Abstract
A sensitive and accurate analytical method based on dispersive liquid-liquid microextraction was developed for the simultaneous determination of selected pesticides, hormones, and endocrine disruptors by GC-MS. The optimum conditions of the extraction procedure were determined using an experimental design of factors significantly affecting the extraction output. Analysis of variance (ANOVA) was used to evaluate the main effects of experimental factors and their interactions. The limit of detection values determined for the analytes under optimum experimental conditions were found to be between 0.30–2.0 ng/mL. The linear calibration plot of analytes span across a wide concentration range and low %RSD values from replicate measurements indicated good precision of the developed method. Spiked recovery tests were also performed on municipal wastewater, well water, lake water, sea water, and tap water matrices to determine the method’s accuracy and applicability to water samples. The recovery results obtained were satisfactory for all water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many centuries, pesticides have been used in agriculture to protect plants against pests. They have also been used to protect humans from pests, especially disease-bearing insects such as mosquitos (Akhgari et al. 2003). The production and application of pesticides on a large scale started after the discovery of dichlorodiphenyltrichloroethane’s (DDT) insecticidal action in the 1940s (Chormey et al. 2017b). Since then, the agricultural field has been a major benefactor with pesticide usage providing high yield of foods and seed, quality food, prolonged storage among others. Pesticides are usually classified according to their target organisms and these include insecticides, nematicides, fungicides, rodenticides, herbicides, and others. Classification of pesticides is also made according to chemical class, mode of action, and mode of application (Aktar et al. 2009; Chormey et al. 2017a).
Parathion ethyl and malathion are known as organophospate insecticides and their mode of action is inhibiting acetylcholinesterase (AChE) in the peripheral and central nervous system of humans and insects (Rathnayake and Northrup 2016; Facure et al. 2017; Bala et al. 2015). Penconazole and fludioxonil are two fungicides that have been used worldwide to intercept and heal fungal infections in crops leading to increased yield (Perdichizzi et al. 2014; Rose et al. 2009). The basic action mode of fungicides is the termination of internal energy and fungal cell membranes (Hutson and Miyamoto 1998; Chormey et al. 2017b). Chlorthiamid, an obsolete benzonitrile herbicide, undergoes degradation in soil to form the product dichlobenil (Sandín-España and Sevilla-Morán 2012). 2,6-dichlorobenzamide is a metabolite of chlorthiamid and dichlobenil and it has been reported as a common contaminant of groundwater with concentrations exceeding the 0.10 μg/L European Union standard in some countries (Pukkila and Kontro 2014). Excessive use of pesticides presents the risk of runoff into surface water and leaching into ground water bodies (Brauns et al. 2018).
Estrone and other estrogenes such as estradiol are natural hormones but they are prescribed as medication to prevent and treat menopausal symptoms in women (Labrie et al. 2009). However, prolonged usage and high doses can lead to reactions such as vomiting, nausea, abdominal pain, and vaginal bleeding (Kopper et al. 2008). Estrogens are naturally excreted into the environment by vertebrates and exposure to aquatic organisms disrupts their reproductive and physiological functions (Cox et al. 2017). Even at trace levels, hormones and pesticides can accumulate in tissues and organs leading to prostate, breast, leukemia, and multiple myeloma cancers (He et al. 2017; Alavanja et al. 2013). It is therefore important to determine pesticides that occur at trace levels in the environment.
Extraction continues to be a very important sample preparation procedure used to separate analytes from a matrix and preconcentrate them into high amounts for trace determinations. However, some extraction methods involve the use of high volumes of organic solvents with varying toxicity levels, the use of expensive sample preparation kits, time consuming processes, and labor intensive. Modern extraction methods have been aimed at mitigating these drawbacks while making major improvements on the advantages. Miniaturization of the conventional liquid-liquid extraction and solid-phase extraction has simplified extraction procedures and significantly cut down the volume of organic solvents used (Turan et al. 2017). These are green methods and examples include solid-phase microextraction (SPME), single drop microextraction (SDME), headspace solid-phase microextraction (HS-SPME), solidified floating organic drop microextraction (SFODME), and hollow fiber liquid phase microextraction (HF-LPME) (Biparva and Matin 2012; Rutkowska et al. 2014). Rezaee et al. developed dispersive liquid-liquid microextraction (DLLME) for the determination of polycyclic aromatic hydrocarbons (Rezaee et al. 2006) and this method has since been successfully applied to a wide range of inorganic and organic analytes in various samples (Primel et al. 2017). DLLME is simple, rapid, inexpensive, and presents very high enrichment factors and analyte recovery (Turan et al. 2017). Another benefit of DLLME is that it can be easily coupled with different instrumentations for the identification and quantification of analyte(s) (Turan et al. 2017). The development of extraction methods have been mainly performed univariately where only one parameter changes while the others are held constant. This mode of method development lacks the interaction of multiple variables from which the most optimum experimental conditions could be found. With an experimental design, several parameter interactions can be studied and a model can be used to predict non-tested parameters and their interactions.
High molecular weight and polar organic compounds are conveniently determined by liquid chromatography (LC), which employs an appropriate isocratic or gradient elution system to separate the individual components of a sample (Crutchfield et al. 2010). Gas chromatography (GC) on the other hand does not require adjusting of mobile phase composition. Separation is achieved by developing a temperature program to suit the vapor pressure of volatile compounds in a sample (Santos and Schug 2017). The coupling of GC to a mass selective detector (MS) is a very useful tool (GC-MS) for the separation, identification, and quantification of analytes based on specific mass-to-charge (m/z) ratios. When GC-MS is operated in the scan mode, it offers selectivity for compounds within a certain m/z ratio range, and specificity for one compound can be achieved using the selected ion monitoring (SIM) mode (Vetter 2012).
This study was aimed at using an experimental design to optimize a dispersive liquid-liquid microextraction method for the determination of selected pesticides and estrone by gas chromatography mass spectrometry.
Materials and methods
Chemicals and reagents
Standard stock solutions of chlorthiamid, malathion, parathion ethyl, penconazole, fludioxonil, and estrone (purity of standards > 98%) were prepared in acetonitrile and aliquots taken from each to prepare a 100 mg/L mix standard solution. Working aqueous standard solutions used for method development were prepared by diluting the mix standard with deionized water obtained from an Ultrapure Water Purification System (resistivity 18.2 Ω.cm). All reagents used in this study were of analytical grade. Ethanol, 2-propanol, methanol, potassium nitrate, barium chloride, potassium iodide, sodium chloride, potassium chloride, chloroform, carbon tetrachloride, 1,2-dichloroethane, and dichloromethane were all obtained from Merck (Germany). A stock solution of deuterated bisphenol A (BPA-D16) (purchased from Dr. Ehrenstorfer - Germany) was prepared in ethanol and used as internal standard for samples and standards.
Apparatus
Separation, determination, and quantification of analytes were achieved using a gas chromatography mass spectrometer system (HP 6890) fitted with a non-polar Agilent HP-5MS column (30 m; 250 μm; 0.25 μm). The mass selective detector was operated in the scan mode for data acquisition. The ion source energy and transfer line temperature were kept at 70 eV and 280 °C, respectively. All injections were performed in the splitless mode using a sample/standard volume of 1.0 μL and an inlet temperature of 250 °C. Helium was used as carrier gas and was kept at a constant flow of 1.0 mL/min. The temperature program used for the separation of analytes started at 70 °C and increased to 180 °C at 60 °C/min, then it increased at 40 °C/min to 300 °C, where it was held for 2.0 min. Quantification of BPA-D16, chlorthiamid, malathion, parathion ethyl, penconazole, fludioxonil, and estrone was done using the ions (m/z) 224, 170, 125, 291, 159, 248, and 270, respectively.
Dispersive liquid-liquid microextraction procedure
A mixture of 1,2-dichloroethane and 2-propanol (200 μL and 3.0 mL) was injected at a moderate speed into a 15 mL centrifuge tube containing 8.0 mL of standard/sample solution. The cloudy solution resulting from fine dispersion of extraction solvent was vortexed for 30 s and then centrifuged for 2.0 min at 3461 g to facilitate phase separation. A sufficient amount (≈ 80 μL) was carefully taken from the bottom 1,2-dichloroethane phase into microliter insert vials for auto injections into the GC-MS system.
Samples
Applicability of the method to real samples was tested on municipal wastewater, well water, lake water, sea water, and tap water matrices. Tap water was sampled directly from the laboratory faucet and taken through the optimum extraction conditions. Municipal wastewater was sampled from an open-to-air aeration pool at a Biological Wastewater Treatment Plant in İstanbul. In order to obtain particulate-free wastewater sample, two successive filtrations were performed using 125-mm regular filter paper and 0.45-μm RC (regenerative cellulose) syringe filter. The wastewater was stored below 5.0 °C. Well water, lake water, and sea water samples were sampled into polypropylene plastic bottles after washing thoroughly with samples and filling to the brim without air spaces.
Results and discussions
Preceding the DLLME method development, analytical performance of the GC-MS system based on analytical figures of merit was determined for the analytes using mixed standard solutions in the range of 0.10–100 mg/L. The entire method optimization process was carried out with 50 ng/mL aqueous standard solutions, and the optimum parameters/values were selected from the highest average of triplicate peak area values. BPA-D16 was used as internal standard by adding low amounts of concentrated standards to calibration and working standards. The final concentration of BPA-D16 as internal standard in direct GC-MS and DLLME analysis were 2.0 mg/L and 50 μg/L, respectively.
Selection of experimental parameters
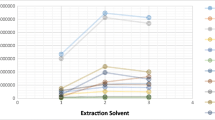
The selection of parameters for experimental design was performed univariately by keeping other parameters constant while different variables of one parameter were tested. The most influential parameters in dispersive liquid-liquid microextraction are the types of extraction and dispersive solvents. Other parameters such as pH, mixing, and salt addition could have an impact on extraction output depending on the type of analyte under study. In the selection of extraction solvent, only solvents with densities higher than 1.0 were tested because phase separation of the microliter volume after extraction could only be observed at the tapered bottom of conical tube used. Two hundred microliters each of four chlorinated hydrocarbon solvents (dichloromethane, chloroform, 1,2-dichloroethane, and carbon tetrachloride) were tested under similar conditions with ethanol as dispersive solvent, and 1,2-dichloroethane recorded the highest output for four out of the six analytes. Figure 1 is a graphical representation of integrated peak areas of extraction solvents but dichloromethane is not shown because it did not produce a settled phase for the volume used. This observation has also been reported in literature and it was attributed to the relatively high percent solubility of dichloromethane (1.6%) in water and this solubility further increases by reason of dispersion (Chormey et al. 2017b). The dispersion efficiencies of 2.0 mL acetone, ethanol, 2-propanol, and acetonitrile (all miscible with water and 1,2-dichloroethane) were then tested with 200 μL 1,2-dichloroethane. 2-Propanol recorded the highest average peak area for all analytes as shown in Fig. 2 and was therefore selected for the experimental design. Salts are added to extraction procedures to induce a salting out effect on analytes and to enhance phase separation. 1.0 g each of potassium nitrate, barium chloride, potassium iodide, sodium chloride, and potassium chloride were therefore tested and compared to a saltless extraction (Fig. 3). The salt added extractions recorded low results relative to the saltless extraction probably due to saturation of solution which hindered mass transfer of analytes. The extraction procedure was therefore continued without adding salts. For the purpose of further distribution of extraction solvent through the aqueous solution, vortex was added to the experimental design to determine its optimum mixing period. Vortex was selected due to its practical usage and repeatability in replicate mixing.
Experimental design and optimization of the extraction process
The Box-Behnken experimental design based on response surface methodology was used to examine the effects of three independent variables (extraction solvent amount (A), dispersive solvent amount (B), and mixing period (C)) on DLLME. The design incorporated three levels (− 1; low, 0; middle and + 1; high) of each factor into a total of 17 combinations with 5 center points. The behavior of the independent variables (X1, X2, and X3) in the design system was explained using the quadratic polynomial expression below (Lazic 2006):
where β0 is constant; β1, β2, and β3 are linear coefficients; β12, β13, and β23 are coefficients of interaction; β11, β22, and β33 are quadratic coefficients, and Y is the output or response.
The design of experimental combinations and average response (R) of integrated peak area for analytes obtained from triplicate measurements are given in Online Resource 1. All experimental design extractions were carried out using 50 ng/mL mixed aqueous standard solutions of the six analytes. The expression below depicts the empirical relationship between the independent variables and response (coefficients determined using Design-Expert 7.0.0 software):
ANOVA was used to ascertain the agreement between the experimental model and the real system. Presented in Table 1 is a summary of ANOVA results for penconazole as an example. The statistical significance of each variable and interaction was tested by the P values. The contribution of each variable and interaction to response variance was also tested by the F value. At 95% confidence level, P values less or equal to 0.05 and F values greater than F critical values are considered to be statistically significant. In the recommended model, the F value (F = 90.35) and P value (P < 0.0001) were determined to be significant. Figure 4 is a normal probability plot depicting the data set’s normal distribution for penconazole. The linear regression coefficient (R2) calculated for the straight line was 97% and this established good agreement between the predicted values and experimented results. The adjusted R2 value of 96% only varied by approximately 1.0% from the experimental data. These therefore confirm the accuracy and reliability of the model.
The Design-Expert 7.0.0 software was used to obtain the coefficients of Eq. 2 and the response surface plots shown in Fig. 5 for penconazole. These were used to examine the main effects and interactions of experimental variables (1,2-dichloroethane, 2-propanol, and vortex mixing). For the main effects, positive main effects (greater values) were obtained for dispersive solvent amount and mixing period while a negative main effect (lower values) was observed for extraction solvent amount. The same effects were observed for all six analytes and the magnitude of each variable was proportional to the effect. From the ANOVA results, the most influential variable was determined as 1,2-dichloroethane amount followed by 2-propanol amount. The different periods of vortex were not significant to the model. The high impact of low 1,2-dichloroethane amount conforms to the logic of preconcentration, where a very low amount (final volume) of extraction solvent yields a high preconcentration factor. The high amount of 2-propanol also agrees with the role of dispersive solvents which increase the surface area between extractant and aqueous sample. Interaction of variables that were significant to the model was amounts of 2-propanol and 1,2-dichloroethane (AB) and 1,2-dichloroethane quadratic effect (A2) as can be seen from Table 1. Optimum parameters obtained from the modeling of main effects and the interaction of variables were found to be 200 μL extraction solvent volume, 3.0 mL dispersive solvent volume, and 30 s of mixing.
Analytical figures of merit
The optimum values obtained for each parameter throughout the optimization studies were applied to aqueous standard solutions between 0.50 and 1000 ng/mL to determine the analytical performance of GC-MS under optimum DLLME conditions as presented in Table 2. The linear dynamic range of each analyte was determined by the coefficient of determination (R2) being greater than 0.999. A direct plot of concentration against integrated peak areas yielded R2 values between 0.9956 and 0.9991 for the analytes. Though satisfactory, plot of the ratios of standard’s peak area to internal standard peak area improved the linearity of calibration as observed by R2 values between 0.9997 and 1.000. Analytical figures of merit used to validate the method were limit of detection (LOD), limit of quantification (LOQ), precision (%RSD), R2, dynamic range, and spiked recovery. LOD and LOQ values of analytes were determined with the expressions 3.SDLC/m and 10.SDLC/m, where SDLC is the standard deviation of the lowest calibration concentration (6 replicates) and m is the slope of linear calibration plot. The limits of detection calculated for the analytes ranged between 0.30 and 3.0 ng/mL and the enhancement in detection power based on LOD values with respect to direct GC-MS analysis was between 44 and 252 times. These detection limits are better or comparable to other studies that determined these analytes with methods such as matrix solid-phase dispersion GC-MS, QuEChERS-UHPLC-MS/MS, and QuEChERS-GC-MS (Chu et al. 2005; Carneiro et al. 2013; Jahanmard et al. 2016; Machado et al. 2017). A wide linear range was obtained for the analytes and the low %RSD values (< 9.0%) also established the precision of the extraction process and instrumental determinations.
Recovery tests
Other components of a sample matrix besides the analyte of interest could inhibit the extraction of an analyte or enhance its migration into the extraction solvent leading to biased quantification of the analyte. It is therefore essential to perform recovery studies to determine the applicability of a developed method in the presence of sample matrices. Wastewater tends to have a very complicated matrix and this limits analyte recovery (Koçoğlu et al. 2017). Spiked recovery studies were therefore performed to assess the effect of the matrices of wastewater, well water, lake water, sea water, and tap water on the analytes’ extraction. Three final spiking concentrations (10, 50, and 100 ng/mL) representing different parts of the linear dynamic range of analytes were selected in this study. Wastewater and tap water were tested at all three spiked concentrations while sea, lake, and well water samples were tested at 50 ng/mL. Calibration standards prepared in deionized water were used to calculate the percent recovery of analytes from triplicate sample extractions. Blank extractions were performed on the samples but none of the analytes was detected according to their respective LODs. Table 3 shows percent recovery values of analytes that are between 78–93% and 86–107% for tap water and wastewater, respectively. The recovery results calculated for sea, lake, and well water ranged between 84 and 111% (Table 4), with sea water recording the lowest results due to its high salinity content and density. These results are satisfactory and suggest that this method can be used for accurate determination of these analytes in the selected matrices.
Conclusion
A sensitive, accurate, and precise dispersive liquid-liquid microextraction method was developed for the simultaneous determination of selected pesticides and hormone by GC-MS. The optimum extraction conditions were determined using an experimental design of factors that have significant impact on extraction output. The detection limits of all analytes were low enough to determine residues of these chemicals that could be present in water samples. The method was applicable over a wide linear concentration range and the precision of the method was confirmed by low %RSD values. Appreciable recovery results from spiked experiments also proved the method’s accuracy for municipal wastewater, well water, lake water, sea water, and tap water matrices. The method is simple, inexpensive, rapid, and can be routinely used for the determination of analytes at trace levels.
References
Akhgari, M., Abdollahi, M., Kebryaeezadeh, A., Hosseini, R., & Sabzevari, O. (2003). Biochemical evidence for free radicalinduced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Human & Experimental Toxicology, 22(4), 205–211. https://doi.org/10.1191/0960327103ht346oa.
Aktar, M. W., Sengupta, D., & Chowdhury, A. (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary Toxicology, 2(1), 1–12. https://doi.org/10.2478/v10102-009-0001-7.
Alavanja, M. C. R., Ross, M. K., & Bonner, M. R. (2013). Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA: a Cancer Journal for Clinicians, 63(2), 120–142. https://doi.org/10.3322/caac.21170.
Bala, R., Sharma, R. K., & Wangoo, N. (2015). Highly sensitive colorimetric detection of ethyl parathion using gold nanoprobes. Sensors and Actuators B: Chemical, 210(Supplement C), 425–430. https://doi.org/10.1016/j.snb.2014.12.123.
Biparva, P., & Matin, A. A. (2012). Microextraction techniques as a sample preparation step for metal analysis. In M. A. Farrukh (Ed.), Atomic absorption spectroscopy (pp. 61–88): InTech.
Brauns, B., Jakobsen, R., Song, X., & Bjerg, P. L. (2018). Pesticide use in the wheat-maize double cropping systems of the North China Plain: assessment, field study, and implications. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2017.10.187.
Carneiro, R. P., Oliveira, F. A. S., Madureira, F. D., Silva, G., de Souza, W. R., & Lopes, R. P. (2013). Development and method validation for determination of 128 pesticides in bananas by modified QuEChERS and UHPLC–MS/MS analysis. Food Control, 33(2), 413–423. https://doi.org/10.1016/j.foodcont.2013.02.027.
Chormey, D. S., Büyükpınar, Ç., Turak, F., Komesli, O. T., & Bakırdere, S. (2017a). Simultaneous determination of selected hormones, endocrine disruptor compounds, and pesticides in water medium at trace levels by GC-MS after dispersive liquid-liquid microextraction. Environmental Monitoring and Assessment, 189(6), 277. https://doi.org/10.1007/s10661-017-6003-6.
Chormey, D. S., Karakuş, Y., Karayaka, S., Özsöyler, Ç., Bozdoğan, A. E., & Bakırdere, S. (2017b). Multivariate optimization of dispersive liquid–liquid microextraction for the determination of paclobutrazol and triflumizole in water by GC–MS. Journal of Separation Science, n/a-n/a, 40, 4541–4548. https://doi.org/10.1002/jssc.201700853.
Chu, X.-G., Hu, X.-Z., & Yao, H.-Y. (2005). Determination of 266 pesticide residues in apple juice by matrix solid-phase dispersion and gas chromatography–mass selective detection. Journal of Chromatography A, 1063(1), 201–210. https://doi.org/10.1016/j.chroma.2004.12.003.
Cox, M. K., Peterson, K. N., Tan, D., Novak, P. J., Schoenfuss, H. L., & Ward, J. L. (2017). Temperature modulates estrone degradation and biological effects of exposure in fathead minnows. Science of the Total Environment, 621, 1591–1600. https://doi.org/10.1016/j.scitotenv.2017.10.069.
Crutchfield, C. A., Lu, W., Melamud, E., & Rabinowitz, J. D. (2010). Chapter 16—Mass spectrometry-based metabolomics of yeast. In Methods in enzymology (Vol. Volume 470, pp. 393–426): Academic Press.
Facure, M. H. M., Mercante, L. A., Mattoso, L. H. C., & Correa, D. S. (2017). Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta, 167(Supplement C), 59–66. https://doi.org/10.1016/j.talanta.2017.02.005.
He, T.-T., Zuo, A.-J., Wang, J.-G., & Zhao, P. (2017). Organochlorine pesticides accumulation and breast cancer: a hospital-based case–control study. Tumor Biology, 39(5), 1010428317699114. https://doi.org/10.1177/1010428317699114.
Hutson, D. H., & Miyamoto, J. (1998). Fungicide classes: chemistry, uses and mode of action. In Fungicidal activity: chemical and biological approaches to plant protection. United Kingdom: Wiley.
Jahanmard, E., Ansari, F., & Feizi, M. (2016). Evaluation of Quechers sample preparation and GC mass spectrometry method for the determination of 15 pesticide residues in tomatoes used in salad production plants. Iranian Journal of Public Health, 45(2), 230–238.
Koçoğlu, E. S., Bakırdere, S., & Keyf, S. (2017). A novel liquid–liquid extraction for the determination of sertraline in tap water and waste water at trace levels by GC–MS. Bulletin of Environmental Contamination and Toxicology, 99(3), 354–359. https://doi.org/10.1007/s00128-017-2118-2.
Kopper, N. W., Gudeman, J., & Thompson, D. J. (2008). Transdermal hormone therapy in postmenopausal women: a review of metabolic effects and drug delivery technologies. Drug Design, Development and Therapy, 2, 193–202.
Labrie, F., Cusan, L., Gomez, J. L., Cote, I., Berube, R., Belanger, P., et al. (2009). Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause-the Journal of the North American Menopause Society, 16(1), 30–36. https://doi.org/10.1097/gme.0b013e31817b6132.
Lazic, Z. R. (2006). Design of experiments in chemical engineering: a practical guide: Wiley.
Machado, I., Gérez, N., Pistón, M., Heinzen, H., & Cesio, M. V. (2017). Determination of pesticide residues in globe artichoke leaves and fruits by GC–MS and LC–MS/MS using the same QuEChERS procedure. Food Chemistry, 227, 227–236. https://doi.org/10.1016/j.foodchem.2017.01.025.
Perdichizzi, S., Mascolo, M. G., Silingardi, P., Morandi, E., Rotondo, F., Guerrini, A., Prete, L., Vaccari, M., & Colacci, A. (2014). Cancer-related genes transcriptionally induced by the fungicide penconazole. Toxicology In Vitro, 28(1), 125–130. https://doi.org/10.1016/j.tiv.2013.06.006.
Primel, E. G., Caldas, S. S., Marube, L. C., & Escarrone, A. L. V. (2017). An overview of advances in dispersive liquid–liquid microextraction for the extraction of pesticides and emerging contaminants from environmental samples. Trends in Environmental Analytical Chemistry, 14, 1–18. https://doi.org/10.1016/j.teac.2017.03.001.
Pukkila, V., & Kontro, M. H. (2014). Dichlobenil and 2,6-dichlorobenzamide (BAM) dissipation in topsoil and deposits from groundwater environment within the boreal region in southern Finland. Environmental Science and Pollution Research, 21(3), 2289–2297. https://doi.org/10.1007/s11356-013-2164-1.
Rathnayake, L. K., & Northrup, S. H. (2016). Structure and mode of action of organophosphate pesticides: a computational study. Computational and Theoretical Chemistry, 1088, 9–23. https://doi.org/10.1016/j.comptc.2016.04.024.
Rezaee, M., Assadi, Y., Milani Hosseini, M.-R., Aghaee, E., Ahmadi, F., & Berijani, S. (2006). Determination of organic compounds in water using dispersive liquid–liquid microextraction. Journal of Chromatography A, 1116, 1), 1–1), 9. https://doi.org/10.1016/j.chroma.2006.03.007.
Rose, G., Lane, S., & Jordan, R. (2009). The fate of fungicide and insecticide residues in Australian wine grape by-products following field application. Food Chemistry, 117(4), 634–640. https://doi.org/10.1016/j.foodchem.2009.04.061.
Rutkowska, M., Dubalska, K., Konieczka, P., & Namieśnik, J. (2014). Microextraction techniques used in the procedures for determining organomercury and organotin compounds in environmental samples. Molecules, 19, 7581–7609. https://doi.org/10.3390/molecules19067581.
Sandín-España, P., & Sevilla-Morán, B. (2012). Pesticide degradation in water. In H. S. Rathore, & L. M. L. Nollet (Eds.) Pesticides: evaluation of environmental pollution (pp. 89): CRC Press.
Santos, I. C., & Schug, K. A. (2017). Recent advances and applications of gas chromatography vacuum ultraviolet spectroscopy. Journal of Separation Science, 40(1), 138–151. https://doi.org/10.1002/jssc.201601023.
Turan, N. B., Chormey, D. S., Büyükpınar, Ç., Engin, G. O., & Bakirdere, S. (2017). Quorum sensing: little talks for an effective bacterial coordination. TrAC Trends in Analytical Chemistry, 91, 1–11. https://doi.org/10.1016/j.trac.2017.03.007.
Vetter, W. (2012). Chapter 3—Polyhalogenated Alkaloids in environmental and food samples. In K. Hans-Joachim (Ed.), The alkaloids: chemistry and biology (Vol. Volume 71, pp. 211–276): Academic Press.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Bulgurcuoğlu, A.E., Yılmaz, B., Chormey, D.S. et al. Simultaneous determination of estrone and selected pesticides in water medium by GC-MS after multivariate optimization of microextraction strategy. Environ Monit Assess 190, 252 (2018). https://doi.org/10.1007/s10661-018-6625-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6625-3