Abstract
Recent studies have shown reproductively arrested gonad development in female Alburnus tarichi (Güldenstädt, 1814) (Cyprinidae) from the eastern coastline of Lake Van, Turkey, due to increasing pollution. In the reproductively arrested females (RAF), oocytes were developmentally blocked and arrested at the previtellogenic stage and gonadosomatic indices (GSI) were very low, while reproductively non-arrested females (RNF) found at the same site displayed relatively normal ovarian development and higher GSI. The present study investigated various oxidative stress biomarkers in the ovaries of RAF and RNF collected from a polluted site at Lake Van at the mid-vitellogenic phase, compared with reference fish from a non-polluted site (Lake Erçek). Ovarian total protein content, biometric indices, and histology were also evaluated. The oxidative stress biomarkers used were levels of lipid peroxidation (LPO) and glutathione (GSH), and activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione-S-transferase (GST). High levels of LPO and GSH and activities of SOD, GPx and GST were found in the ovaries of RAF compared with the reference fish. GSH content and activities of GPx and GST were also higher in the RNF. The total protein content and biometric indices decreased significantly in the RAF compared with the RNF and reference fish. The histology of the ovaries revealed atresia, melano-macrophage centers, encapsulated follicle cysts, and severe fibrosis in the RAF. The results of this study suggest that abnormalities in the ovaries of A. tarichi are causally related to increased oxidative stress as a result of pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing amounts of pollutants discharged into aquatic environments from industrial and agricultural facilities and anthropogenic sources have been reported to cause deleterious effects on fish health (Lukin et al. 2011). In particular, environmental pollutants possessing endocrine activities such as pesticides, heavy metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, alkylphenolic compounds, phthalates, and synthetic and natural hormones are capable of disrupting the reproductive physiology of fish populations (Blazer 2002; Agbohessi et al. 2015). Studies reported that gonadal abnormalities, reduced gonadosomatic index values, altered hormone levels, increased plasma vitellogenin levels, and delay in gametogenesis occurred in fish found in areas receiving wastewater containing agricultural and industrial chemicals (Hassanin et al. 2002; Kavanagh et al. 2004; Moharram et al. 2011; Gilroy et al. 2012).
Recent evidence indicates that environmental pollutants generate reactive oxygen species (ROS) which are responsible for toxicity in aquatic organisms living in contaminated regions (Valavanidis et al. 2006). Cellular antioxidant defense systems are depleted in the living organisms when exposed to these environmental pollutants, and excessive ROS production can overwhelm endogenous defenses, causing oxidative damage to cellular components (Livingstone 2003). Increased ROS levels may be responsible for impairment in the physiological functions of tissues and organs, and assayed antioxidant system parameters can serve as biomarkers of oxidative stress status (Van der Oost et al. 2003).
Lake Van is in the Eastern Anatolia Region of Turkey. It is the largest soda lake in the world, with highly alkaline water (Sari 2008). Alburnus tarichi, (Güldenstädt, 1814) (Cyprinidae), is a fish species endemic to the Lake Van basin. The fish is anadromous, migrating to freshwater inlets for spawning during the reproduction phase (from mid-April to mid-June) (Danulat and Selcuk 1992; Kaptaner and Kankaya 2013). About 11,000 tons of the fish are caught annually, indicating that it is economically important in the region. Studies conducted in the last decade show that Lake Van has been undergoing a process of environmental degradation causally related to pollutants from domestic discharge, agricultural runoff, and industrial effluents. Various chemical families (e.g., heavy metals (Bilgili et al. 1995; Oğuz and Yeltekin 2014), pesticides (Unal et al. 2014) and natural and synthetic hormones (Oğuz and Kankaya 2013)) have been detected in the surface waters and sediment samples of the lake. In recent years, increasing pollution has begun to exert pressure on A. tarichi, which is known to be the only vertebrate species in the lake (Kaptaner et al. 2014). Ünal et al. (2007) described a subset of female A. tarichi, collected from the eastern coastline of the lake, showing disturbances in reproductive physiology that resulted in a significant reduction in gonad mass, poor gonadosomatic index values, ovaries having arrested oocyte development, and lower plasma 17β-estradiol level. This group of fish was termed reproductively arrested females (RAF) by the authors. Other female fish in the same environment were reported to have normal ovaries, relatively higher plasma 17β-estradiol and GSI values, and were termed reproductively non-arrested females (RNF). The authors reported 43 % of the 150 females sampled to be RAF and hypothesized that A. tarichi might be exposed to endocrine disrupting chemicals. Subsequently, Unal et al. (2014) showed that RAF possessed lower hepatic and ovarian estrogen receptor α and vitellogenin messenger RNA (mRNA) levels than RNF found in the same environment and concluded that RAF were a segment of the population more sensitive to endocrine-disrupting chemicals.
The present study aimed to determine a suite of biomarkers to reveal disturbances in the reproductive physiology of A. tarichi. Here, we report antioxidant defense system profiles in the ovarian tissue of RAF and RNF of A. tarichi collected from a polluted region of Lake Van, compared with reference fish from Lake Erçek. In addition to oxidative stress parameters, biometric measurements, total protein content, and histological alterations in the ovarian tissue are presented.
Materials and methods
Study area
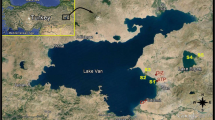
Lake Van is the largest lake in Turkey, with a surface area of 3574 km2. Settlements of various sizes such as Van, Erciş, Gevaş, Edremit, Tatvan, Adilcevaz, and Ahlat all discharge their wastewater into the lake. Van is a large city on the eastern coastline of Lake Van. Because of the extended domestic, industrial, and agricultural activities along the eastern side, various pollutants reach the lake from different points in Van city. Near this part of the lake are cement and industrial factories, a wastewater sewage treatment plant, agricultural areas, and suburban discharge sources (Fig. 1). Pollution in the form of pesticides, endocrine-disrupting chemicals, and heavy metals enter the lake mainly from this side. Recent studies have reported gonadal and hepatic histopathologies and endocrine disturbances in A. tarichi sampled from the eastern coastline of the lake (Kaptaner et al. 2014; Ünal et al. 2007, Unal et al. 2014). Lake Erçek is 20 km east of Lake Van. It has a surface elevation of about 1890 m, an area of 95.20 km2, and a maximum depth of 38 m (Adızel and Durmuş 2009). In the present study, fish samples were obtained from two sites. These were selected because the first one, Lake Erçek (S1: 43° 34′ E, 38° 40′ N), was considered to be less affected by pollution, if at all, and thus was chosen as a reference site. This site is 20–25 km outside Van city. The second site is on the eastern coastline of Lake Van (S2: 43° 13′ E, 38° 26′ N) and assumed to be polluted (Fig. 1). Physicochemical parameters of sampling sites such as temperature, pH, dissolved oxygen, conductivity, and salinity were measured (Table 1).
Geographic locations of the study sites (S1: reference site on the Lake Erçek; S2: contaminated site on Lake Van). Some facilities are present on the eastern side of Lake Van. CF cement factory, STP sewage treatment plant of Van Metropolitan Municipality, OIZ organized industry zone (Source: Google Earth)
Fish sampling and processing
A total of 26 sexually mature female A. tarichi (Güldenstädt, 1814) were sampled from S1 and S2 by professional fishermen using gill nets, between February 24 and March 01, 2015. Sampling time was selected according to the reproductive period of A. tarichi. At this time, A. tarichi presents the mid-vitellogenic period of its reproductive cycle (Ünal et al. 1999). In addition, at this time, RAF were more easily separated by morphologic gonadal examination than were RNF. Ten of the sampled female fish were from S1 and the remainder (n = 16) from Lake Van. After sampling, the live fish were transferred to the laboratory in aerated coolers. Body weight (W) and total length (L) were individually measured and recorded to calculate the Fulton conditioning factor (CF = 100 × W ÷ L 3). The fish were then sacrificed and their ovaries were removed. Ovary weights (OWs) were recorded for calculation of gonadosomatic index (GSI = OW ÷ W × 100). Prior to sacrifice, the fish were anesthetized with 2-phenoxyethanol. Ethical regulations were followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments. The ovaries of each fish were macroscopically examined after dissection to identify abnormalities. RAF were determined according to the gonadal features (lower ovary weight and GSI and reduced ovaries with oocytes developmentally blocked before the vitellogenic stage) described by Unal et al. (2014). Eight of the fish sampled from the polluted site of Lake Van were determined to be RAF, and others were RNF (n = 8).
Histology
The mid-fragments of the ovaries were fixed in 10 % neutral buffered formalin solution for up to 48 h and then washed with phosphate buffered saline (pH 7.4). The tissues were then dehydrated through graded ethanol series and embedded in paraffin. The 10-μm-thick sections from the paraffinized tissues were placed on polylysine-coated slides. Next, the sections were deparaffinized in xylene, and after rehydration with graded ethanol concentrations, they were stained with hematoxylin and eosin (H&E) and Mallory’s trichrome for examination of the developmental stages and histological alterations. The slides were examined under a Leica DMI 6000 B model microscope and photographed.
Biochemical analyses
The ovarian tissues were rapidly frozen after removal and stored at −20 °C. Prior to the analyses, the tissues were thawed and homogenized for 5 min in 50 mM ice-cold KH2PO4 solution (1:10 w/v) using a glass porcelain ultrasonic homogenizer (Jencons Scientific Co., UK). The homogenates were centrifuged at 10,000 g for 30 min. All processes were carried out at 4 °C. Supernatant fractions were removed and used to determine the lipid peroxidation and antioxidant defenses.
Lipid peroxidation (LPO) was determined by measuring the malondialdehyde (MDA) content, a product of lipid peroxidation, in the samples. The MDA concentration was measured spectrophotometrically at 532 nm, using the method described by Jain et al. (1989), based on thiobarbituric acid reactivity. Concentration of MDA was calculated using the molar extinction coefficient (1.56 × 105 mol/L/cm) according to the formula, A = ε × C × L, where A is absorbance, ε is molar extinction coefficient, and L is path length.
The superoxide dismutase (SOD) activity was measured using a commercial kit (Ransod, Randox Lab., UK) at 505 nm and 37 °C according to the manufacturer’s instructions. In this method, a xanthine-xanthine oxidase system was used to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. SOD activity was then measured by the degree of inhibition of this reaction. One unit of SOD is that which causes a 50 % inhibition of the rate of reduction of INT under the conditions of the assay.
Glutathione peroxidase (GPx) activity was measured using a commercial kit (Ransel, Randox Lab., UK) at 340 nm and 37 °C according to the manufacturer’s instructions. This assay is based on the method of Paglia and Valentine (1967). GPx catalyzes the oxidation of glutathione by cumene hydroperoxide. In the presence of glutathione reductase and NADPH, the oxidized glutathione (GSSG) is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The GPx activity was calculated from the decreases in absorbance values.
The glutathione (GSH) content was measured spectrophotometrically at 412 nm using the method described by Beutler (1984). The GSH levels were obtained by using a standard curve derived from external GSH standards.
Glutathione-S-transferase (GST) activity was measured using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate (Habig et al. 1974). The assay was carried out in a quartz cuvette containing 0.1 M phosphate buffer (pH 6.5), 1 mM CDNB, and 1 mM GSH. The reaction was started by adding a 50-μL sample. Absorbance values of the CDNB-glutathione conjugate were assayed spectrophotometrically at 340 nm.
The total protein content in the supernatant fractions was assayed spectrophotometrically using the method of Bradford (1976), with bovine serum albumin as a standard. The SOD activity was expressed as unit per milligram protein, GPx activity was expressed as unit per gram protein, GST activity was expressed as nanomole of CDNB-glutathione conjugate per minute per milligram protein, and the MDA and GSH content was expressed as nanomole per milligram protein. The total protein content values for the ovarian tissue were expressed as milligram per gram wet weight of the tissue.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences software, version 16.0. Differences among the data were analyzed using a one-way analysis of variance with a post hoc Duncan’s multiple comparison test. The results were expressed as the mean ± standard deviation (SD). Values P ≤ 0.05 were considered to be statistically significant.
Results
Biometric indices
All sampled female fish were sexually mature. As shown in Table 2, length and weight of the RAF and RNF fish at S2 was significantly lower than that of reference fish at S1. The CF values of RAF at S2 were very low compared with both the RNF and reference fish. The lowest GSI values were found in RAF. The GSI of RNF collected from S2 was also significantly lower than in the reference fish collected from S1.
Histology
The ovarian sections of reference fish and RNF indicated that A. tarichi was at mid-vitellogenic phase of ovarian development. A higher proportion of oocytes at mid-vitellogenic stage and lower rates of cortical alveolar and perinucleolar oocytes were observed in the ovaries of these fish (Fig. 2a, b). In contrast, mostly perinucleolar stage oocytes were observed in RAF (Fig. 2c). Few oocytes in cortical alveolar and early vitellogenic stages were observed in two individuals of RAF. Several types of histological alterations were also observed in the ovaries of RAF. These were atresia in perinucleolar oocytes (Fig. 3a), melano-macrophage centers (Fig. 3b), encapsulated follicle cysts (Fig. 3c), and severe fibrosis (Fig. 3d).
Representative light micrographs of ovary sections of reference fish from S1 (a) and RNF from S2 showing normal ovarian organization (b), and RAF from S2 showing arrested ovarian development (c). pn perinucleolar oocyte, ca cortical alveolar oocyte, eVo early vitellogenic oocyte, mVo mid-vitellogenic oocyte. H&E stain
Light micrographs of ovary sections of RAF showing histological alterations. Atresia in perinucleolar follicle (ao) (H&E stain) (a); melano-macrophage centers (mmc) (H&E stain) (b); encapsulated follice cysts (efc) (H&E stain) (c); severe fibrosis (sf) in which collagen fibers stained in blue by Mallory’s trichrome stain (d)
GSI values and histological examinations confirmed that 8 individuals of a total of 16 females sampled from S2 displayed reproductively arrested development in their ovaries.
Biochemical assays
The total protein content, LPO, and endogenous antioxidants in the ovary of A. tarichi are presented in Table 3.
Levels of total protein in the ovary of fish captured at S2 were significantly lower than in those recorded at the reference site, S1. The lowest levels of total protein content were observed in the ovaries of RAF.
LPO levels were found to be significantly higher in the ovarian tissue samples of RAF than in those of RNF from S2 and reference fish from S1.
Ovarian SOD activity in RAF was significantly higher than in the RNF and reference fish. GPx activities in the ovarian tissues of RAF and RNF were significantly higher than in ovaries of the reference fish. GST activity increased significantly in ovaries of both RAF and RNF fish compared with the reference fish. Activity of GST in the RAF ovaries was significantly higher than values observed in the ovaries of RNF. The ovarian GSH content was significantly higher in RAF and RNF than in reference fish; however, the GSH content was found to be higher in RAF than in RNF.
Discussion
Biometric indices
Recent studies have shown that the eastern coastline of Lake Van is undergoing environmental pollution by contaminants arising from agricultural and industrial facilities and other anthropogenic sources existing along this shore (Fig. 1). Previous reports showed that metals increased in the Lake Van environment and accumulated in various tissues of A. tarichi, including the ovaries (Bilgili et al. 1995; Oğuz and Yeltekin 2014). It was reported that municipal sewage treatment plant effluent, containing endocrine disrupting chemicals and flowing into the lake, is a major source of pollution in the sediment and surface waters of Lake Van. Some pesticide residues and phthalates in the fish ovarian tissue and lake sediment samples were also reported at this site (Unal et al. 2014). The present study was intended to focus on the biomarkers of oxidative stress, histology, and biometric indices in the RAF and RNF in this polluted area of Lake Van compared with the reference fish from an unpolluted site (Lake Erçek).
In female A. tarichi, length, weight, CF, and GSI varied considerably between sites. The biometric indices of fish sampled from Lake Van were generally lower than those of reference fish. This effect might be related to niche changes, the use of available food resources, and pollutants existing in the lake environment (Nwabueze and Ekelemu 2011; Gilroy et al. 2012). RAF displayed significantly lower CF, an indication of physiological status related to well-being, than those of RNF and reference fish. In accordance, the poor CF was also recorded in fish exposed to industrial effluent (Adeogun et al. 2012). Fish found in sites contaminated with pesticides were reported to have decreased CF regardless of the season, species, and sex (Agbohessi et al. 2015). A low GSI was observed in RAF from Lake Van compared with RNF and reference fish. Elp and Çetinkaya (2000) reported that GSI is well correlated with oocyte development in A. tarichi. Decrease in the GSI could be attributed to the presence of mainly previtellogenic oocytes showing arrested development instead of mid-vitellogenic stage oocytes in the ovaries (Ünal et al. 2007). Unal et al. (2014) reported that the presence of previtellogenic oocytes in the ovaries of RAF is most likely to be a consequence of exposure to contaminants. Such a reduced GSI has also been observed in other fish species exposed to various types of contaminants (Singh and Singh 2008; Mdegela et al. 2010; Lal et al. 2013).
Histology
Histological examination showed that RNF from Lake Van and reference fish from Lake Erçek possessed mainly mid-vitellogenic oocytes in their ovaries, whereas mainly previtellogenic oocytes were observed in RAF. Such an observation was termed “reproductively arrested gonad development” in previous studies (Ünal et al. 2007, Unal et al. 2014). It was hypothesized that regressed ovaries in A. tarichi might arise because of accumulation of endocrine disrupting chemicals such as phthalates and pesticides (Unal et al. 2014), and heavy metals (Oğuz and Yeltekin 2014) in the ovaries. In the present study, some histological alterations including atresia, melano-macrophage centers, encapsulated follicle cysts, and severe fibrosis in addition to the regressed ovarian development were observed. Similar to our findings, atresia and melano-macrophage centers were described in a previous study (Ünal et al. 2007); however, encapsulated follicle cysts and severe fibrosis were first determined here. This result suggested that increasing pollution in the lake environment raised its pressure on A. tarichi as time progressed. Many factors can cause atresia in fish ovaries, and atresia, especially in previtellogenic oocytes as in the present study, is an important indicator of pathological conditions. When fish are exposed to environmental contaminants, the rate of atretic oocytes has been reported to increase (Kaptaner and Ünal 2011). Melano-macrophage centers are pigment-containing macrophage aggregates that play a role in the storage of foreign material, and increases in size and frequency of these centers in environmentally stressful conditions suggest chemical pollution (Agius and Roberts 2003). Consistently, Agbohessi et al. (2015) have reported melano-macrophage centers in the ovaries of fish inhabiting a cotton basin impacted by pesticides. Encapsulated follicle cysts are abnormal structures in which follicular cell layers proliferate and form a nonkeratinized multilayer epithelium (Witthames et al. 2010; Dominguez-Petit et al. 2011). These structures in the fish ovary presumably occurred as a result of pollution. Severe fibrosis observed in the ovary of A. tarichi might be a chronic tissue response to contaminant injury (Blazer 2002).
Biochemistry
The total protein content of ovaries in fish sampled from Lake Van displayed a general decrease compared with those sampled from the reference site; however, it was remarkably lower in the RAF than in RNF and reference fish. Studies showed when fish were exposed to the toxicants, the protein content of the tissues was decreased (Nagaraju and Rathnamma 2013). It has been reported that the ovarian protein content increased as vitellogenesis progressed, depending on the reproductive cycle of fish; however, it could be decreased in the presence of pollutants (Assem et al. 2005; Kumari 2012). A remarkable reduction of the protein content in the ovaries of RAF can be attributed to the unfavorable process of vitellogenesis. The key product in vitellogenesis is a lipophosphoprotein called vitellogenin, which is synthesized by liver in response to circulating 17β-estradiol. After synthesis, vitellogenin is transported to the ovary by blood and incorporated into developing oocytes. However, some external (environmental) and internal (sex hormones) factors may negatively impact this process (Khan et al. 1991). Previous studies reported that RAF have reduced circulating 17β-estradiol levels (Ünal et al. 2007) and lower vitellogenin mRNA levels in their livers and ovaries (Unal et al. 2014).
LPO is a complex process in which polyunsaturated fatty acids in biological membranes undergo changes by chain reactions and form lipid hydroperoxides that decompose double bonds of unsaturated fatty acids and destroy membrane lipids (Janero 1990). A wide variety of environmental contaminants have been shown to produce free radicals and initiate LPO, and free radicals cause oxidative damage in cells by attacking main cellular components such as proteins, carbohydrates, and nucleic acids (Van der Oost et al. 2003). In the present study, significantly increased levels of LPO in the ovaries of RAF collected from a polluted region of Lake Van indicate oxidative injury and provide an indicator of pollution. This result coincides with a previous study demonstrating elevated LPO in gonadal tissue of fish (Pomadys jubelini) collected from polluted coastal sites (Ekaete 2014). Increased LPO levels were also reported in various tissues of different fish living in rivers (Farombi et al. 2007) and lakes (Abdel-Moneim et al. 2013) contaminated by environmental pollutants.
SOD plays an important role against oxygen toxicity by catalyzing the transformation of superoxide radical to hydrogen peroxide and water and catalase which further degrades hydrogen peroxide to water. Exposure to environmental pollutants has been reported to increase SOD activity in fish (Dimitrova et al. 1994; Dautremepuits et al. 2004; Zagal and Mazmanci 2011). In the present study, SOD activity was found to be enhanced in the ovaries of RAF compared with the RNF and reference fish, suggesting pollution-induced production of superoxide radicals. Similar to the present study, SOD activity was reported by several authors to be higher in fish from polluted sites (Avci et al. 2005; Abdel-Moneim et al. 2013; Gül et al. 2004).
The results showed that GPx activity increased significantly in the ovaries of RAF and RNF. GPx is an important enzyme that converts hydrogen peroxide into hydrogen oxide using GSH as a substrate. Increased GPx activity has been described in fish treated with certain environmental pollutants (Almeida et al. 2002; Sayeed et al. 2003; Sanchez et al. 2005; Kankaya et al. 2015). The observed increase in the GPx activity might be a protective response to overproduction of hydrogen peroxide in the ovary under pollutant-induced LPO. Consistent with our results, some researchers also reported high levels of GPx activity in the tissues of other fish collected from polluted waters (Pandey et al. 2003; Abdel-Moneim et al. 2013).
GST is as a major phase II detoxification enzyme that plays a role in catalyzing the electrophilic substrates to GSH. The enzyme protects cells against endogenous and exogenous toxic chemicals (Sheehan et al. 2001). The activity of GST was found to be significantly increased in the ovaries of fish collected from Lake Van as compared with reference fish. Elevated GST activity is possibly related to increased free radical production from the metabolism of environmental pollutants. Our results coincide with those of Lu et al. (2011) who reported increased GST activity in caged fish at Lake Taihu, which is known to be contaminated with estrogenic chemicals. Increased GST activity has also been demonstrated in fish species as a result of exposure to certain toxicants (Otto and Moon 1996; Farombi et al. 2007; Peebua et al. 2007; Lu et al. 2009).
GSH protects cells against the toxicity of xenobiotics and oxidants and plays a central role in maintaining cellular redox status (Stephensen et al. 2002). GSH levels were significantly increased in the ovaries of fish from Lake Van. This increase was more evident in the RAF. These results agreed with Pandey et al. (2003); Farombi et al. (2007), and Doherty et al. (2010), who also found an increase in GSH level together with elevated lipid peroxidation in the tissues of fish collected from polluted waters. GSH itself is a free radical scavenger and known to be a cofactor substrate for GPx and GST. The increase in GSH level might be due to an adaptive and protective role of this biomolecule against oxidative stress as a result of chronic exposure to environmental contaminants (Dickinson and Forman 2002).
In conclusion, the present study demonstrated evidence of elevated oxidative stress in the ovaries of RAF from the eastern coastline of Lake Van, which is known to be polluted with various contaminants. RNF developed antioxidant defenses to remove or transform free radicals and reduce oxidative damage. By making a comparative study of two distinct sites, we have shown that oxidative stress parameters and physiological responses such as CF, GSI and ovarian histology are linked. Thus, abnormalities in the ovaries of A. tarichi are causally related to increased oxidative stress as a result of elevated levels of pollution in Lake Van.
References
Abdel-Moneim, A. M., Essawy, A. E., El-Din, N. K. B., & El-Naggar, N. M. (2013). Biochemical and histopathological changes in liver of the Nile tilapia from Egyptian polluted lakes. Toxicology and Industrial Health. doi:10.1177/0748233713503374.
Adeogun, A. O., Ogidan, I. M., Ibor, O. R., Chukwuka, A. V., Adedara, I. A., & Farombi, E. O. (2012). Long-term exposure to industrial effluent induces oxidative stress and affects growth in Clarias gariepinus. Research Journal of Environmental and Earth Sciences, 4, 738–746.
Adızel, Ö., & Durmuş, A. (2009). A study on bird species under threat and avifauna of Erçek Lake (Van-Turkey). Scientific Research and Essays, 4, 1006–1011.
Agbohessi, P. T., Toko, I. I., Ouedraogo, A., Jauniaux, T., Mantiki, S. N. M., & Kastemont, P. (2015). Assessment of the health status of wild fish inhabiting a cotton basin heavily impacted by pesticides in Benin (West Africa). Science of the Total Environment, 506−507, 567–584.
Agius, C., & Roberts, R. J. (2003). Melano-macrophage centres and their role in fish pathology. Journal of Fish Diseases, 26, 499–509.
Almeida, J. A., Diniz, Y. S., Marques, S. F. G., Faine, L. A., Ribas, B. O., Burneik, R. C., & Novelli, E. L. B. (2002). The use of oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environment International, 27, 673–679.
Assem, S. S., El-Serafy, S. S., El-Garabawy, M. M., Elabsawy, M. E. G., & Kaldus, S. K. (2005). Some biochemical aspects of reproduction in female Trachinotus ovatus (Carangidae). Egyptian Journal of Aquatic Research, 31, 315–327.
Avci, A., Kaçmaz, M., & Durak, İ. (2005). Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicology and Environmental Safety, 60, 101–105.
Beutler, E. (1984). Red cell metabolism. A manual of biochemical methods. Third ed. New York: Grune and Startton, pp 105–106.
Bilgili, A., Sağmanlıgil, H., Çetinkaya, N., Yarsan, E., & Türel, I. (1995). The natural quality of Van Lake and the levels of some heavy metals in grey mullet (Chalcalburus tarichi, Pallas 1811) samples taken from this lake. Veterinary Journal of Ankara University, 42, 445–450.
Blazer, V. S. (2002). Histopathological assessment of gonadal fish tissue in wild fishes. Fish Physiology and Biochemistry, 26, 85–101.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biocheistry, 72, 248–254.
Danulat, E., & Selcuk, B. (1992). Life history and environmental conditions of the anadromous Chalcalburnus tarichi (Cyprinidae) in the highly alkaline lake Van, Eastern Anatolia, Turkey. Archiv für Hydrobiologie, 126, 105–125.
Dautremepuits, C., Paris-Palacios, S., Betoulle, S., & Vernet, G. (2004). Modulation in hepatic and head kidney parameters of carp (Cyprinus carpio L) induced by copper and chitosan. Comparative Biochemistry and Physiology C, 137, 325–333.
Dickinson, D. A., & Forman, H. J. (2002). Cellular glutathione and thiols metabolism. Biochemical Pharmacology, 64, 1019–1026.
Dimitrova, M. S. T., Tsinova, V., & Velcheva, V. (1994). Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio). Comparative Biochemistry and Physiology C, 108, 43–46.
Doherty, V. F., Ogunkuade, O. O., & Kanife, U. C. (2010). Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in some selected fishes in Lagos, Nigeria. American-Eurasian Journal of Agricultural and Environmental Sciences, 7, 359–365.
Dominguez-Petit, R., Alonso-Fernandez, A., & Saborido-Rey, F. (2011). Incidence and significance of cystic structures in the ovaries of gadoid fish. Scientia Marina, 75, 359–368.
Ekaete, A. G. (2014). Oxidative stress in fish living in coastal water polluted with sawdust and wood waste along Lagos lagoon, Nigeria. Researcher, 6, 1–5.
Elp, M., & Çetinkaya, O. (2000). İnci kefali (Chalcalburnus tarichi Pallas, 1811)’nin üreme biyolojisi üzerine bir araştırma. IV. Su ürünleri Sempozyumu, 28–30 Haziran 2000 Erzurum 51–66.
Farombi, E. O., Adelowo, O. A., & Ajimoko, Y. R. (2007). Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun river. International Journal of Environmental Research and Public Health, 4, 158–165.
Gilroy, E. A. M., McMaster, M. E., Parrot, J. L., Hewitt, L. M., Park, B. J., Brown, S. B., & Sherry, J. P. (2012). Assessment of the health status of wild fih from the Wheathley Harbour area of concern, Ontario, Canada. Environmental Toxicology and Chemistry, 31, 2798–2811.
Gül, S., Belge-Kurutaş, E., Yıldız, E., Şahan, A., & Doran, F. (2004). Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environment International, 30, 605–609.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry, 249, 7130–7139.
Hassanin, M., Kuwahara, S., Nurdihayat, Tsukamoto, Y., Ogawa, K., Hiramatsu, K., & Sasaki, F. (2002). Gonadosomatic index and testis morphology of common carp (Cyprinus carpio) in rivers contaminated with estrogenic chemicals. The Journal of Veterinary Medical Science, 64, 921–926.
Jain, S. K., McVie, R., Duett, J., & Herbst, J. J. (1989). Erythrocyte membrane lipid peroxidation and glycolylated hemoglobin in diabetes. Diabetes, 38, 1539–1543.
Janero, D. R. (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnistic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicie, 9, 515–540.
Kankaya, E., Kaptaner, B., Doğan, A., & Çelik, İ. (2015). Toxicity of bisphenol A during the early life stages of Chalcalburnus tarichi (Pallas, 1811). Fresenius Environmental Bulletin, 24, 977–985.
Kaptaner, B., & Kankaya, E. (2013). Analysis of germ cell proliferation, apoptosis, and androgenesis in the Lake Van fish (Chalcalburnus tarichi) during testicular development. Fish Physiology and Biochemistry, 39, 1165–1679.
Kaptaner, B., & Ünal, G. (2011). Effects of 17α-ethynylestradiol and nonylphenol on liver and gonadal apoptosis and histopathology in Chalcalburnus tarichi. Environmental Toxicology, 26, 610–622.
Kaptaner, B., Kankaya, E., Doğan, A., & Çelik, İ. (2014). Histopathology and oxidative stress in the liver of Chalcalburnus tarichi living in lake Van, Turkey. Life Science Journal, 11, 66–77.
Kavanagh, R. J., Balch, G. C., Kiparissis, Y., Niimi, A. J., Sherry, J., Tinson, C. I., & Metcalfe, C. D. (2004). Endocrine disruption and altered gonadal development in white perch (Morone americana) from the lower Great Lakes region. Environmental Health Perspectives, 112, 898–902.
Khan, E. A., Sinha, M. P., Saxena, N., Panday, P. N., & Mehrotra, P. N. (1991). Biochemical variation during ovarian vitellogenic growth in a hill stream teleost Garra mullya (Sykes) due to cadmium toxicity. Journal of the Indian Fisheries Association, 21, 11–14.
Kumari, M. (2012). Effects of organophosphate pesticide abate on the ovary of the cat fish, Heteropneustes fossilis (Bloch.). Bangladesh Journal of Zoology, 40, 207–212.
Lal, B., Sarang, M. K., & Kumar, P. (2013). Malathion exposure induces the endocrine disruption and growth retardation in the catfish Clarias batrachus (Linn). General and Comparative Endocrinology, 181, 139–145.
Livingstone, D. R. (2003). Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue de Médecine Vétérinaire, 154, 427–430.
Lu, G., Yan, Z., & Wang, Y. (2011). Assesstment of estrogenic contamination and biological effects in Lake Taihu. Ecotoxicology, 20, 974–981.
Lu, G. H., Wang, C., & Zhu, Z. (2009). The dose-response relationships for EROD and GST induced by polyaromatic hydrocarbons in Carassius auratus. Bulletin of Environmental Contamination and Toxicology, 82, 194–199.
Lukin, A., Sharova, J., Belicheva, L., & Camus, L. (2011). Assessment of fish health status in the Pechora river: effects of contamination. Ecotoxicology and Environmental Safety, 74, 355–365.
Mdegela, R. H., Braathen, M., Mosha, R. D., Skaare, J. U., & Sandvik, M. (2010). Assessment of pollution in sewage ponds using biomarker responses in wild African sharptooth catfish (Clarias gariepinus) in Tanzania. Ecotoxicology, 19, 722–734.
Moharram, S. G., Wahbi, O. M., & El-Greisy, Z. A. (2011). Effect of polluted water from the Egyptian Eastern Mediterranean coast on reproductive, toxicological and hematological characteristics of Siganus rivulatus. Pakistan. Journal of Biological Sciences, 14, 668–681.
Nagaraju, B., & Rathnamma, V. V. (2013). Effect of profenofos an organophosphate on protein levels in some tissues of fresh water fish Labeo rohita (Hamilton). International Journal of Pharmacy and Pharmaceutical Sciences, 5, 276–279.
Nwabueze, A. A., & Ekelemu, J. K. (2011). Growth and survival of Clarias gariepinus (Burchell, 1822) fingerlings in different concentrations of domestic leachate. ARPN Journal of Agricultural and Biological Science, 6, 25–29.
Oğuz, A. R., & Kankaya, E. (2013). Determination of selected endocrine disrupting chemicals in Lake Van, Turkey. Bulletin of Environmental Contamination and Toxicology, 91, 283–286.
Oğuz, A. R., & Yeltekin, A. (2014). Metal levels in the liver, muscle, gill, intestine, and gonad of Lake Van fish (Chalcalburnus tarichi) with abnormal gonad. Biological Trace Element Research, 159, 219–223.
Otto, D. M. E., & Moon, T. W. (1996). Phase I and II enzymes and antioxidant responses in different tissues of brown bullheads from relatively polluted and nonpolluted systems. Archives of Environmental Contamination and Toxicology, 31, 141–147.
Paglia, D. E., & Valentine, W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70, 158–169.
Pandey, S., Parvez, S., Sayeed, I., Haque, R., Hafeez, B. B., & Raisuddin, S. (2003). Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Science of the Total Environment, 309, 105–115.
Peebua, P., Kosiyachinda, P., Pokethitiyook, P., & Kruatrachue, M. (2007). Evaluation of alachlor herbicide impacts on Nile tilapia (Oreochromis niloticus) using biochemical biomarkers. Bulletin of Environmental Contamination and Toxicology, 78, 128–131.
Sanchez, W., Palluel, O., Meunier, L., Coquery, M., Porcher, J.-M., & Ait-Aissa, S. (2005). Copper induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environmental Toxicology and Pharmacology, 19, 177–183.
Sari, M. (2008). Threatened fishes of the world: Chalcalburnus tarichi (Pallas 1811) (Cyprinidae) living in the highly alkaline Lake Van, Turkey. Environmental Biology of Fishes, 81, 21–23.
Sayeed, I., Parvez, S., Pandey, S., Bin-Hafeez, B., Haque, R., & Raisuddin, S. (2003). Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicology and Enviromental Safety, 56, 295–301.
Sheehan, D., Meade, G., Foley, V. M., & Dowd, C. A. (2001). Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochemical Journal, 360, 1–16.
Singh, P. B., & Singh, V. (2008). Pesticide bioaccumulation and plasma sex steroids in fishes during breeding phase from north India. Environmental Toxicology and Pharmacology, 25, 342–350.
Stephensen, E., Sturve, J., & Forlin, L. (2002). Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comparative Biochemistry and Physiology, 133, 435–442.
Ünal, G., Çetinkaya, O., & Elp, M. (1999). Histological investigation of gonad development of Chalcalburnus tarichi P., 1811). Turkish Journal of Zoology, 23, 329–338.
Ünal, G., Türkoğlu, V., Oğuz, A. R., & Kaptaner, B. (2007). Gonadal histology and some biochemical characteristics of Chalcalburnus tarichi (Pallas, 1811) having abnormal gonads. Fish Physiology and Biochemistry, 33, 153–165.
Unal, G., Marquez, E. C., Feld, M., Stavropoulos, P., & Callard, I. P. (2014). Isolation of estrogen receptor subtypes and vitellogenin genes: expression in female Chalcalburnus tarichi. Comparative Biochemistry and Physiology C, 172−173, 67–73.
Valavanidis, A., Vlahogianni, T., Dassenakis, M., & Scoullos, M. (2006). Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Enviromental Safety, 64, 178–189.
Van der Oost, R., Beyer, J., & Vermeulen, N. P. E. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology, 13, 57–149.
Witthames, P. R., Thorsen, A., & Kjesbu, O. S. (2010). The fate of vitellogenic follicles in experimentally monitored Atlantic cod Gadus morhua (L.): application to stock assessment. Fisheries Research, 104, 27–37.
Zagal, A., & Mazmanci, B. (2011). Oxidative stress response in Nile tilapia (Oreochromis niloticus) exposed to textile mill effluent. Toxicology and Industrial Health, 27, 81–85.
Acknowledgments
The author wishes to thank Dr. Ertuğrul Kankaya and research assistant Hüseyin Eroğlu for their help during fish sampling. Special thanks to Gurbet Ceylan Kaptaner for her dedicated support during the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaptaner, B. Relation between increased oxidative stress and histological abnormalities in the ovaries of Alburnus tarichi in Lake Van, Turkey. Environ Monit Assess 187, 702 (2015). https://doi.org/10.1007/s10661-015-4936-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4936-1