, Roger NN (2011) Evaluation of groundwater quality and its suitability for drinking, domestic, and agricultural uses in the Banana Plain (Mbanga, Njombe, Penja) of the Cameroon Volcanic Line. Environ Geochemisty and Health 33:559–575
Al-Khashman OA, Jaradat AQ (2014) Assessment of groundwater quality and its suitability for drinking and agricultural uses in arid environment. Stochastic Environ Resour Risk Assess 28:743–753
American Public Health Association (APHA) (1995) Standard methods for the examination of water and wastewater, 19th edn. APHA, Washington DC, USASS
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. Balkerma, Amsterdam
Drever JI (1997) The geochemistry of natural Waters. Prentice Hall, Upper Saddle River, NJ
Du SH, Su XS, Zhang WJ (2013) Effective storage rates analysis of groundwater reservoir with surplus local and transferred water used in Shijiazhuang City, China. Water Environ J 27:157–169
El-Fadel M, Tomaszkiewicz M, Adra Y, Sadek S, Abou Najm M (2014) GIS-based assessment for the development of a groundwater quality Index towards sustainable aquifer management. Water Resour Manag 28:3471–3487
Erdlenbruch K, Tidball M, Zaccour G (2014) Quantity-quality management of a groundwater resource by a water agency. Environ Sci Pol 44:201–214
Fetter CW (2014) Applied hydrogeology. CBS Publishers and Distributors, New Delhi
Fu CC, Zhang WJ, Zhang SY, Su XS, Lin XY (2014) Identifying key hydrochemical processes in a confined aquifer of an arid basin using multivariate statistical analysis and inverse modeling. Environ Earth Sci 72:299–310
Garg VK, Suthar S, Singh S, Sheoran A, Meenakshi G, Jai S (2009) Drinking water quality in villages of southwestern Haryana, India: assessing human health risks associated with hydrochemistry. Environ Geol 58:1329–1340
Ghislain TYJ, Roger F, Joseph W, Emmanuel EG, Ghislain DM (2012) Evaluation fo groundwater suitability for domestic and irrigational purposes: a case study from Mingoa River basin, Yaounde, Cameroon. J Water resour protection 4:285–293
Gorelick SM, Zheng CM (2015) Global change and the groundwater management challenge. Water Resour Res 51:3031–3051
Huan H, Wang JS, Teng YG (2012) Assessment and validation of groundwater vulnerability to nitrate based on a modified DRASTIC model: a case study in Jilin City of northeast China. Sci Total Environ 440:14–23
Huan H, Wang JS, Lai DS, Teng YG, Zhai YZ (2015) Assessment of well vulnerability for groundwater source protection based on a solute transport model: a case study from Jilin City, Northeast China. Hydrogeol J 23:581–596
Jang CS, Chen SK, Kuo YM (2012) Establishing an irrigation management plan of sustainable groundwater based on spatial variability of water quality and quantity. J Hydrol 414:201–210
Katz BG, Coplen TB, Bullen TD, Davis JH (1997) Use of chemical and isotopic tracers to characterize the interactions between ground water and surface water in mantled karst. Ground Water 35:1014–1028
Kumar K, Bharani SR, Magesh NS, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci 4:341–350
Kura NU, Ramli MF, Sulaiman WNA, Ibrahim S, Aris AZ, Mustapha A (2013) Evaluation of factors influencing the groundwater chemistry in a small tropical Island of Malaysia. Int J Environ Res Public Health 10:1861–1881
Mayo AL, Loucks MD (1995) Solute and isotopic geochemistry and ground water low in the central Wasatch Range, Utah. J Hydrol 172:31–59
Ministry of Health of the People's Republic of China (2006) Chinese standards for drinking water quality. GB5749. Beijing
Mukherjee A, Bhattacharya P, Shi F, Fryar AE, Mukherjee AB, Xie ZM, Jacks G, Bundschuh J (2009) Chemical evolution in the high arsenic groundwater of the Huhhot basin (Inner Mongolia, PR China) and its difference from the western Bengal basin (India). Appl Geochem 24:1835–1851
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess 171:289–308
Nazzal Y, Ahmed I, Al-Arifi NSN, Ghrefat H, Zaidi FK, El-Waheidi MM, Batayneh A, Zumlot T (2014) A pragmatic approach to study the groundwater quality suitability for domestic and agricultural usage, Saq aquifer, northwest of Saudi Arabia. Environ Monit Assess 186:4655–4667
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analyses, transactions. American Geophysical Union 25: 914–928
Rao NS, Subrahmanyam A, Kumar SR, Srinivasulu N, Rao GB, Rao PS, Reddy GV (2012) Geochemistry and quality of groundwater of Gummanampadu sub-basin, Guntur District, Andhra Pradesh, India. Environ Earth Sci 67:1451–1471
Rao SN (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49:413–429
Re V, Sacchi E, Mas-Pla J, Mencio A, Amrani NE (2014) Indentifying the effects of human pressure on groundwater quality to support water management strategies in coastal regions: A multi-tracer and statistical approach (Bou-Areg region, Morocco). Sci Total Environ 500-501:211–223
Su X, Xu W, Du SH (2014a) In situ infiltration test using a reclaimed abandoned river bed: managed aquifer recharge in Shijiazhuang City, China. Environ Earth Sci 71:5017–5025
Su X, Xu W, Du SH (2014b) Responses of groundwater vulnerability to artificial recharge under extreme weather conditions in Shijiazhuang City, China. Journal of Water Supply Research and Technology—Aqua 63:224–238
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Wang S (2013) Groundwater quality and its suitability for drinking and agricultural use in the Yanqi Basin of Xinjiang Province, Northwest China. Environ Monit Assess 185:7469–7484
Wayland KG, Long DT, Hyndman DW, Pijanowski BC, Woodhams SM, Haack SK (2003) Identifying relationships between baseflow geochemistry and land use with synoptic sampling and R-mode factor analysis. J Environ Qual 32:180–190
WHO (2011) Guidelines for drinking water quality. World Health Organization, Geneva
Zhai YZ, Lei Y, Zhou J, Li MZ, Wang JS, Teng YG (2015) The spatial and seasonal variability of the groundwater chemistry and quality in the exploited aquifer in the Daxing District, Beijing, China. Environ Monit Assess 187:43
Zhang WJ, Lin XY, Su XS (2010) Transport and fate modeling of nitrobenzene in groundwater after the Songhua River Pollution Accident. J Environ Manag 91:2378–2384
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(XLS 137 kb)
Rights and permissions
About this article
Cite this article
Xiao, C., Liu, J., Liang, X. et al. Hydrogeochemistry characteristics of groundwater and its suitability for water supply and irrigation in Jilin City, China. Arab J Geosci 9, 434 (2016). https://doi.org/10.1007/s12517-016-2447-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-016-2447-1