Abstract
We describe a nanosized Hg(II)-imprinted polymer that was prepared from methacrylic acid as functional monomer, ethyleneglycol dimethacrylate as cross-linker, 2,2′-azobisisobutyronitrile (AIBN) as radical initiator, 2, 2′-di pyrydyl amine as a specific ligand, and Hg (II) as the template ions by precipitation polymerization method in methanol as the progeny solvent. Batch adsorption experiments were carried out as a function of pH, Hg (II) imprinted polymer amount, adsorption and desorption time, volume, and concentration of eluent. The synthesized polymer particles were characterized physically and morphologically by using infrared spectroscopy, thermogravimetric analysis, X-ray diffraction, and scanning electron microscopic techniques. The maximum adsorption capacity of the ion-imprinted and non-imprinted sorbent was 27.96 and 7.89 mg g−1, respectively. Under optimal conditions, the detection limit for mercury was 0.01 μg L−1 and the relative standard deviation was 3.2 % (n = 6) at the 1.00 μg L−1. The procedure was applied to determination of mercury in fish and water samples with satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the release of various harmful heavy metal ions into the environment has attracted great attention worldwide because of their toxicity and widespread use. Mercury (II) is among those which are of great concern (Buyuktiryakis et al. 2007). It is a widely distributed environmental pollutant in aqueous environments. Its toxicity in humans and animals even at low concentrations is dangerous. Mercury (II) is included in all lists of priority pollutants; as a result, different regulations and guidelines have been developed for monitoring its levels in water and sediments (Hayes 1997). Its existence in water would be a potential hazard to human health due to its accumulation and amplification along aquatic food chain. Long-term exposure to even very low levels of mercury in water is dangerous for the human health (Safavi et al. 2010). Considering the extreme toxicity of mercury, the United States Environmental Protection Agency (EPA) has mandated an upper limit of 10 nM (2000 ng/L) for Hg2+ in drinking water (EPA 2001).
Long-term uptake of Hg (II)-contaminated water causes damage to central nervous system, impairment of kidney function, chest pain and dyspnea (Huang et al. 2009). These features have made the mercury of great research interest in terms of both total content and analytical speciation using high sensitive techniques (Leopold et al. 2010). On the other hand, the toxicity level of mercury is becoming lower and lower; therefore, the direct for determination of mercury at sub-microgram per liter levels suffers from the matrix interferences. It is evident that the application of separation and preconcentration procedures is still necessary before the determination step. The most common methods for determination of mercury are high-performance liquid chromatography (HPLC) (Jones and Hardy 1997), ICP mass spectrometry (Lin and Jiang 2013), cold vapor atomic absorption spectroscopy (CVAAS) (Adlnasab et al. 2014), X-ray fluorescence (XRF) (O’Meara et al. 2000), furnace atomic absorption spectrometry (Tuzen 2003), flame atomic absorption spectrometry (FAAS) (Ghaedi et al. 2007), capillary zone electrophoresis-inductively coupled plasma mass spectrometry (Tu et al. 2000), voltammetry (Locatelli and Melucci 2012), and inductively coupled plasma (ICP) (Li et al. 2011). Among them, vapor generation techniques (cold vapor and hydride generation) coupled with atomic absorption spectrometry has been extensively used for the determination of mercury and hydride-forming elements in several liquid and digested samples due to its simplicity, high sensitivity, and relative freedom from interference (Moreda-Pińeiro et al. 2002).
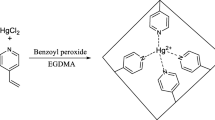
In recent years ion-imprinted polymers (IIPs), as selective sorbents for a particular chemical form of the given element, have received much attention (Ebrahimzadeh et al. 2013; Abbasi et al. 2015; Roushani et al. 2015; Kalate Bojdi et al. 2014, 2015; Behbahani et al. 2014; Shamsipur et al. 2014 and Vatanpour et al. 2011) . In the present work, we are interested to develop a novel type of ion-imprinted polymer nanoparticle using Hg (II) ion-2,2′-2,2′-di pyrydyl amine complex (Hg (II)-PA) as a template molecule, methacrylic acid (MAA) as a monomer, ethylene glycol dimethacrylate (EGDMA) as a cross-linker and 2,2′-azobisisobutyronitrile (AIBN) as an initiator. The advantages of this work are high sorption capacity and remarkable selectivity in Hg(II) ion separation from mixture of metal ions by new interpenetrating polymer network. The method of preparation of the IIP is simple, rapid, low cost, and environment friendly due to the use of aqueous environment. Furthermore, a new method is presented for the separation and preconcentration and determination of trace amount of mercury in fish and environmental water samples, with satisfactory results.
Experimental
Materials
Methacrylic acid (MAA), ethyleneglycoldimethacrylate (EGDMA), and 2,2′-azobisisobutyronitrile (AIBN) were obtained from Aldrich (Milwaukee, WI, USA). Ethanol was of reagent grade from Merck Chemical Company (Darmstadt, Germany) and was used as received. 2,2′-Dipyrydyl amine (PA), reagent grade HgCl2 (Aldrich), and nitrate or chloride salts of other cations (all from Merck) were used without any further purification.
Apparatus
Hg absorbance was measured with an Aanalyst 800 (Perkin-Elmer). No background correction system was used. Electrodeless discharge lamp operated at 183 mA was also utilized. The provided line and the spectral bandwidth was 253.7 and 0.7 nm, respectively. A MSH-10 hydride Generation System (Perkin-Elmer) was employed for cold vapor generation. A T-shaped quartz tube had a specification of 150 mm tube length, 2 mm wall thickness, and 10 mm outer diameter (FIAS-100, Perkin-Elmer, Germany), while the instrumental parameters were those recommended by the manufacturer. All signals were processed in the peak height mode. A 780 pH Meter (Metrohm), equipped with a combined Ag/AgCl glass electrode, was used for pH measurement. Scanning electron micrographs were recorded by means of a Philips XL30 series instrument using a gold film for loading the dried particles on the instrument. Gold films were prepared by a Sputter Coater model SCD005 made by BAL-TEC (Switzerland). A Varian model 300 Bio equipped with 10 mm quartz cell was used for recording the UV–vis spectra at 25.0 ± 0.1 °C. The FTIR spectra (4000–400 cm−1) were recorded on a Bruker (Germany) FTIR Vertex 70 spectrometer. Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were carried out using a Stanton Redcroft, STA–780 series with an aluminum crucible, and applying the heating rate of 10 °C/min in a temperature range of 50–600 °C, under air atmosphere with the flow rate of 50 mL min−1. The utilized sample mass was about 3.0 mg. The sample mass used was about 3.0 mg. Eppendorf Varied-Pipettes (10–100, 100–1000 μL) were used to deliver accurate volumes. All glassware and storage bottles were soaked in 10 % HNO3 overnight and thoroughly rinsed with deionized water prior to use.
Preparation of Hg2+-ion-imprinted polymeric nanoparticles
The mercury-ion-imprinted nanobeads were prepared by precipitation polymerization technique (Shamsipur et al. 2013). PA (1.0 mmol) was mixed with HgCl2 (1.0 mmol) in 25.0 mL of methanol as progeny solvent. This mixture was added to MAA (4.0 mmol), EGDMA (30.0 mmol), and AIBN (0.4 mmol) as initiator. The polymerization was carried out at 60 °C with constant stirring under a nitrogen atmosphere. The obtained polymer was washed with ethanol and water to remove unreacted monomer until neutral pH. The imprint ion, i.e., Hg2+ ions, was leached from the above polymer material by stirring with 50 mL of HCl (50 %; v/v) for about 18 h (Behbahani et al. 2012), and after centrifugation, the mercury contents of the supernatant solutions were determined by cold vapor atomic absorption spectrometer. This step was carried out three times until the supernatant solution was free from Hg2+ ions. The bulk polymer obtained was dried, sieved, and weighed. Non-imprinted polymer networks were also prepared using the same procedure without metal ions (Scheme. 1). Powder nanoparticles of 40–120 nm in diameter were obtained and then applied for future sorption and desorption studies.
Extraction procedure
Adsorption experiments were performed in batch set-up. Aliquots of mercury solution (100 mL) in the concentration range of 0.03–2.70 μg L−1 were treated with 30.0 mg of polymer particles in the pH range of 3.0–12.0 for 20 min. The pH of the solution was adjusted to desired values by adding 0.1 M sodium hydroxide or hydrochloric acid solutions. At the end of predetermined time intervals, polymer was separated by centrifugation. Adsorbed Hg (II) ions were eluted by treatment with 5.0 mL of 2.0 M HCl. The suspensions were then centrifuged and eluent solutions containing Hg2+ ions were removed from the nanoparticles. The resulting solutions were centrifuged and the mercury contents of the solutions were determined by cold vapor atomic absorption spectrometry (CVAAS). Extraction percent of Hg2+ was calculated by the following equation (Rajabi et al. 2013):
C i and C f are the concentrations of Hg2+ ion before and after extraction in the solution.
The distribution ratio (mL g−1) of Hg2+ ions between the IIP particles and aqueous solution is defined by the following equation:
where V is the volume of initial solution and m is the mass of IIP materials. Selectivity coefficients and relative selectivity coefficients (k′) for Hg2+ ions relative to foreign ions in the solution are defined as:
where \( {k}_{\mathrm{d}}^{{\mathrm{Hg}}^{2+}} \) and \( {k}_{\mathrm{d}}^{{\mathrm{M}}^{n+}} \) are the distribution ratios of Hg2+ and foreign ion, respectively.
Real sample preparation
Determination of mercury in water samples
The water samples, such as river water and mineral water, were centrifuged, filtered, and subjected to UV digestion for 2 h. After adjusting the pH samples to 9.5, they were stored in a cool place and analyzed, according to the procedure given in “Extraction procedure” section.
Determination of mercury in fish
The fishes were collected from a commercial market located at some local fishing port in Tonekabon, Iran in summer 2014. The samples were placed in clean plastic bags and stored on ice in an ice chest. They were then transported to a laboratory, where they were identified and kept in a freezer at 20 °C prior to preparation for chemical analysis. The samples were washed with distilled water and dried in tissue paper after defrosting in the laboratory. A portion of the edible muscle tissue was removed from the dorsal part of each fish, which was then homogenized and stored in clean-capped glass vials and kept in a freezer until the time of analysis. A part of the muscles was taken out quickly and was dried in an oven at 70 °C for 48 h. After grinding the dry tissue, 0.5 g of each sample was digested with 5.0 mL of concentrated HNO3 in a Teflon beaker for 4 h at 100 °C (Najafi et al. 2013). The resulted mixture was filtered into a 100-mL Erlenmeyer flask and then was diluted with double distillated water up to 100 mL. Finally, the mercury content of the sample was analyzed following the procedure given in “Extraction procedure” section.
Results and discussion
Characterization of Hg (II)-ion-imprinted polymer
FTIR spectra
The resulting imprinted particles were characterized by FTIR spectroscopy. The FTIR spectra of unleached and leached IIP were recorded using KBr pellet method. Figure 1 shows the FTIR spectra of the unleached (Fig. 1a) and leached (Fig. 1b) IIP nanoparticles. The similarity between these IR spectra shows that these polymers have a similar backbone (Daniel et al. 2005). In the IR spectra, the absorption was observed at 1394.0 cm−1 due to N═C (Dehno Khalaji et al. 2013). This band at 1394.0 cm−1 in unleached IIP was shifted to 1388.9 in leached IIP. This amount of reduction in band frequency indicates that the Hg2+ ions have been coordinated with non-bonding electron pairs of nitrogen in N–C groups in PA, and consequently the presence of Hg2+ ions in unleached IIP structure is evident. Moreover, the presence of C═N and N–H bands in the IR spectra of these materials indicated that PA had been sufficiently immobilized in the polymer matrix.
Scanning electron microscopic
The scanning electron microscopic (SEM) pattern of mercury-IIP is shown in Fig. 2 to describe the surface morphology. As seen, the resulted precipitation polymerization appeared as nanometer-sized particles. It is clear that the imprinted polymeric nanobeads with pore structure have been formed in the polymerization condition with a size range of 30–60 nm in diameter, which are slightly irregular in shape. Moreover, there are many micro-pores on the spherical surface of the imprinted sorbent, facilitating the fast binding of template ions.
X-ray diffraction
The X-ray diffraction (XRD) patterns of the unleached (a), leached (b), and NIP (c) nanosized ion-imprinted polymers are shown in Fig. 3. The XRD patterns of the unleached (a) and leached (b) IIP particles indicated similar patterns, except for the peaks corresponding to mercury (2θ values of 10.8860, 12.2683, 20.4572, and 38.3353). These peaks were absent in the curve of leached IIP, indicating the complete removal of Hg (II) ions from the structure. In all of the above cases, the XRD of leached IIPs was similar to the corresponding XRD patterns of control polymers.
Results of thermal analysis
Thermal stability of the leached and unleached IIP and NIP polymer particles was evaluated by TGA and DTA techniques. Figure 4 represents TGA/DTA curves for the IIP and NIP nanoparticles. As seen in Fig. 4a, an endothermic event was observed in the DTA curve of the NIP sample. This peak is observed due to the starting thermal decomposition of the ligand around 385 °C. This peak is followed by a continuous exothermic peak which is responsible for the main decomposition of the complex. On the other hand, TGA thermogram of this sample showed a main mass loss step for the decomposition of nanoparticles. This step was started at about 200 °C and continued until 530 °C. The TGA curve indicates about 85 % total mass loss for the thermal decomposition of this sample in this temperature range. Furthermore, the DTA curve reveals another exothermic peak at higher temperature which is compatible with 5 % mass loss in the TGA curve of the sample.
Figure 4b, c shows TGA and DTA plots for unleached and leached ion-imprinted polymer respectively. In DTA plot of Hg (II)-imprinted polymer, exothermic peaks were observed at 260.0 and 270.5 (for unleached) and 225.4.5 and 305.3 °C (for leached). As it is shown in TGA plot for unleached ion-imprinted particles, weight loss for Hg (II)-IIP was about 82 %, and this amount of reduction in weight is related to the presence of Hg (II) ions in polymer bead. While decrease in weight for leached imprinted polymer up to 89 % is due to the absence of Hg (II) ions in polymer. Also, as seen in TGA/DTA curves, thermal stability of unleached sample is significantly different from leached and NIP nanoparticles. These observations indicate that the formation of Hg (II)-imprinted polymer and elution of Hg (II) ions from the polymer was performed successfully.
Sorption/desorption experiments
Effect of pH
As the pH could change the amine groups in PA ligand, it seems to be one of the most controlling parameters for mercury adsorption (Taty-Costodes et al. 2003). The effect of pH on the adsorption of mercury ions was tested by equilibrating 100 mL of 10.00 μg L −1 solution of mercury with 30.0 mg of imprinted sorbent under different pH conditions from pH = 3.0 to pH = 12.0 for 10 min. The extraction efficiency of Hg (II) increased as the pH increased, from a low value at pH 3.0 to its maximum value at pH 9.5. The reason may be that the protonation of amine group in PA at low pH decreased the extent for the adsorption of Hg (II) ions. When the pH increased, the protonation weakened and the sorbent surface became less positively charged, therefore, adsorption of Hg (II) ions was more favorable. It can be inferred from the above results that pH solution should be adjusted to pH 9.5 to obtain the optimal adsorption efficiency.
Effect of type and concentration of eluent
As the ions are not removed from the sorbent completely with an inappropriate eluent, the eluent solution is an important factor for elution efficiency and recovery. Since in acidic solutions, the coordination cites of PA will be protonated and consequently it cannot coordinate with mercury, a series of acidic eluent solutions such as HCl, HNO3, and H2SO4 in different concentrations were used with elute mercury ions from ion-imprinted polymer. After the leaching of bounded mercury using 5.0 mL of 1.0 M of each acid on the IIPs nanoparticles, effluent mercury was determined by cold vapor atomic absorption spectrometer. The experimental results showed that 5.0 mL of hydrochloric acid 1.0 M can accomplish the quantitative elution of the mercury ion from the IIP nanobeads. High efficiency of HCl in the leaching of Hg2+ is probably due to the reason that the Hg (II) ion imprinted PA exhibited a low affinity in acidic medium as a result of the protonation of PA. Thus, 1.0 M HCl was selected as the eluent for future studies.
After choosing the proper eluent, several 5.0 mL portions of hydrochloric acid solutions with different concentrations (i.e., 0.1, 0.5, 1.0, and 2.0 M) were used for the elution of Hg2+ ions from the imprinted sites in the polymer network in order to study the optimum eluent concentration. Quantitative elution of mercury (II) was obtained when HCl concentration was greater than 2.0 M. This is most probably due to the increased protonation of the nitrogen atoms as the donor atoms in the PA structure of imprinted polymer. Thus, 5.0 mL of 2.0 M hydrochloric acid was selected as the optimal eluent solution.
Effect of sorbent amount
Again, the imprinting effect was noticed with different weights of polymer particles. As low as 30.0 mg of IIP particles was enough for quantitative enrichment of mercury ion from dilute aqueous solutions. Hence, 30.0 mg of IIP particles was used for the enrichment of mercury ion.
Effect of adsorption and desorption times
The effect of sorption and desorption times on the absorption of Hg2+ ions was investigated in a batch system while other parameters were kept in optimum conditions. In a typical uptake kinetics test, 30.0 mg of the sorbent was added to 100.0 mL of a 10.00 μg L−1 solution of Hg2+ at pH 9.5. The resulting suspension was stirred for different periods of time (i.e., from 5 to 30 min) under magnetic stirring. After the centrifugation, the supernatant solution was removed and the Hg2+ ions were determined by cold vapor atomic absorption spectrometer. The results indicated that at least 20 min is needed for completing an extraction. Therefore, the optimum adsorption time of 20 min was selected and used in all subsequent studies. To investigate the optimum elution time, the researchers repeated this procedure for a different range of elution time; the results showed that the minimum time needed for a complete elution is 5 min.
Preconcentration factor
The effect of aqueous phase volume on the preconcentration of mercury ion from dilute aqueous solutions was studied in the range of 25.0–800.0 mL. The results demonstrate that the dilution effect was not significant for sample volumes up to 600 mL for mercury ions; thus, an enrichment factor of 120 was enabled. However, at higher sample volumes, the recovery decreased significantly.
The adsorption capacity
The adsorption capacity (maximum amount of mercury ion adsorbed for 1.00 g of IIP particles) is an important factor to evaluate the IIPs (He et al. 2008). To evaluate this factor, the researchers equilibrated imprinted or non-imprinted polymers (50.0 mg) with Hg2+ solutions (100.0 mL) within the concentration range of 0.10–70.00 μg mL−1 at pH 9.5. Consequently, the adsorption value increased with the increase of the concentration of Hg2+ (0.10–30.00 μg mL−1), and a saturation value was achieved in the concentration range of 30.00–70.00 μg mL −1 (Fig. 5). The adsorption capacity increased with an increase in concentration of Hg (II) in both IIP and NIP and reached a plateau at 27.96 and 7.89 mg g−1, respectively. Obviously, the capacity of imprinted polymers was larger than that of non-imprinted polymers. The significant difference in adsorption capacities indicates the role of ion imprinting in determining the adsorption property of the adsorbent. Ion-binding cavities resulted from imprinting essentially activated the polymer surface for extensive ion uptake, whereas the interactions of PA, lacking ion-recognition ability, remained unspecific. After the removal of Hg2+, the imprinted cavities and specific binding sites of functional groups in a predetermined orientation were formed; however, no such specificity was found in non-imprinted polymers (He et al. 2008). Thereby, the memory effect owned by imprinted materials to the template metal ion allows them to possess a shorter response time and higher adsorption capacity to metal ions than NIP.
Regeneration and reusability
The repeated use of a commercial adsorbent is likely to be a key factor in industrial applications. In order to examine the reusability of the prepared ion-imprinted polymer particles, it was subjected to several loading and elution operations by using the same particles. The results showed that Hg2+ imprinted particles could be repeatedly used without any significant loss in the initial binding affinity. Hg (II)-ion-imprinted polymer has a superior reusability, which can be repeated in 9 cycles with no less than 95 % recovery. These studies clearly indicate the availability of the reversible nature of binding sites for picking Hg2+ ions using mercury-IIP nanoparticles. The prepared ion-imprinted polymer sorbent was repeatedly used and regenerated for at least 3 months. No significant decrease was observed in the sorbent affinity over this period of time, and the sorbent efficiency clearly remained stable. This stability of the sorbent without any evidence for polymer destruction is most likely due to the strong hydrogen bonding attachment of polymer chains to the monomers in the polymer network (Rajabi et al. 2013). It can be concluded that the IIP nanobeads can be used many times without decreasing their adsorption capacities significantly.
Selectivity study
The imprinted polymers are also characterized by a uniform distribution of chelating sites (Molochnikov et al. 2003). In IIPs, the cavities created after the removal of the template are complementary to the imprint ion in size and coordination geometries. Furthermore, the unique shape of the mercury ions may be expected due to much greater selectivity for the mercury ion by ion imprinting. In comparison with other metal ions, in the case of non ion-imprinted sorbent, the random distribution of ligand functionalities in the polymeric network results in no specificity in rebinding affinities. In order to assess the selectivity of imprinted polymer, Ni2+, Fe3+, Pb2+, Al3+, Cd2+, Zn2+, Ca2+, Mg2+, Cu2+, and Co2+ are chosen as the interfering ions as they have the same charge and similar size. Both the imprinted and non-imprinted polymers were used to obtain two sets of experimental data. Polymer material (30.0 mg) was added to 100 mL aqueous solutions containing 0.10 μg mL−1 Hg2+/Mn+. The pH was then adjusted accordingly to pH 9.5. After the adsorption-equilibrium, the mixtures were filtered and the concentration of each ion in the remaining solution was measured by atomic absorption spectroscopy. The measured values gave the concentrations of the unextracted ions, from which extraction efficiency was evaluated. Table 1 summarizes the distribution ratios (K d), selectivity coefficients (k) and relative selectively coefficients (k′) calculated using Eqs. (2, 3, and 4), respectively. It is clear from the results that the quantitative separation of mercury ions from Ni2+, Pb2+, Fe3+, Cu2+, Zn2+, Mg2+, Cd2+, Co2+, and Al3+ are possible. In comparison with other metal ions, whereas in the case of non ion-imprinted sorbent, the random distribution of ligand functionalities in the polymeric network results in no specificity in rebinding affinities.
Linearity, sensitivity, and precision
The calibration was performed using Hg (II) at different concentrations in the range of 0.03 to 2.70 μg L−1. The obtained linear regression equation and correlation coefficient (R 2) for Hg (II) were y = 0.3314 C Hg2+ + 0.0118; and R 2 = 0.9986, correspondingly; where y is the absorbance and C Hg2+ is the amount of Hg (II) in micrograms per liter of sample solution. According to the IUPAC definition, the detection limit (3 s of the blank signal intensity divided by slope of the calibration curve) of this method was 0.01 μg L−1 with the preconcentration step. The relative standard deviation for six separate batch experiments with 30 mg of sorbent for the determination of 1.00 μg L−1 Hg (II) in 100.0 mL of double-distilled water was calculated to be 3.2 % which indicated that the method had good precision for the analysis of trace Hg(II) ion in solution samples. A comparison of the proposed method with others in the literature is given in Table 2. The comparison of the present work with the results presented in this table shows good analytical figures of merit compared with other studies.
Real sample analysis
The applicability of the ion-imprinted polymer for preconcentration of trace levels of Hg2+ was tested using river water, mineral water, and fish samples. The mentioned samples were prepared according to the “Real sample preparation” section. For the preconcentration procedure, the researchers adjusted the pH of real samples to 9.5; these samples were spiked with Hg2+ ions and used in the extraction procedure. The concentration of sorbed Hg2+ ions was determined based on standard addition method and recovery tests. The relative recoveries are presented in Table 3. It was found that the quantitative extraction of Hg2+ ions was performed successfully by the Hg (II)-IIP even in the presence of various diverse ions.
Conclusion
Ion-imprinted polymers have attracted widespread attention as highly selective adsorbents for removing target metal ions selectively in the presence of other metal ions. In this study, a Hg (II)-ion-imprinted polymer with exceedingly high performance was successfully prepared. The present method provide fast equilibration-adsorption kinetics, very large relative selectivity coefficients, and high extraction efficiency percentages of the targeted ion (Hg2+) even in the presence of other closely related ions. The polymer was simple and relatively easy to prepare. The Hg (II)-imprinted particles can be used repeatedly with no significant decrease in binding affinities. Due to relatively high preconcentration factor, trace mercury ions at trace levels in high-volume samples can be determined and separated by Hg(II)-imprinted polymer. The high selectivity of this sorbent toward mercury ions, easy separation, and the high capacity factor make it a confidential sorbent for extraction of mercury ions. As a conclusion, the performance of the method was excellent in the extraction of trace amounts of Hg (II) in different food samples with high selectivity.
References
Abbasi, S., Roushani, M., Khani, H. A., Sahraei, R., & Mansouri, G. (2015). Synthesis and application of ion-imprinted polymer nanoparticles for the determination of nickel ions. Spectrochimica Acta A, 140, 534–543.
Adlnasab, L., Ebrahimzadeh, H., Asgharinezhad, A. A., Nasiri Aghdam, M., Dehghani, A., & Esmaeilpour, S. (2014). A preconcentration procedure for determination of ultra-trace mercury (II) in environmental samples employing continuous-flow cold vapor atomic absorption spectrometry. Food Analytical Methods, 7, 616–628.
Andac, M., Mirel, S., Senel, S., Say, R., Ersoz, A., & Denizli, A. (2007). Ion-imprinted beads for molecular recognition based mercury removal from human serum. International Journal of Biological Macromolecules, 40, 159–166.
Batlokwa, B. S., Chimuka, L., Tshentu, Z., Cukrowska, E., & Torto, N. (2012). An ion-imprinted polymer for the selective extraction of mercury (II) ions in aqueous media. Water SA, 38, 255–260.
Behbahani, M., Taghizadeh, M., Bagheri, A., Hosseini, H., Salarian, M., & Tootoonchi, A. (2012). A nanostructured ion-imprinted polymer for the selective extraction and preconcentration of ultra-trace quantities of nickel ions. Microchimica Acta, 178, 429–437.
Behbahani, M., Salarian, M., Bagheri, A., Tabani, H., Omidi, F., & Fakhari, A. (2014). Synthesis, characterization and analytical application of Zn(II)-imprinted polymer as an efficient solid-phase extraction technique for trace determination of zinc ions in food samples. Journal of Food Composition and Analysis, 34, 81–89.
Buyuktiryakis, S., Say, R., Denizli, A., & Ersoz, A. (2007). Mimicking receptor for methylmercury preconcentration based on ion-imprinting. Talanta, 71, 699–705.
Dakova, I., Karadjova, I., Georgieva, V., & Georgiev, G. (2009). Ion-imprinted polymethacrylic microbeads as new sorbent for preconcentration and speciation of mercury. Talanta, 78, 523–529.
Dakova, I., Yordanova, T., & Karadjova, I. (2012). Non-chromatographic mercury speciation and determination in wine by new core-shell ion-imprinted sorbents. Journal of Hazardous Materials, 231–232, 49–56.
Daniel, S., Rao, P., & Rao, T. P. (2005). Investigation of different polymerisation methods on the analytical performance of palladium (II) ion imprinted polymer materials. Analytica Chimica Acta, 536, 197–206.
Dehno Khalaji, D., Mehrani, S., Eigner, V., & Dusek, M. (2013). Synthesis, experimental and theoretical studies on its crystal structure and FT-IR spectrum of new thiosemicarbazone compound E-2-(4-isopropylbenzylidene)thiosemicarbazone. Journal of Molecular Structure, 1047, 87–94.
Ebrahimzadeh, H., Behbahani, M., Yamini, Y., Adlnasab, L., & Asgharinezhad, A. A. (2013). Optimization of Cu(II)-ion imprinted nanoparticles for trace monitoring of copper in water and fish samples using a Box–Behnken design. Reactive & Functional Polymers, 73, 23–29.
EPA (Environmental Protection Agency, United States) (2001). Mercury update: Impact of fish advisories. In: EPA Fact Sheet EPA823-F-01-011, Office of Water, USEPA, Washington, DC.
Fan, Z. (2006). Hg (II)-imprinted thiol-functionalized mesoporous sorbent micro-column preconcentration of trace mercury and determination by inductively coupled plasma optical emission spectrometry. Talanta, 70, 1164–1169.
Firouzzare, M., & Wang, Q. (2012). Synthesis and characterization of a high selective mercury (II)-imprinted polymer using novel aminothiol monomer. Talanta, 101, 261–266.
Ghaedi, M., Ahmadi, F., & Shokrollahi, A. (2007). Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. Journal of Hazardous Materials, 142, 272–278.
Hayes, R. B. (1997). The carcinogenicity of metals in humans. Cancer Causes and Control, 8, 371–385.
He, Q., Chang, X. J., Zheng, H., Jiang, N., Hu, Z., & Wang, X. Y. (2008). Determination of chromium (III) and total chromium in natural waters using a surface ion-imprinted silica gel as selective adsorbent. International Journal of Environmental Analytical Chemistry, 88, 373–384.
Huang, X., Liao, X., & Shi, B. (2009). Hg (II) removal from aqueous solution by bayberry tannin-immobilized collagen fiber. Journal of Hazardous Materials, 170, 1141–1148.
Jones, P., & Hardy, S. (1997). Development of a capillary electrophoretic method for the separation and determination of trace inorganic and organomercury species utilizing the formation of highly absorbing water soluble dithizone sulphonate complexes. Journal of Chromatography A, 765, 345–352.
Kalate Bojdi, M., Behbahani, M., Sahragard, A., Golrokh Amin, B., Fakhari, A., & Bagheri, A. (2014). A palladium imprinted polymer for highly selective and sensitive electrochemical determination of ultra-trace of palladium ions. Electrochimica Acta, 149, 108–116.
Kalate Bojdi, M., Behbahani, M., Najafi, M., Bagheri, A., Omidi, F., & Salimi, S. (2015). Selective and sensitive determination of uranyl ions in complex matrices by ion imprinted polymers-based electrochemical sensor. Electroanalysis, 27, 1–11.
Leopold, L., Foulkes, M., & Worsfold, P. G. (2010). Methods for the determination and speciation of mercury in natural waters—a review. Analytica Chimica Acta, 663, 127–138.
Li, S. X., Zheng, F. Y., Cai, S. J., & Cai, T. S. (2011). Determination of mercury and selenium in herbal medicines and hair by using a nanometer TiO2-coated quartz tube atomizer and hydride generation atomic absorption spectrometry. Journal of Hazardous Materials, 189, 609–613.
Lin, M. L., & Jiang, S. J. (2013). Determination of as Cd, Hg and Pb in herbs using slurry sampling electrothermal vaporisation inductively coupled plasma mass spectrometry. Food Chemistry, 141, 2158–2162.
Liu, Y., Changa, X., Yang, D., Guob, Y., & Meng, S. (2005). Highly selective determination of inorganic mercury (II) after preconcentration with Hg(II)-imprinted diazoaminobenzene–vinylpyridine copolymers. Analytica Chimica Acta, 538, 85–91.
Locatelli, C., & Melucci, D. (2012). Voltammetric determination of ultra-trace total mercury and toxic metals in meals. Food Chemistry, 130, 460–466.
Molochnikov, L. S., Kovalyova, E. G., Zagorodni, A. A., Muhammed, M., Sultanov, Y. M., & Efendiev, A. A. (2003). Coordination of Cu (II) and Ni(II) in polymers imprinted so as to optimize amine chelate formation. Polymer, 44, 4805–4815.
Moreda-Pińeiro, J., López-Mahıa, P., Muniategui-Lorenzo, S., Fernández-Fernández, E., & Prada-Rodrıguez, D. (2002). Direct as, Bi, Ge, Hg and Se (IV) cold vapor/hydride generation from coal fly ash slurry samples and determination by electrothermal atomic absorption spectrometry. Spectrochimica Acta B, 57, 883–895.
Najafi, E., Aboufazeli, F., Lotfi Zadeh Zhad, H. R., Sadeghi, O., & Amani, V. (2013). A novel magnetic ion imprinted nano-polymer for selective separation and determination of low levels of mercury (II) ions in fish samples. Food Chemistry, 141, 4040–4045.
O’Meara, J. M., Brjesson, J., & Chettle, D. R. (2000). Improving the in vivo X-ray fluorescence (XRF) measurement of renal mercury. Applied Radiation and Isotopes, 53, 639–646.
Rajabi, H. R., Shamsipur, M., & Pourmortazavi, S. M. (2013). Preparation of a novel potassium ion imprinted polymeric nanoparticles based on dicyclohexyl 18C6 for selective determination of K+ ion in different water samples. Materials Science and Engineering C, 33, 3374–3381.
Roushani, M., Abbasi, S., & Khani, H. (2015). Synthesis and application of ion-imprinted polymer nanoparticles for the extraction and preconcentration of copper ions in environmental water samples. Environmental Monitoring and Assessment, 187, 219–232.
Safavi, A., Maleki, N., & Doroodmand, M. M. (2010). Fabrication of a selective mercury sensor based on the adsorption of cold vapor of mercury on carbon nanotubes: determination of mercury in industrial wastewater. Journal of Hazardous Materials, 173, 622–629.
Shamsipur, M., Rajabi, H. R., Beyzavi, M. H., & Sharghi, H. (2013). Bulk polymer nanoparticles containing a tetrakis (3-hydroxyphenyl) porphyrin for fast and highly selective separation of mercury ions. Microchimica Acta, 180, 791–799.
Shamsipur, M., Rajabi, H. R., Pourmortazavi, S. M., & Roushani, M. (2014). Ion imprinted polymeric nanoparticles for selective separation and sensitive determination of zinc ions in different matrices. Spectrochimica Acta A, 117, 24–33.
Singh, D. K., & Mishra, S. (2010). Synthesis and characterization of Hg (II)-ion-imprinted polymer: kinetic and isotherm studies. Desalination, 257, 177–183.
Taty-Costodes, V. C., Fauduet, H., Porte, C., & Delacroix, A. (2003). Removal of Cd (II) and Pb (II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. Journal of Hazardous Materials, 105, 121–142.
Tu, Q., Qvarnstrm, J., & Frech, W. (2000). Determination of mercury species by capillary zone electrophoresis-inductively coupled plasma mass spectrometry: a comparison of two spray chamber–nebulizer combinations. Analyst, 125, 705–710.
Tuzen, M. (2003). Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chemistry, 80, 119–123.
Vatanpour, V., Madaenia, S. S., Zinadinia, S., & Rajabi, H. R. (2011). Development of ion imprinted technique for designing nickel ion selective membrane. Journal of Membrane Science, 373, 36–42.
Wang, Z., Wu, G., & He, C. (2009). Ion-imprinted thiol-functionalized silica gel sorbent for selective separation of mercury ions. Microchimica Acta, 165, 151–157.
Wu, G., Wang, Z., Wang, J., & He, C. (2007). Hierarchically imprinted organic–inorganic hybrid sorbent for selective separation of mercury ion from aqueous solution. Analytica Chimica Acta, 582, 304–310.
Xua, S., Chen, L., Li, J., Guan, Y., & Lu, H. (2012). Novel Hg2+-imprinted polymers based on thymine–Hg2+–thymine interaction for highly selective preconcentration of Hg2+ in water samples. Journal of Hazardous Materials, 237–238, 347–354.
Acknowledgments
The authors are grateful to the Ilam University Research Council for financing the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roushani, M., Abbasi, S. & Khani, H. Synthesis and application of ion-imprinted polymer nanoparticles for the extraction and preconcentration of mercury in water and food samples employing cold vapor atomic absorption spectrometry. Environ Monit Assess 187, 601 (2015). https://doi.org/10.1007/s10661-015-4820-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4820-z