Abstract
Recent reports have demonstrated gonadal abnormalities in the Lake Van fish (Alburnus tarichi) from Lake Van caused by increasing pollution. In the present study, the fish was collected from an area of Lake Van receiving mainly sewage treatment plant effluent at prespawning period (April) and from a river (Karasu) which is close to the polluted area of the lake and where the fish migrates at spawning period (May). Collected specimens were examined for testicular alterations, gonadosomatic index (GSI), condition factor (CF), and antioxidant defense system biomarkers based on comparison with a reference lake (Erçek) and a reference freshwater inlet (Memedik River). Histological examinations of the testes of fish from the polluted area and the connected river showed various alterations consisting of macrophage aggregates, vacuolation, pyknosis, germ cell degeneration, seminiferous tubule dilation, disorganization of tubules, reduced spermatozoa, and fibrosis. A lower GSI and CF were also observed. Moreover, alterations in the antioxidant system biomarkers were determined in the testis tissues of fish from the Lake Van and Karasu River, indicating oxidative stress. These results suggest that the abnormalities in the testes are causally related to the increased oxidative stress, and pollution in Lake Van may have adversely affected the reproductive health of the lake Van fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic environments are continously being contaminated with hazardous pollutants originating from domestic sewage, wastewater treatment plant effluent, and industrial and agricultural activities (Au 2004). A wide variety of contaminants including pesticides, heavy metals, synthetic and natural estrogens, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and phthalates can reach water bodies from those sources and induce negative impacts on the aquatic organisms. As inhabitants of aquatic systems, fish are inevitably exposed to such chemicals (Scott and Sloman 2004). Many works have reported that histological alterations may occur in organs of fish living in or near contaminated systems (Simpson et al. 2000; Ayas et al. 2007; Triebskorn et al. 2008; Lukin et al. 2011; Oliva et al. 2013; Dane and Şişman 2015). Adverse effects detected in fish that have been exposed to pollutants found in aquatic environments include induction of oxidative damage in tissues as a consequence of overproduction of reactive oxygen species (ROS) (Gül et al. 2004; Ameur et al. 2012). Ecotoxicological studies have revealed that the reproductive systems of fish also suffer from increasing concentrations of toxic compounds in aquatic environments. Gonadal abnormalities (Louiz et al. 2009), reduced gonadosomatic index (GSI) values (Hassanin et al. 2002), altered sex hormone levels (Gilroy et al. 2012), and induction of female-specific vitellogenin in males and/or feminization (Carballo et al. 2005; Penaz et al. 2005; Blazer et al. 2014) are the most often reported reproductive disturbances in fish inhabiting areas receiving wastewater worldwide.
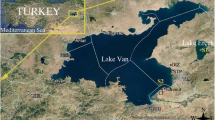
Alburnus tarichi is an endemic Cyprinid fish found in the Lake Van basin of Turkey. This anadromous fish species migrates to freshwater inlets feeding the lake to spawn during the reproductive period (April–June), after which it returns to the lake (Danulat and Selcuk 1992). Apart from its interesting biological properties, it is also a commercially important species, with 10,000 t/year being harvested (TÜİK 2013). Studies have shown that Lake Van has been undergoing environmental degradation caused by pollutants from domestic discharge, agricultural runoff, and industrial effluents and that A. tarichi in the lake may be adversely affected. Bilgili et al. (1995) analyzed water quality and some metals in the water of Lake Van and muscle tissue of A. tarichi and found that the water quality parameters were below the quality limits. Moreover, they reported concentrations of lead high enough to pose a risk to human health in the muscle tissue of A. tarichi as a result of terrestrial contamination. An investigation by Aksoy et al. (2011), conducted in some fish and mussel species collected from Lake Van and connected rivers, revealed the presence of organochlorine compounds (e.g., gamma-HCH, beta-HCH, hexachlorobenzene, and PCB 28) and a pesticide (4,4′ DDE) in A. tarichi. Field studies indicated histopathological lesions, such as melanomacrophage centers, glycogen depletion, and lipidosis in the liver of A. tarichi from the Van-Edremit coast of Lake Van, which receives domestic, sewage wastewater plant, and industrial effluents (Fig. 1) (Kaptaner et al. 2014). Ünal et al. (2007) reported decreased gonadosomatic index values, abnormalities in testicular and ovarian tissues, inhibition of liver acetylcholinesterase activity, and reduced plasma 17β-estradiol levels in A. tarichi from the same area. Analyses of sediment samples from the Van-Edremit coast of the lake have shown organic contaminants such as bis(2-ethylhexyl) phtalate and 4,4′-DDT and reproductive disturbances in female A. tarichi (Unal et al. 2014). In a last study, the relationship between histological alterations and increased oxidative stress in the ovary of A. tarichi as a result of pollution was indicated (Kaptaner 2015).
Histopathological changes have long been preferred as biomarkers of exposure to pollutants (Greenfield et al. 2008) and provide direct evidence of the adverse effects of contamination in fish (Fricke et al. 2012).
Many environmental pollutants generate ROS and subsequently induce oxidative stress, resulting in toxicity toward aquatic organisms inhabiting in contaminated waters (Valavanidis et al. 2006). The antioxidant defense system that constitutes antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST) as well as nonenzymatic antioxidants [e.g., glutathione (GSH)] serve as an important biological defense against the toxicity of ROS. Malondialdehyde is a main product of lipid peroxidation and one of the most frequently observed biomarkers indicating lipid peroxidation in the tissues (Tabrez and Ahmad 2009). All of these markers have been extensively used in numerous field studies and are known to be involved in pathology and the etiology of fish diseases (Gül et al. 2004; Abdel-Moneim et al. 2013).
Testes development has been well described in A. tarichi, and spermatogenesis is known to occur in three stages: (1) recrudescence (July–April), (2) spawning (May–June), and (3) postspawning (July). GSI, germ cell proliferation, and plasma androgens have been reported to be well correlated during testes development (Ünal et al. 1999; Kaptaner and Kankaya 2013). Although pollution-related disturbances in the ovary of A. tarichi have been thoroughly investigated, their effects in males are poorly understood. Therefore, in the present study, male A. tarichi were sampled from sites of Lake Van receiving mainly sewage treatment plant effluent and other effluents, as well as a river (Karasu) entering the lake near these sites. Collected specimens were then analyzed for changes in histological characteristics and antioxidant systems in the testes based on comparison to reference fish from an uncontaminated lake (Erçek) and river (Memedik River).
Materials and methods
Study area
Van is a metropolitan city on the eastern side of Lake Van. Settlements of various sizes discharge their wastewater into the Lake Van. Due to the extended domestic, industrial, and agricultural activities, various pollutants reach the lake from different points of the Van city. A cement factory, a wastewater sewage treatment plant, an organized industrial zone, and domestic discharges all exist between the Van and Edremit districts (Fig. 1). Pollutants such as pesticides, endocrine-disrupting chemicals, and heavy metals mainly reach the lake from this area (Bilgili et al. 1995; Aksoy et al. 2011; Ünal et al. 2007; Unal et al. 2014). Karasu River is a freshwater inlet that enters Lake Van, in which A. tarichi engages in spawning activity. Lake Erçek has similar physicochemical properties to Lake Van. Memedik River is the only freshwater inlet entering the lake in which A. tarichi spawn (Akkuş and Sarı 2013) (Fig. 1). Five sampling sites were selected for this study. The physicochemical parameters were measured by a multiparameter meter kit (Thermo Scientific, Orion 5 Star). The site description, sampling dates, geographical positions, and physicochemical parameters of surface water samples from the sampling sites are summarized in Table 1. Briefly, the first site (S1) mainly receives sewage water from the Van metropolitan city wastewater treatment plant and is assumed to be polluted. The second site is close to S1 and represents a migration route of the A. tarichi to the freshwater. The third site is the Karasu River, in which A. tarichi conduct reproductive activity. The Karasu River is located close to the first two sites. Lake Erçek (S4) and the Memedik River (S5), which are uncontaminated locations 30–35 km outside Van, were seleced as reference sites (Fig. 1).
Fish sampling and processing
Sixty-six sexually mature male A. tarichi were caught from five sites using gill nets in April and May of 2015. These sampling times (Table 1) were selected because histological abnormalities such as delays in spermatogenesis could be more easily discernable by gonadal examination. In the lake population in April, males of A. tarichi are in the late recrudescence period, while May is the spawning period of testicular development in which seminiferous tubules are full of spermatozoa (Ünal et al. 1999; Kaptaner and Kankaya 2013). After sampling, the fish were anesthetized by using 2-phenoxyethanol and sacrificed, after which the total body weight (W) and total length (L) were individually measured and recorded to calculate the Fulton conditioning factor (CF = 100 × W ÷ L 3). Testes were excised from each side, weighed, and noted for calculation of the gonadosomatic index (GSI = testes weight ÷ W × 100). Testes fragments from the middle parts were fixed in Bouin’s fixative for 48 h. The remaining parts of the tissues are stored at −20 °C. All procedures were performed in agreement with national and institutional regulations for the protection of animal welfare during the study. This work was approved by the Animal Experiments Ethics Committee of Yuzuncu Yil University for the ethical concerns.
Histology
All histological procedures were performed as described by Lavado et al. (2004). The fixed tissues were dehydrated through graduated ethanol series and embedded in paraffin wax. Then, the consecutive sections (7 μm) were taken from the tissues and placed on adhesive silane-coated slides. The sections were deparaffinized in xylene, rehydrated with graded ethanol concentrations, and then stained with hematoxylin and eosin (H&E), periodic acid Schiff and hematoxylin (PAS&H), and Mallory’s trichrome stainings. The preparations were examined under a microscope (Leica DMI 6000 B, Germany), and photographs were taken.
Biochemical analyses
Prior to analyses, the frozen tissues were thawed and homogenized for 5 min in 50 mM ice-cold KH2PO4 solution (1:10 w/v) using a glass-porcelain ultrasonic homogenizer (Jencons Scientific Co., Bedfordshire, UK). The homogenates were centrifuged at 10,000×g for 30 min at 4 °C, after which the supernatant fractions were removed and used to determine the lipid peroxidation and antioxidant defenses.
The testicular malondialdehyde (MDA) concentration was measured spectrophotometrically at 532 nm using the method described by Jain et al. (1989) based on the thiobarbituric acid reactivity. The results were expressed as nanomoles per gram of tissue.
The SOD activity was measured spectrophotometrically at 505 nm using a commercial kit (Ransod, Randox Lab., UK) according to the direction of manufacturer’s procedures. The SOD activity was expressed as units per milligram of tissue.
The CAT activity was measured spectrophotometrically according to Aebi (1974). The activity of CAT was determined by assaying the decrease in absorbance (hydrogen peroxide consumption) at 240 nm. The results were expressed as units per gram of tissue.
The GPx activity was measured spectrophotometrically using a commercial kit (Ransel, Randox Lab.) at 340 nm and 37 °C according to the direction of manufacturer’s procedures. The GPx activity was calculated from the decreases in absorbance values and expressed as units per gram of tissue.
The GSH content was measured spectrophotometrically at 412 nm using the method described by Beutler (1984). The results were expressed as miligrams GSH per gram of tissue.
The GR activity was measured spectrophotometrically according to Carlberg and Mannervik (1975) based on the decrease in absorbance of NADPH at 340 nm. The results were expressed as units per milligram of tissue.
GST activity was measured spectrophotometrically according to Habig et al. (1974). The results were expressed as units per milligram of tissue.
Statistical analysis
All data were analyzed using one-way analysis of variance with SPSS 16.0 for Windows statistical packet program. A post hoc Scheffe’s multiple range test was used for the determination of differences between groups. The results were presented as the mean ± standard deviation (SD). Significance was accepted at P ≤ 0.05 level.
Results
Histology and biometric indices
Reference fish from S4 and S5 exhibited normal testis texture. Some individuals from S1, S2, and S3 had no histological alterations and showed normal tissue organization in their testes. Histological changes determined in the testes of fish from Lake Van (S1 and S2) and the Karasu River (S3) are summarized in Tables 2 and 3 and illustrated in Figs. 2 and 3, respectively. Histological examinations showed that A. tarichi sampled from lake environment (S1, S2, and S4) was in the late phase of the recrudescence period. The seminiferous tubules in individuals with normal testis structure in the reference fish from Lake Erçek and Lake Van contained spermatogonia, spermatocyte cysts, spermatid cysts, and residual spermatozoa (Fig. 2a, b). In the river environment (S5, S3), the fish exhibited mature testes that were full of spermatozoa, as well as a few spermatonia, spermatocytes, and spermatid cysts in the semiferous tubule epithelium (Fig. 3a, b). Testicular alterations were determined in the fish sampled from Lake Van (S1, S2) and Karasu River (S3) when compared to the reference sites (S4, S5). Seven of 11 fish collected from S1 exhibited histological alterations, including PAS-positive macrophage aggregates that were usually observed usually near the blood vessels and stained in purple (Fig. 2c), seminiferous tubule dilation (Fig. 2d), increased pycnotic and degenerated germ cells in the germinal epithelium (Fig. 2e), and infiltration of adipocytes (Fig. 2f). Histological alterations were more evident in fish sampled from the Karasu River (S3), with 10 of 22 fish found to have these changes. The PAS-positive macrophage aggregates (Fig. 3c) and large vacuoles close to the tubule wall (vacuolation) (Fig. 3d, e) were the most prevalent histological alterations. Other alterations observed in the testes included disorganization of seminiferous tubules (Fig. 3f), degeneration of germ cells (Fig. 3g), delayed spermatogenesis (Fig. 3h), reduced spermatozoa (Fig. 3i), thickening in the wall of blood vessels (Fig. 3j), thickening of the tubule walls (Fig. 3k), and fibrosis (Fig. 3l). Other fish (n = 12) collected in the same place (S3) were observed to have normal testis architecture as observed in the reference fish (S5). Accordingly, fish possessing testes with histological alterations were assessed seperately in terms of biometric indices and biochemical analyses.
Histological sections of testes of A. tarichi sampled from the reference lake (Erçek, S4) (a) and from a polluted area of Lake Van (S1) (b–f) during the prespawning period (April). a Testes tissue of reference fish showing normal structure and all germ cell stages (Sg spermatogonia, Sc spermatocyte, Sd spermatid, Sz spermatozoa) (H&E). b Normal testes showing no histological alteration from S1 (H&E). c Testes showing PAS-positive macrophage aggregate (ma) near a blood vessel (bv) (PAS&H). d Testes showing seminiferous tubule dilation (asterisks) (H&E). e Testes showing pyknosis (arrowheads) and degeneration (arrows) in germ cells (H&E). f Testes showing infiltrated adipocytes (H&E)
Histological sections of testes of A. tarichi sampled from a reference freshwater inlet, (Memedik, S5) (a), and from Karasu River (b-l) close to the polluted area of Lake Van in which the fish migrated (S3) during spawning period (May). a Testes tissue of reference fish showing normal structure with the lumens of the seminiferous tubules having full of spermatozoa (Sz) (H&E). b Normal testes showing no histological alterations from S3 as in the reference site (H&E). c Testes showing PAS-positive macrophage aggregate (ma) and vacuolation (v) (PAS&H). d Testes showing large vacuoles (v) in the germinal epithelium (H&E). e Testes showing high degree of vacuolation (arrows) (H&E). f Testes showing seminiferous tubule disorganization (circle) (Mallory’s trichrome). g Testes showing degenerated germ cells (arrowheads) (H&E). h Testes with delayed spermatogenesis (PAS&H). i Testes having reduced spermatozoa (Rs) (Mallory’s trichrome). j Thickening of the wall of blood vessels in testes (two-headed arrow) (H&E). k Testes showing thickening of the tubule wall (two-headed arrow) (H&E). l Testes showing fibrosis (f) in the interstitial area (Mallory’s trichrome).
Biometric indices of male fish sampled from the lake (S1, S2, and S4) and river are given in Tables 4 and 5, respectively. CF values were similar in fish groups sampled from the lake (S1, S2, and S4). Lower GSI values were found in fish at S1 than S2 and the reference fish S4 (P < 0.05). In the river, male fish with histological alterations in the testicular tissue from S3 had low CF and GSI values than fish with normal testis histology collected from the same enviroment and reference fish from S5 (P < 0.05).
Biochemical assays
The MDA levels and enzymatic and nonenzymatic antioxidants in the testes of the fish sampled from the lake and river environment are presented in Tables 6 and 7, respectively. MDA levels were found to be unchanged in testes of fish from Lake Van (S1, S2). In samples from the river (S3, S5), MDA levels were determined to be significantly higher in the testes of male fish with histological alterations than in reference fish from S5 and fish displaying normal testis histology from the same environment (S3).
Testicular GSH content and SOD, CAT, GPx, and GST activities at S1 were all significantly higher than those in fish from S2 and reference fish from S4 (P < 0.05). Higher SOD and CAT activitives were also observed in fish from S2 than in the reference fish from S4 (P < 0.05).
The GSH content remained unchanged in the testis of fish from the river (S3) comparing to the fish from the reference river (S5). The CAT, GPx, and GST activities were significantly higher in fish from the river (S3) possessing histological alterations than in fish with normal testis histology present in the same environment and reference fish from S5 (P < 0.05). Conversely, the activity of GR was significantly lower in the testes of fish showing histological alterations than in fish with normal histology and the reference fish. The CAT, GPx, and GST activities were also higher in fish with normal histology from S3 than in reference fish from S5 (P < 0.05).
Discussion
Environmental monitoring studies have documented heavy metal, organochlorine compound, pesticide, and phthalate contamination in Lake Van (Bilgili et al. 1995; Aksoy et al. 2011; Unal et al. 2014). Additionally, many studies have displayed that xenobiotics can disrupt structural organization and function of the gonads of fish inhabiting in contaminated regions (Louiz et al. 2009; Allner et al. 2010; Blazer et al. 2014). The present study investigated reproductive disturbances in the testes of A. tarichi from contaminated sites of Lake Van (S1, S2), which receives sewage effluent from a wastewater treatment plant and a connected river (S3; the Karasu River) to which fish migrate for spawning. Biometric indices, histological alterations, and oxidative stress biomarkers were investigated based on comparison with fish from an uncontaminated lake (S4; Erçek) and a freshwater inlet (S5; Memedik River).
Histological examination of the testes of fish from the reference lake (S4) and river (S5) demonstrated normal testis structure. Fish from S4 showed spermatogonia, spermatocyte, and spermatid cysts and residual spermatozoa in the tubules (Fig. 2a). Fish from S5 appeared to have mature testes full of spermatozoa in the tubules (Fig. 3a). Some of the fish collected from Lake Van (S1 and S2) and the Karasu River (S3) had normal testes as observed in reference fish, but others showed various pathological changes, indicating testicular regression. The histological alterations observed in those sites were PAS-positive macrophage aggregates, seminiferous tubule dilation, increased pycnosis and degeneration in germ cells, infiltration of adipocytes, vacuolation, disorganization of seminiferous tubules, thickening of blood vessel walls, delayed development of germ cells, reduced spermatozoa, thickening of interstitial tissue, and fibrosis. The histopathological alterations in the testes of 10 of 22 fish from the Karasu River (S3) were more remarkable and easily discernable. This is because all fish normally in the river population usually exhibit mature testes with seminiferous tubules full of spermatozoa (Ünal et al. 1999; Kaptaner and Kankaya 2013). Observation of histopatologic individuals in the river suggests that those fish were exposed to contaminants in the lake and migrated to freshwater with healthy ones. These fish might be more sensitive to contaminants (Unal et al. 2014). The other fish with normal testes histology in the same area may have migrated to the river from cleaner sites in Lake Van or been less affected by contaminants.
One of the most prevalent alterations observed in the testes of A. tarichi was PAS-positive macrophage aggregates. These pigment-containing cells were generally observed near blood vessels. The size and frequency of these cells have been reported to increase in fish as a nonspecific response of the immune system, in response to environmental stress and poor water quality caused by contaminants (Agius and Roberts 2003; Marty et al. 2003). This alteration has also been reported in the liver and ovaries of A. tarichi collected from the same region (Van-Edremit coast) (Kaptaner et al. 2014; Kaptaner 2015). Similar to our findings, Lavado et al. (2004) reported macrophage aggregates in the testis of carp (Cyprinus carpio) from portions of the Ebro River (Spain) receiving sewage treatment plant effluent. Louiz et al. (2009) identified these cell aggregates in the testes of black goby (Gobius niger) inhabiting the Bizerta Lagoon (Tunisia), which is contaminated with organic pollutants. The presence of these structures has also been observed in the testes of the Sacremento splittail (Pogonichthys macrolepidotus) in a river containated by organochlorine contaminants and metals (Greenfield et al. 2008). Vacuolization in the germinal epithelium of seminiferous tubules is another histological alteration commonly found in the testes of A. tarichi from river samples. Both field (Bjerregaard et al. 2006; Louiz et al. 2009; Agbohessi et al. 2015) and laboratory (Sangalang et al. 1981; Santos et al. 2015) studies have revealed testiscular vacuolization. Thus, vacuolization is not a specific response to a sole chemical compound and can be caused by xenobiotics that may lead to cytotoxic effects on gametes and change the hormonal environment during gamete development (Kime 1999; Bjerregaard et al. 2006). Interstitial fibrosis was also observed around some tubules. This was also reported in some fish sampled from polluted waters (Lavado et al. 2004; Agbohessi et al. 2015). The large vacuoles and fibrosis in the testes of A. tarichi may be signs of degenerative and necrotic processes (Da Cuña et al. 2013). Vacuolated necrotic areas containing fatty material in the testes lobules of cod (Gadus morhua) because of polychlorinated biphenyl (Aroclor 1254) exposure have been observed to lead to fatty degeneration and fibrosis (Sangalang et al. 1981). Conversely, chemical messengers from macrophages are responsible for production of extracellular matrix elements such as collagen, glycoproteins, and proteoglycans that result in fibrosis (Highleyman and Franciscus 2011; Lech and Anders 2013; Da Cuña et al. 2013). Seminiferous tubule dilation is characterized by increased luminal diameter of tubules and appears to be related to chemical toxicity (Creasy 2001). Other hispathological changes include pyknosis and degenerated germ cells in the testes of A. tarichi. Necrotic germ cells in fish testes are characterized based on nuclear changes including pyknosis characterized by shrinking and darkening of the cell. Arrest of germ cell maturation during the process of spermatogenesis may cause such an effect, eventually leading to degeneration. A similar observation has also been reported in the testes of the fish collected from Lake Michigan (Blazer 2002). Germ cell pyknosis has been observed in the testes of Mosambique tilapia (Orechromis niloticus) exposed to DDT (Mlambo et al. 2009) and yellow tail lambari (Astyanax aff. bimaculatus) exposed to zinc (Santos et al. 2015). The disorganization of seminiferous tubules and thickening of tubule walls have also been found in fish species exposed to pesticides (Sangalang et al. 1981; Mlambo et al. 2009; Agbohessi et al. 2015) and inhabiting in the waters contaminated by endocrine disrupters (Simpson et al. 2000). Reduced spermatozoa in the lumina of tubules has also been determined in the testes of freshwater fish (Rasbora dandia) chronically treated with mercury that might have arisen from arrested spermatogenesis and resulted in impaired reproductive success (Rajan and Kuzhivelil 2013). The findings of this work also indicate that alterations determined in the testes of A. tarichi are general tissue injuries as a result of contaminant exposure.
CF reflects the well-being and physiological status in fish. In the present study, fish from the river (S3) displaying histological alterations also had a significantly lower mean CF than reference fish, while fish from the same site with normal testes histology were not different from the reference fish. The lower CF with the presecence of histological changes in the testes of the fish suggests a connection and requires further investigation. Nwabueze and Ekelemu (2011) also reported a lower CF in Clarias gariepinus exposed to different concentrations of domestic leachate. Additionally, long-term exposure to industrial effluents has been reported to affect physiological parameters and cause poor CF in C. gariepinus (Adeogun et al. 2012). Similar to our findings, fish found in regions contaminated with wastewater treatment plant effluent (Lavado et al. 2004; Blazer et al. 2014) and pesticides (Agbohessi et al. 2015) were observed to have decreased CF values.
GSI analyses showed significant decreases in fish from S1 and S3. A low GSI has also been related with gonadal histopathologies (Mills et al. 2001; Holm et al. 2006; Louiz et al. 2009; Mlambo et al. 2009). Additionally, reduced GSI values have been observed in fish living in aquatic environments contaminated by sewage effluents (Hassanin et al. 2002; Lavado et al. 2004; Carballo et al. 2005), pesticides (Agbohessi et al. 2015), and organic pollutants (Louiz et al. 2009; Gilroy et al. 2012). Thus, decreases in GSI could be attributed to regressive histological changes such as degeneration and arrrested spermatogenesis.
Toxic compounds in the aquatic environments have been reported to disrupt the prooxidant/antioxidant balance of fish cells. The generation of ROS was enhanced by heavy metals, organic contaminants, polycyclic aromatic hydrocarbons, and pesticides. Because of cytotoxic agents, ROS attack critical macromolecules in cells such as lipids, carbohydrates, nucleic acids, and proteins, which may lead to tissue damage (Livingstone et al. 2003). Lipid peroxidation (LPO) is an indicator of cellular damage that contributes to oxidative stress (Frenzilli et al. 2001). Environmental contaminants have been reported to lead LPO in fish tissues (Van der Oost et al. 2003). Therefore, measurement of MDA, a product of LPO, is a widely used biomarker of oxidative injury (Tabrez and Ahmad 2009). Our results show a significant increase in the MDA level of the testes of fish with histological alterations from the Karasu River (S3). Contistently, increased MDA levels together with histopathological damage were reported in the tissues of fish caught from polluted sites (Gül et al. 2004; Belge-Kurutaş et al. 2009; Ameur et al. 2012; Ruiz-Picos and López-López 2012; Abdel-Moneim et al. 2013). A recent study by Kaptaner (2015) showed a correlation between increased MDA level and ovarian abnormalities in A. tarichi from a contaminated region of Lake Van. Our results are also concordant with those of a previous work reporting high LPO levels in the gonads of the fish, caught from Lagos lagoon polluted with sawmill industry discharge (Ekaete 2014). The SOD-CAT system functions the primary line of defense mechanism against oxidative stress. The endogenous scavenger, SOD, catalyzes dismutation of the highly reactive superoxide anions to hydrogen peroxide, whereas role of CAT is to degradate of hydrogen peroxide, a precursor of hydroxyl radical, to water (Livingstone 2003). The simultaneous increases in the activities of these two enzymes are usually observed in response to environmental contaminants (Dimitrova et al. 1994; Belge Kurutaş et al. 2009). In the current study, similar relationship was also found in fish from Lake Van (S1, S2). The increased activity of SOD in the liver, kidney, gills, and gonads of fish living in the polluted areas has been also detected by different researchers (Pandey et al. 2003; Avci et al. 2005; Bagnyukova et al. 2006; Farombi et al. 2007; Belge Kurutaş et al. 2009; Abdel-Moneim et al. 2013; Ekaete 2014; Kaptaner 2015), suggesting a high formation of superoxide anions. CAT removes hydrogen peroxide occured as a result of SOD activity and its activity was frequently increased by ROS production (Hermes-Lima 2004; El-Shenawy et al. 2012). The elevated activities of CAT in testes of fish from both lakes and rivers in this study may be attributable to the accumulation of peroxide radicals and be an adaptive and protective role of this enzyme against ROS (Ahmad et al. 2006; Belge Kurutaş et al. 2009; Zagal and Mazmanci 2011). GPx has major role in the reduction of lipid hydroperoxides. Specifically, this enzyme protects cells against damage induced by oxyradical formation (Orbea et al. 2000). The treatment of fish to certain chemical compounds has been reported to induce GPx activity in response to hydrogen peroxide production (Almeida et al. 2002; Sayeed et al. 2003; Sanchez et al. 2005). In this work, activity of the enzyme was increased in the testes of fish in which histological changes where observed (S1, S3). A high level of GPx activity was also reported in the ovaries of A. tarichi having histopathological changes (Kaptaner 2015). Our findings are also in agreement with the results of previous studies reporting elevated levels of GPx activity in the tissues of fish living in waters polluted by different types of contaminants (Pandey et al. 2003; Ruiz-Picos and López-López 2012; Abdel-Moneim et al. 2013). GSH is an effective protectant cabaple of quenching free radicals that is known to be a cofactor of GPx and GST (Stephensen et al. 2002). Similarly, Di Giulio et al. (1993), Pandey et al. (2003), Farombi et al. (2007), Doherty et al. (2010), and Mahboob et al. 2014 observed high levels of GSH in fish exposed to polluted waters. Therefore, the higher testicular GSH content may result from adaptive and protective function of this molecule against oxidative stress induced by environmental toxic compounds (Dickinson and Forman 2002). In this work, fish with histopathological testes showed unchanged GSH levels. GSH is regenerated from glutathione disulfite (GSSG) by the enzyme GR. The decrease in GR activity in these individuals may cause unchanged levels of GSH that the reducing equivalent of GR (NADPH) is provided by the glucose 6-phoshate dehydrogenase. Some researchers have reported that the decreased GR activity in fish living in polluted waters might result from utilization of the enzyme to produce GSH or from a lack of NADPH (Pandey et al. 2003; Abdel-Moneim et al. 2013). GST is a phase II enzyme that detoxifies toxic substances and provides crucial defending against products of oxidative stress. This enzyme catalyzes the conjugation of GSH with a wide variety of xenobiotics (Hayes and Pulford 1995). The increased activity of GST in the testis may be associated to formation of free radicals that arised from the metabolism of environmental contaminants (Otto and Moon 1996; Peebua et al. 2007; Lu et al. 2009; 2011); thus, the existence of an altered antioxidant system may reflect the reasons for histological changes in the testes.
In conclusion, the present study provides new data regarding testicular alterations in A. tarichi sampled from a site of Lake Van that receives sewage treatment plant effluent and other types of anthropogenic pollutants, as well as from a river (Karasu) near this site to which fish migrate and spawn. Comparison with reference sites (Lake Erçek and a freshwater inlet of Memedik River) revealed that alterations in antioxidant system parameters, physiological responses (CF and GSI), and histology are linked. Our results indicate that multiple contaminants in the polluted area are possibly responsible for the detected impacts in the testes. Thus, pollution in Lake Van may pose a serious risk to the fitness and reproductive capacity of A. tarichi.
References
Abdel-Moneim, A. M., Essawy, A. E., El-Din, N. K. B., & El-Naggar, N. M. (2013). Biochemical and histopathological changes in liver of the Nile tilapia from Egyptian polluted lakes. Toxicology and Industrial Health. doi:10.1177/0748233713503374.
Adeogun, A. O., Ogidan, I. M., Ibor, O. R., Chukwuka, A. V., Adedara, I. A., & Farombi, E. O. (2012). Long-term exposure to industrial effluent induces oxidative stress and affects growth in Clarias gariepinus. Research Journal of Environmental and Earth Sciences, 4, 738–746.
Aebi, H. (1974). Catalase. In: Methods of enzymatic analysis. In H. U. Bergemeyer (Ed.), (pp. 673–684). Academic Press: NewYork.
Agbohessi, P. T., Toko, I. I., Ouedraogo, A., Jauniaux, T., Mantiki, S. N. M., & Kastemont, P. (2015). Assessment of the health status of wild fish inhabiting a cotton basin heavily impacted by pesticides in Benin (West Africa). Science of the Total Environment, 506–507, 567–584.
Agius, C., & Roberts, R. J. (2003). Melano-macrophage centres and their role in fish pathology. Journal of Fish Diseases, 26, 499–509.
Ahmad, İ., Pacheco, M., & Santos, M. A. (2006). Anguilla anguilla L. oxidative stress biomarkers: an in situ study of freshwater wetland ecosystem (Pateira de Fermentelos, Portugal). Chemosphere, 65, 952–962.
Akkuş, M., & Sarı, M. (2013). A research on estimating of carrying capacity of lake Erçek with the remote sensing method. Proc. ESA Living Planet Syposium 9–13 September 2013 (ESA-SP722, December 2013).
Aksoy, A., Das, Y. K., Yavuz, O., Guvenc, D., Atmaca, E., & Agaoglu, S. (2011). Organochlorine pesticide and polychlorinated biphenyls levels in fish and mussel in Van Region, Turkey. Bulletin of Environmental Contamination and Toxicology, 87, 65–69.
Allner, B., von der Gönna, S., Griebeler, E.-M., Nikutowski, N., Weltin, A., & Stahlschmidt-Allner, P. (2010). Reproductive functions of wild fish as bioindicators of reproductive toxicants in the aquatic evironment. Environmantal Science and Pollution Research International, 17, 505–518.
Almeida, J. A., Diniz, Y. S., Marques, S. F. G., Faine, L. A., Ribas, B. O., Burneik, R. C., & Novelli, E. L. B. (2002). The use of oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environment International, 27, 673–679.
Ameur, B. W., de Lapuente, J., El Megdiche, Y., Barhoumi, B., Trabelsi, S., Camps, L., Serret, J., Ramos-López, D., Gonzalez-Linares, J., Driss, M. R., & Borràs, M. (2012). Oxidative stress, genotoxicity and histopathology biomarker responses in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) liver from Bizerte Lagoon (Tunisia). Marine Pollution Bulletin, 64, 241–251.
Au, D. W. T. (2004). The application of histo-cytopathological biomarkers in marine pollution monitoring: a review. Marine Pollution Bulletin, 48, 817–834.
Avci, A., Kaçmaz, M., & Durak, İ. (2005). Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicology and Environmental Safety, 60, 101–105.
Ayas, Z., Ekmekci, G., Ozmen, M., & Yerli, S. V. (2007). Histopathological changes in the livers and kidneys of fish in Sariyer Reservoir, Turkey. Environmental Toxicology and Pharmacology, 23, 242–249.
Bagnyukova, T. V., Chahrak, O. I., & Lushchak, V. I. (2006). Coordinated response of gold fish antioxidant defenses to environmental stress. Aquatic Toxicology, 78, 325–331.
Belge Kurutaş, E., Şahan, A., & Altun, T. (2009). Oxidative stress biomarkers in liver and gill tissues of spotted barb (Capoeta barroisi Lortet, 1894) living in the river Ceyhan, Adana, Turkey. Turkish Journal of Biology, 33, 275–282.
Beutler, E. (1984). Red cell metabolism. A manual of biochemical methods (3rd ed., pp. 105–106). New York: Grune and Startton.
Bilgili, A., Sağmanlıgil, H., Çetinkaya, N., Yarsan, E., & Türel, I. (1995). The natural quality of Van Lake and the levels of some heavy metals in grey mullet (Chalcalburus tarichi, Pallas 1811) samples taken from this lake. Veterinary Journal of Ankara University, 42, 445–450.
Bjerregaard, L. B., Madsen, A. H., Korsgaard, B., & Bjerregaard, P. (2006). Gonad histology and vitellogenin concentrations in brown trout (Salmo trutta) from Danish streams impacted by sewage effluent. Ecotoxicology, 15, 315–327.
Blazer, V. S. (2002). Histopathological assessment of gonadal fish tissue in wild fishes. Fish Physiology and Biochemistry, 26, 85–101.
Blazer, V. S., Iwanowicz, D. D., Walsh, H. L., Sperry, A. J., Iwanowicz, L. R., Alvarez, D. A., Brightbill, R. A., Smith, G., Foreman, W. T., & Manning, R. (2014). Reproductive health indicators of fishes from Pennsylvania watersheds: association with chemicals of emerging concern. Environmental Monitoring and Assessment, 86, 6471–6491.
Carballo, M., Aguayo, S., De la Torre, A., & Munoz, M. J. (2005). Plasma vitellogenin levels and gonadal morhology of wild carp (Cyprinus carpio L.) in a receiving rivers downstream of sewage treatment plants. Science of the Total Environment, 341, 71–79.
Carlberg, I., & Mannervik, B. (1975). Purification and characterization of the flavoenzyme glutathione reductase from rat liver. Journal of Biological Chemistry, 250, 5475–5480.
Creasy, D. M. (2001). Pathogenesis of male reproductive toxicity. Toxicologic Pathology, 29, 64–76.
Da Cuña, R. H., Pandolfi, M., Genovese, G., Piazza, Y., Ansaldo, M., & Lo Nostro, F. L. (2013). Endocrine disruptive potential of endosulfan on the reproductive axis of Cichlasoma dimerus (Perciformes, Cichlidae). Aquatic Toxicology, 126, 299–305.
Dane, H., & Şişman, T. (2015). Histopathological changes in gill and liver of Capoeta capoeta living in the Karasu River, Erzurum. Environmental Toxicology, 30, 904–917.
Danulat, E., & Selcuk, B. (1992). Life history and environmental conditions of the anadromous Chalcalburnus tarichi (Cyprinidae) in the highly alkaline lake Van, Eastern Anatolia, Turkey. Archiv für Hydrobiologie, 126, 105–125.
Di Giulio, R. T., Habig, C., & Gallagher, E. P. (1993). Effects of Blach Rock Harbor sediments on indices of biotransformation, oxidative stress, and DNA integrity in channel catfish. Aquatic Toxicology, 26, 1–22.
Dickinson, D. A., & Forman, H. J. (2002). Cellular glutathione and thiols metabolism. Biochemical Pharmacology, 64, 1019–1026.
Dimitrova, M. S. T., Tsinova, V., & Velcheva, V. (1994). Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio). Comparative Biochemistry and Physiology. C, 108, 43–46.
Doherty, V. F., Ogunkuade, O. O., & Kanife, U. C. (2010). Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in some selected fishes in Lagos, Nigeria. American-Eurasian Journal of Agricultural and Environmental Sciences, 7, 359–365.
Ekaete, A. G. (2014). Oxidative stress in fish living in coastal water polluted with sawdust and wood waste along Lagos lagoon, Nigeria. Researcher, 6, 1–5.
El-Shenawy, N. S., Mohammedden, A., & Al-Fahmie, Z. H. (2012). Using the enzymatic and non-enzymatic antioxidant system of the land snail Eobania vermiculata as biomarkers of terrestrial heavy metal pollution. Ecotoxicology and Environmental Safety, 84, 347–354.
Farombi, E. O., Adelowo, O. A., & Ajimoko, Y. R. (2007). Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun river. International Journal of Environmental Research and Public Health, 4, 158–165.
Frenzilli, G., Nigro, M., Scarcelli, V., Gorbi, S., & Regoli, F. (2001). DNA integrity and total oxyradical scavenging capacity in the Mediterranean mussel, Mytilus galloprovincialis: a field study in a highly eutrophicated coastal lagoon. Aquatic Toxicology, 53, 19–32.
Fricke, N. F., Stentiford, G. D., Feist, S. W., & Lang, T. (2012). Liver histopathology in Baltic eelpout (Zoarces viviparus)—a baseline study for use in marine environmental monitoring. Marine Environmental Research, 82, 1–14.
Gilroy, E. A. M., McMaster, M. E., Parrot, J. L., Hewitt, L. M., Park, B. J., Brown, S. B., & Sherry, J. P. (2012). Assessment of the health status of wild fih from the Wheathley Harbour area of concern, Ontario, Canada. Environmental Toxicology and Chemistry, 31, 2798–2811.
Greenfield, B. K., Teh, S. J., Ross, J. R. M., Hunt, J., Zhang, G., Davis, J. A., Ichikawa, G., Crane, D., Hung, S. S. O., Deng, D., Teh, F.-C., & Green, P. G. (2008). Contaminant concentrations and histopathological effects in Sacramento splittail (Pogonichthys macrolepidotus). Archives of Environmental Contamination and Toxicology, 55, 270–281.
Gül, S., Belge-Kurutaş, E., Yıldız, E., Şahan, A., & Doran, F. (2004). Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environment International, 30, 605–609.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry, 249, 7130–7139.
Hassanin, M., Kuwahara, S., Nurdihayat, Tsukamoto, Y., Ogawa, K., Hiramatsu, K., & Sasaki, F. (2002). Gonadosomatic index and testis morphology of common carp (Cyprinus carpio) in rivers contaminated with estrogenic chemicals. The Journal of Veterinary Medical Science, 64, 921–926.
Hayes, J. D., & Pulford, D. J. (1995). The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical Reviews in Biochemistry and Molecular Biology, 30, 445–600.
Hermes-Lima, M. (2004). Oxygen in biology and biochemistry: role of free radicals. In: Storey, K.B. (Ed). Functional metabolism: regulation adaptation, pp. 319–368.
Highleyman, L., Franciscus, A. (2011). Disease progression: what is fibrosis? Hcsp-FACTsheet, http://www.hcvadvocate.org/, accessed November 2015.
Holm, G., Norrgren, L., & Lindén, O. (2006). Reproductive and histopathological effects of long-term experimental exposure to bis(tributyltin)oxide (TBTO) on the threespined stickleback, Gasterosteus aculeatus Linnaeus. Journal of Fish Biology, 38, 373–386.
Jain, S. K., McVie, R., Duett, J., & Herbst, J. J. (1989). Erythrocyte membrane lipid peroxidation and glycolylated hemoglobin in diabetes. Diabetes, 38, 1539–1543.
Kaptaner, B. (2015). Relation between increased oxidative stress and histological abnormalities in the ovaries of Alburnus tarichi in Lake Van, Turkey. Environmental Monitoring and Assessment, 187, 702.
Kaptaner, B., & Kankaya, E. (2013). Analysis of germ cell proliferation, apoptosis, and androgenesis in the Lake Van fish (Chalcalburnus tarichi) during testicular development. Fish Physiology and Biochemistry, 39, 1165–1679.
Kaptaner, B., Kankaya, E., Doğan, A., & Çelik, İ. (2014). Histopathology and oxidative stress in the liver of Chalcalburnus tarichi living in lake Van, Turkey. Life Science Journal, 11, 66–77.
Kime, D. E. (1999). A strategy for assessing the effects of xenobiotics on fish reproduction. Science of the Total Environment, 225, 3–11.
Lavado, R., Thibaut, R., Raldua, D., Martin, R., & Porte, C. (2004). First evidence of endocrine disruption in feral carp from the Ebro River. Toxicology and Applied Pharmacology, 196, 247–257.
Lech, M., & Anders, H. J. (2013). Macrophages and fibrosis: How resident and infiltraing mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochimica et Biophysica Acta, 1832, 989–997.
Livingstone, D. R. (2003). Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue de Médecine Vétérinaire, 154, 427–430.
Louiz, I., Ben-Attia, M., & Ben-Hassine, O. K. (2009). Gonadosomatic index and gonad histopathology of Gobius niger (Gobiidea, Teleost) from Bizerta lagoon (Tunisia): evidence of reproduction disturbance. Fisheries Research, 100, 266–273.
Lu, G., Yan, Z., & Wang, Y. (2011). Assesstment of estrogenic contamination and biological effects in Lake Taihu. Ecotoxicology, 20, 974–981.
Lu, G. H., Wang, C., & Zhu, Z. (2009). The dose–response relationships for EROD and GST induced by polyaromatic hydrocarbons in Carassius auratus. Bulletin of Environmental Contamination and Toxicology, 82, 194–199.
Lukin, A., Sharova, J., Belicheva, L., & Camus, L. (2011). Assessment of fish health status in the Pechora River: effects of contamination. Ecotoxicology and Environmental Safety, 74, 355–365.
Mahboob, S., Alkkahem Al-Balwai, H. F., Al-Ghanim, K. A., Al-Misned, F., Ahmed, Z., & Suliman, E.-A. M. (2014). Biomarkers of oxidative stress as indicators of water pollution in Nile tilapia (Oreochromis niloticus) from a water reservoir in Riyadh, Saudi Arabia. Toxicological and Environmental Chemistry, 96, 624–632.
Marty, G. D., Quinn, T. J., II, Carpenter, G., Meyers, T. R., & Willits, N. H. (2003). Role of disease in abundance of a Pacific herring (Clupea pallasi) population. Canadian Journal of Fisheries and Aquatic Sciences, 60, 1258–1265.
Mills, L. J., Gutjahr-Gobell, R., Haebler, R., Horowitz, D., Jayaraman, S., Pruell, R. J., McKinney, R. A., Gardner, G. R., & Zaroogian, G. E. (2001). Effects of estrogenic (o,p′-DDT; octylphenol) and anti-androgenic (p,p′-DDE) chemicals on indicators of endocrine status in juvenile male summer flounder (Paralichthys dentatus). Aquatic Toxicology, 52, 157–176.
Mlambo, S. S., van Vuren, J. H. J., Barnhoorn, I. E. J., & Bornman, M. S. (2009). Histopathological changes in the reproductive system (ovaries and testes) of Oreochromis mossambicus following exposure to DDT. Environmental Toxicology and Pharmacology, 28, 133–139.
Nwabueze, A. A., & Ekelemu, J. K. (2011). Growth and survival of Clarias gariepinus (Burchell, 1822) fingerlings in different concentrations of domestic leachate. ARPN Journal of Agricultural and Biological Science, 6, 25–29.
Oliva, M., Vicente-Martorell, J. J., Galindo-Riano, M. D., & Perales, J. A. (2013). Histopathological alterations in Senegal sole, Solea Senegalensis, from a polluted Huelva estuary (SW, Spain). Fish Physiology and Biochemistry, 39, 523–545.
Orbea, A., Fahimi, H. D., & Cajaraville, M. P. (2000). Immunolocalization of four antioxidant enzymes in digestive glands of mollusks and crustaceans and fish liver. Histochemistry and Cell Biology, 114, 393–404.
Otto, D. M. E., & Moon, T. W. (1996). Phase I and II enzymes and antioxidant responses in different tissues of brown bullheads from relatively polluted and nonpolluted systems. Archives of Environmental Contamination and Toxicology, 31, 141–147.
Pandey, S., Parvez, S., Sayeed, I., Haque, R., Hafeez, B. B., & Raisuddin, S. (2003). Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Science of the Total Environment, 309, 105–115.
Peebua, P., Kosiyachinda, P., Pokethitiyook, P., & Kruatrachue, M. (2007). Evaluation of alachlor herbicide impacts on Nile tilapia (Oreochromis niloticus) using biochemical biomarkers. Bulletin of Environmental Contamination and Toxicology, 78, 128–131.
Penaz, M., Svobodova, Z., Barus, V., Prokes, M., & Drastichova, J. (2005). Endocrine disruption in a barbel, Barbus barbus population from the river Jihlava, Czech Republic. Journal of Applied Ichthyology, 21, 420–428.
Rajan, P., & Kuzhivelil, B. T. (2013). Mercury induced histopathological changes in the testis of the freshwater fish, Rasbora dandia. International Journal of Science and Research, 4, 252–254.
Ruiz-Picos, R., & López-López, E. (2012). Gill and liver histopathology in Goodea atripinnis Jordan, related to oxidative stress in Yuriria lake, Mexico. International Journal of Morphology, 30, 1139–1149.
Sanchez, W., Palluel, O., Meunier, L., Coquery, M., Porcher, J.-M., & Ait-Aissa, S. (2005). Copper induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environmental Toxicology and Pharmacology, 19, 177–183.
Sangalang, G. B., Freeman, H. C., & Crowell, R. (1981). Testicular abnormalities in cod (Gadus morhua) fed Aroclor 1254. Archives of Environmental Contamination and Toxicology, 10, 617–626.
Santos, D. C. M., Cupertino, M. C., Matta, S. L. P., Oliveira, J. A., & Santos, J. A. D. (2015). Histological alterations in liver and testis of Astyanax aff. bimaculatus caused by acute exposition to zinc. Revista Ceres, 62, 133–141.
Sayeed, I., Parvez, S., Pandey, S., Bin-Hafeez, B., Haque, R., & Raisuddin, S. (2003). Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicology and Enviromental Safety, 56, 295–301.
Scott, G. R. & Sloman K. A. (2004). The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquatic Toxicology, 68, 369–392.
Simpson, M. G., Parry, M., Kleinkauf, A., Swarbreck, D., Walker, P., & Leah, R. T. (2000). Pathology of the liver, kidney and gonad of flounder (Platichthys flesus) from a UK estuary impacted by endocrine disrupting chemicals. Marine Environmental Research, 50, 283–287.
Stephensen, E., Sturve, J., & Forlin, L. (2002). Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comparative Biochemistry and Physiology, 133, 435–442.
Tabrez, A., & Ahmad, M. (2009). Effect of wastewater intake on antioxidant and marker enzymes of tissue damage in rat tissues: implications for the use of biochemical markers. Food and Chemical Toxicology, 47, 2465–2478.
Triebskorn, R., Telcean, I., Casper, H., Farkas, A., Sandu, C., Stan, G., Colărescu, O., Dori, T., & Köhler, H.-R. (2008). Monitoring pollution in River Mureş, Romania, part II: metal accumulation and histopathology in fish (2008). Environmental Monitoring and Assessment, 141, 177–188.
Turkish Statistical Institute (TÜİK). (2013). Fishery statistics. Ankara: publication number: 4349, ISSN 1013–6177, pp 66.
Unal, G., Marquez, E. C., Feld, M., Stavropoulos, P., & Callard, I. P. (2014). Isolation of estrogen receptor subtypes and vitellogenin genes: expression in female Chalcalburnus tarichi. Comparative Biochemistry and Physiology. C, 172–173, 67–73.
Ünal, G., Çetinkaya, O., & Elp, M. (1999). Histological investigation of gonad development of Chalcalburnus tarichi P., 1811). Turkish Journal of Zoology, 23, 329–338.
Ünal, G., Türkoğlu, V., Oğuz, A. R., & Kaptaner, B. (2007). Gonadal histology and some biochemical characteristics of Chalcalburnus tarichi (Pallas, 1811) having abnormal gonads. Fish Physiology and Biochemistry, 33, 153–165.
Valavanidis, A., Vlahogianni, T., Dassenakis, M., & Scoullos, M. (2006). Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Enviromental Safety, 64, 178–189.
Van der Oost, R., Beyer, J., & Vermeulen, N. P. E. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology, 13, 57–149.
Zagal, A., & Mazmanci, B. (2011). Oxidative stress response in Nile tilapia (Oreochromis niloticus) exposed to textile mill effluent. Toxicology and Industrial Health, 27, 81–85.
Acknowledgments
This study was funded by the Directorate of Scientific Research Projects of Yuzuncu Yil University, under project number 2015-FEN-B098. The authors wish to thank Dr. Süleyman Mesut Pınar, research assistant Hüseyin Eroğlu, and MSc student Şahin Acar for their assistance during fish sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaptaner, B., Kankaya, E., Dogan, A. et al. Alterations in histology and antioxidant defense system in the testes of the lake Van fish (Alburnus tarichi Güldenstädt, 1814). Environ Monit Assess 188, 474 (2016). https://doi.org/10.1007/s10661-016-5476-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5476-z