Abstract
The concentrations of cadmium, lead, manganese and zinc were determined in the fish species Micropogonias manni captured in Budi Lake, Araucanía Region (Chile). The measurements were made by atomic absorption spectroscopy, and the analysis considered the sex, weight and size of the species; the representative samples were taken from the liver and muscle tissue. The method was validated using certified reference material (DOLT-1). The ranges of concentrations found in the muscle tissue were: Cd, not determinate (n.d.)–0.26; Pb, n.d.–1.88; Mn, 0.02–12.17 and Zn, 0.48–39.04 mg kg−1 (dry weight). The concentrations in muscle tissue were generally lower than those found in the liver. With respect to the average concentrations recorded for each metal in the edible part of the fish (muscle tissue), it was found that the levels of Cd, Pb, Mn and Zn are within the ranges published by other authors in similar works and below the maximum concentration limits permitted by current legislation (FAO/WHO 2004; EU 2001) and do not constitute a health hazard for consumers of this species. The results were subjected to statistical analysis to evaluate the correlations between the content of the various metals and the sex, weight and size of each sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incorporation of heavy metals into aquatic ecosystems (e.g. lakes, rivers, estuaries) may occur through the erosion of geological strata, atmospheric deposit or human sources such as industrial or domestic waste (Forstner and Wittman 1981; Eisler 1988; Nimmo et al. 1998; Alam et al. 2002). Unlike organic contaminants, heavy metals are not degraded either biologically or chemically in nature. They remain in the ecosystem and their stability allows them to be transferred over considerable distances, becoming bioaccumulated in aquatic organisms of the trophic chain and thus constituting a hazard for human health (Gobert et al. 1992; Chiang 1998; Sivaperumal et al. 2007).
Fish can accumulate high concentrations of metals absorbed from the water and their food (Hadson 1988; Alam et al. 2002; Mansour and Sidky 2002); however, bioaccumulation will depend principally on the concentration of the metals in the water and their exposure period, although other factors such as the temperature, pH, salinity and hardness of the water also play an important role in the accumulation of metals (Canli and Atli 2003).
Metals play an important role in various biological processes, some of them being essential, e.g. iron, copper, zinc and manganese, while others are classified as non-essential, such as mercury, cadmium, lead and chromium (Tüzen 2003; Celik and Oehlenschläger 2004). The metals considered as essential may also be toxic when the quantity ingested exceeds the concentration limits permitted by current legislation (EU 2001; FAO/WHO 2004), principally due to their cumulative behaviour (Matta et al. 1999). Heavy metals in the aquatic medium may be found in solution or in suspension, or precipitated in the sediment, from where they are absorbed and incorporated by organisms (Topcuoglu et al. 2002).
Aquatic media include lakes, rivers, estuaries and coastal lagoons. The latter are little represented in Chile, with Budi Lake (Araucanía Region) and Huillinco and Cucao Lakes (Los Lagos Region) cited as some of the most representative (Stuardo et al. 1989; Bertrán et al. 2006, 2010).
Budi Lake is a coastal lagoon with intermittent connection to the sea, containing fauna with estuarine characteristics (Bertrán et al. 2010). Its basin presents intensive land use, which has generated significant changes in the dynamic of the landscape (Peña-Cortés et al. 2006). These systems are characterised by a very particular internal dynamic in their chemical, physical and biological variables, which are largely determined by the effects of the rivers and the entry of sea water and sediment (Stuardo et al. 1989; Bertrán et al. 2006, 2010).
In the present investigation, the content was determined of cadmium, lead, manganese and zinc present in the liver and muscle tissue of the species Micropogonias manni (huaiquil), a principal source of fish protein for the population dwelling around the shores of Budi Lake, since previous studies have indicated high contents of Cd, Pb, Mn and Zn in the sediments of this coastal lagoon (Lizana 2005; Tapia et al. 2006).
Materials and methods
Sample taking
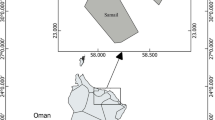
Samples were taken in Budi Lake (38°52′ S, 73°18′ W) (Fig. 1). The samples were collected directly from the fish landing and sales point in Puerto Domínguez, Araucanía Region. The taking of samples was done on two monitoring campaigns at Lake Budi; the first was in July (winter) and the second in October (spring) of the year 2004. The sizes of fish are commercial size and used like food by community of Puerto Domínguez, Araucanía Region.
Methods for sample preparation and analysis were similar to those used by Tapia et al. (2006, 2009, 2010), weighing and measuring all the specimens obtained. The fish were gutted and sexed using plastic material, and part of the liver and muscle tissue was extracted. The samples were stored at −20°C in pre-treated, labelled plastic containers until analysis in the laboratory.
Chemical analysis
The reagents used were of high purity (Suprapur, Merck, Darmstadt, Germany). The cleaning of the material was fundamental to guarantee the optimum result in analysis. The plastic and glass material were washed with non-ionic detergent and abundant deionised water, then treated with a solution of nitric acid (HNO3) 10% v/v for 48 h and finally rinsed three times with bidistilled water. The samples of liver and muscle tissue were lyophilized to a constant weight, using a Labconco lyophilizer. The samples were subsequently homogenised and kept in pre-treated plastic containers for later analysis. For digestion, 1.0 g of sample was weighed out and 10 mL of Suprapur nitric acid was added. The samples were then dried almost completely under an extractor fan, with constant stirring, using a heating plate set to 90°C. The resulting solution was filtered and washed with bidistilled water, and made up to a final volume of 25 mL in a pre-treated volumetric flask. The analyses were done in duplicate with a control solution for each. The measurements were done by flame atomic absorption spectroscopy (air/acetylene), using a Unicam spectrophotometer mod. 969 with deuterium background corrector.
Validation methodology
The method of analysis was validated using DOLT-1 certified reference material (dogfish liver), supplied by the National Research Council, Canada, NRC, Division of Chemistry. The validation results are shown at Table 1.
Statistical analysis
An analysis of co-variance was done (statistical significance was accepted at P < 0.05), using the weight or length of the animals as the categorical variable, the sex as the co-variable and the concentrations of Cd, Pb, Mn and Zn as dependent variables.
Results and discussion
Table 1 shows the results of the determination of Cd, Pb, Mn and Zn in the DOLT-1 reference material (dogfish liver). Replications of the reference material showed good exactness with relative errors varying between 2.49% (Zn) and 8.82% (Pb), and recovery percentages ranging from 93.2% (Mn) to 108.8% (Pb).
Tables 2 and 3 record the results of the concentrations of trace metals (Cd, Pb, Mn and Zn) based on dry weight in representative samples of liver and muscle from male and female Micropogonias manni, respectively. The results indicate that the concentrations of the metals analysed are considerably higher in the samples of liver than in the muscle tissue samples, in both male and female Micropogonias manni; these results agree with those recorded in similar works (Henry et al. 2004; Marcovecchio 2004; Dural et al. 2007; Yilmaz et al. 2007). According to the data recorded in Tables 1 and 2, zinc presented the highest concentrations in both tissues, followed by manganese, lead and cadmium. No significant differences were found between males and females.
The higher concentrations of Cd, Pb, Mn and Zn recorded in the liver samples, as compared with the muscle tissue samples, are attributable to the fact that the liver is the principal metabolic organ and thus a good indicator of the presence of contaminants in the medium (Galindo et al. 1986; WHO 1993; Harrison and Klaverkamp 1990). It also plays an important role in the storage, redistribution, detoxification and transformation of contaminants (Evans et al. 1993; López-Galindo et al. 2010a, b).
On analysing the content in the liver, no significant difference was observed for Cd when the ratio between sex and length was analysed (P > 0.05), unlike Pb, Mn and Zn. On the other hand, considering the weight of the sample and the concentration of metals in the liver, significant differences were determined in all the metals analysed (Fig. 2).
In the analysis of muscle tissue, no significant differences were observed for Pb in relation to length, but they were observed for Cd, Mn and Zn. When weight was considered, significant differences were only found for Pb, Mn and Zn (Fig. 3).
The values obtained for Cd in muscle (Tables 2 and 3), ranged between (no determinate) n.d.–0.26 μg/g dry weight, while the levels reported for other species of fish from different geographical zones were in the range of 0.01–4.16 μg/g dry weight in the species Saurida undosquamis, Sparus aurata and Mullus barbatus from Iskenderun Bay (Türkmen et al. 2005); 0.09–0.48 μg/g dry weight in fish samples of the middle Black Sea (Turkey) (Tüzen 2003); and 0.010–0.084 μg/g wet weight in tissues of Leucis cephalus and Lepomis gibbosus captured from Saricay, South-West Anatolia (Yilmaz et al. 2007). This places our results within the ranges cited previously and within permitted limits. The maximum established for Cd is 0.49 mg per week for a person weighing 70 kg (WHO 1989). If it is estimated that the daily average fish consumption per person is 0.02 kg (FAO 2005), equivalent to 0.14 kg fish consumption per person per week, and if we consider the range described for Cd in muscle of Micropogonias manni, the maximum consumption of Cd would be 0.036 mg per week, well below the 0.49 mg indicated by WHO (op cit).
The concentrations of Pb in muscle (Tables 2 and 3), ranged from n.d.–0.50 μg/g, dry weight, while the values reported for other species of fish expressed in microgrammes per gramme of dry weight range between 0.09 and 6.95 in fish species like Saurida undosquamis, Sparus aurata and Mullus barbatus from Iskenderun Bay (Türkmen et al. 2005); 0.22 and 0.85 μg/g dry weight in fish samples from the middle Black Sea (Turkey) (Tüzen 2003); and 0.068 and 0.920 μg/g wet weight in tissues of Leucis cephalus and Lepomis gibbosus captured from Saricay, South-West Anatolia (Yilmaz et al. 2007). This shows that the levels of Pb are within the ranges found previously. The permitted limit for Pb established by FAO/WHO (2004) is 1.725 mg/week for a person weighing 70 kg, and considering fish consumption of 0.14 kg per person per week (FAO 2005) and that the maximum limit for Pb is 0.50 mg/kg, the maximum amount of Pb which a person might ingest by fish consumption would be 0.07 mg per week, which is within the limits permitted by FAO/WHO (2004).
The levels of Mn in muscle varied between 0.02 and 12.17 μg/g dry weight, while those reported in the literature are in the range of 1.56–3.76 μg/g dry weight in fish samples from the middle Black Sea (Turkey) (Tüzen 2003); 0.05–4.64 μg/g dry weight in fish species like Saurida undosquamis, Sparus aurata and Mullus barbatus from Iskenderun Bay (Türkmen et al. 2005); and 8.8–23.5 μg/g dry weight in fish samples from Dhanmondi Lake (Begum et al. 2005). The US National Academy of Sciences (1980) recommends 2.5–5 mg per day of manganese and WHO (1994) recommends 2–9 mg per day for an adult.
Finally, the concentrations of Zn in muscle ranged from 0.48–39.04 μg/g dry weight, very similar to those recorded previously, which range between 0.60 and 11.57 μg/g dry weight in fish species like Saurida undosquamis, Sparus aurata and Mullus barbatus from Iskenderun Bay (Türkmen et al. 2005); 47.2–73.4 μg/g dry weight in fish samples from Dhanmondi Lake (Begum et al. 2005); and 2.1–8.7 μg/g wet weight in 19 fish samples from the Northeast Atlantic (Celik and Oehlenschläger 2004). The concentrations of Zn recorded are within the ranges permitted by FAO/WHO (2004), which approve a maximum of 490 mg per person weighing 70 kg each week. Considering that a person consumes 0.14 kg of fish per week (FAO 2005), and that the maximum level recorded is 39.04 μg/g (Table 3), when the weekly consumption is calculated, it would not exceed 5.46 mg, well below the value indicated by FAO/WHO (2004).
Conclusion
According to the results obtained, it may be concluded that the concentrations of Cd, Pb, Mn and Zn in samples of liver and muscle tissue in Micropogonias manni collected in Budi Lake, Araucanía Region (Chile) are similar to those recorded in the literature in fish from various geographical locations. Our results showed that the concentrations of the metals investigated were lower in muscle tissue than in liver samples because the liver is the principal metabolic and reserve organ of the organism.
The concentrations of cadmium, lead, manganese and zinc recorded in the edible part of the species Micropogonias manni are well below the limits proposed for fish by FAO/WHO (2004) and by the EU (2001). Although the levels of metals are not high, a potential danger may be generated by the human activity (agriculture and discharge of domestic waste) carried on in the area around Budi Lake.
References
Alam, M. G. M., Tanaka, A., Allison, G., Laureson, L. J. B., Stagnitti, F., & Snow, E. T. (2002). A comparison of trace element concentration in cultured and wild carp (Cyprinus carpio) of lake Kasumigaura, Japan. Ecotoxicology Environment Safety, 53, 348–354.
Begum, A., Amin, Md. N., Kaneco, S., & Ohta, K. (2005). Selected elemental composition of the muscle tissue of three species of fish, Tilapia nilotica, Cirrhina mrigala and Clarius batrachus, from fresh water Dhanmondi Lake in Bangladesh. Food Chemistry, 93, 439–443.
Bertrán, C., Vargas-Chacoff, L., Peña-Cortés, F., Mulsow, S., Tapia, J., Hauenstein, E., et al. (2006). Benthic macrofauna of three saline lake wetlands on the coastal rim of southern Chile. Cienc Mar, 32, 589–596.
Bertrán, C., Vargas-Chacoff, L., Peña-Cortés, F., Schlatter, R., Tapia, J., & Hauenstein, E. (2010). Distribución de la macrofauna bentónica en el lago costero Budi, Sur de Chile. Review on Biological Marine Oceanography, 45, 235–243.
Canli, M., & Atli, G. (2003). The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environmental Pollution, 121, 129–136.
Celik, U., & Oehlenschläger, J. (2004). Determination of zinc and copper in fish samples collected from Northeast Atlantic by DPSAV. Food Chemistry, 87, 343–347.
Chiang, J. (1998). Contaminación del mar y el futuro de la pesca en Chile. Rev Amb Desar, 4, 67–72.
Dural, M., Göksu, M. Z. L., & Özak, A. A. (2007). Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chemistry, 102, 415–421.
Eisler, R., (1988). Zinc hazards to fish, wildlife and invertebrates: a synoptic review. US Fish Wildlife Service Biology of Reproduction, 85.
EU (2001). Commission regulation as regards heavy metals, Directive 2001/22/EC, No: 466/2001
Evans, D. W., Doo, D. K. D., & Hanson, P. (1993). Trace element concentration in fish livers: implication of variations with fish size in pollution monitoring. Marine Pollution Bulletin, 26, 329–354.
FAO (2005). Statistic division, food security statistic, food consumption. http://www.fao.org/es/ESS/faotast/foodsecurity/index.en.htm. Accessed October 2010.
FAO/WHO (2004). Summary of evaluations performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956–2003) (First through sixty-first meetings). Washington, DC: ILSI Press International Life Sciences Institute.
Forstner, U., & Wittman, G. T. W. (1981). Metal pollution in the aquatic environment. Berlin: Springer Verlag.
Galindo, L., Hardisson, A., & Montelongo, F. G. (1986). Correlation between lead, cadmium, copper, zinc and iron concentrations in frozen tuna fish. Bulletin of Environmental Contamination and Toxicology, 36, 595–599.
Gobert, S., Daemers-Lambert, C., & Bouquegneau, J. M. (1992). Etat physiologique et contamination en Metaux Lourds des Moules Mytilus edulis Sur La Cote Belge. Bulletin Society of Royal Science Liège Proc. Royal Society, 61, 177–194.
Hadson, P. V. (1988). The effect of metabolism on uptake, disposition and toxicity in fish. Aquatic Toxicology, 11, 3–18.
Harrison, S. E., & Klaverkamp, J. F. (1990). Metal contamination in liver and muscle of northern pike (Esox lucius) and white sucker (Castomus commersoni) and in sediments from lake near smelter at Flin Flon, Manitoba. Environmental Toxicology and Chemistry, 9, 941–956.
Henry, F., Amara, R., Coucort, L., Lacouture, D., & Bertho, M.-L. (2004). Heavy metals in four fish species from the French coast of the Eastern English Channel and Southern Bight of the North Sea. Environment International, 30, 675–683.
Lizana, C., (2005). Estudio de metales tóxicos en sedimentos del borde costero de la IX Región, Chile. Tesis de Grado de Tecnología Médica. Facultad de Ciencias de la Salud, Universidad de Talca, Talca, Chile.
López-Galindo, C., Vargas-Chacoff, L., Nebot, E., Casanueva, J. F., Rubio, D., Solé, M., et al. (2010a). Biomarker responses in Solea senegalensis exposed to sodium hypochlorite used as antifouling. Chemosphere, 78, 885–893.
López-Galindo, C., Vargas-Chacoff, L., Nebot, E., Casanueva, J. F., Rubio, D., Solé, M., et al. (2010b). Sublethal effects of the organic antifoulant Mexel®432 in the flatfish Solea senegalensis. Chemosphere, 79, 78–85.
Mansour, S. A., & Sidky, M. M. (2002). Ecotoxicological studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chemistry, 78, 15–22.
Marcovecchio, J. E. (2004). The use of Micropogonias furnieri and Mugil liza as bioindicators of heavy metals pollution in La Plata river estuary, Argentina. Science of the Total Environment, 323, 219–226.
Matta, J., Milad, M., Manger, R., & Tosteson, T. (1999). Heavy metals, lipid peroxidation, and cigateratoxicity in the liver of the Caribbean barracuda (Sphyraena barracuda). Biological Trace Element Research, 70, 69–79.
National Academy of Sciences. (1980). Recommended dietary allowances (9th ed.). Washington: NAS.
Nimmo, D. R., Willox, M. J., Lafrancois, T. D., Chapman, P. L., Brinkman, S. F., & Grene, J. C. (1998). Effects of metal mining and milling on boundary waters of Yellowstone National Park, USA. Environmental Management, 22, 913–926.
Peña-Cortés, F., Rebolledo, G., Hermosilla, K., Hauenstein, E., Bertrán, C., Schlatter, R., et al. (2006). Dinámica del paisaje para el período 1980–2004 en la cuenca costera del Río-Lago Budi, Chile. Consideraciones para la conservación de sus humedales. Review of Ecology Australia, 16, 183–196.
Sivaperumal, P., Sankar, T., Viswanathan, V., & Nair, P. G. (2007). Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chemistry, 102, 612–620.
Stuardo, J., Valdovinos, C., & Dellarosa, V. (1989). Caracterización General del Lago Budi: Una Laguna Costera Salobre de Chile Central. CONA, 13, 57–69.
Tapia, J., Duran, E., Peña-Cortés, F., Hauenstein, E., Bertrán, C., Schlatter, R., et al. (2006). Micropogonias manni as a bioindicador for copper in lake Budi (IX Región Chile). Journal of the Chilean Chemical Society, 51, 901–904.
Tapia, J., Bertrán, C., Astudillo, M. J., Vargas-Chacoff, L., Carrasco, G., Valderrama, A., et al. (2009). Study of copper, chromium and lead contents in Mugil cephalus and Eleginops maclovinus species taken in the mouths of Maule and Mataquito rivers (VII region, Chile). Journal of the Chilean Chemical Society, 54, 36–39.
Tapia, J., Vargas-Chacoff, L., Bertrán, C., Torres, F., Pinto, R., Urzúa, S., et al. (2010). Study of the content cadmium, chromium and lead in bivalve molluscs of the Pacific Ocean (Maule Region, Chile). Food Chemistry, 121, 666–671.
Topcuoglu, S., Kırbasoglu, C., & Gungor, N. (2002). Heavy metals in organisms and sediments from the Turkish Coast of the Black Sea. Environment International, 1069, 1–8.
Türkmen, A., Türkmen, M., Tepe, Y., & Akyurt, I. (2005). Heavy metals in three commercially valuable fish species from Iskenderum Bay, Northern East Mediterranean Sea, Turkey. Food Chemistry, 91, 167–172.
Tüzen, M. (2003). Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chemistry, 80, 119–123.
WHO. (1989). Evaluation of certain food additives and contaminants (thirty-third report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series No. 776. Geneva: World Health Organization.
WHO. (1993). Toxicological evaluation of certain food additives and contaminants. Cambridge: Cambridge University Press.
WHO. (1994). Quality directive of potable water (2nd ed., p. 197). Geneva: World Health Organization.
Yilmaz, F., Özdemir, N., Demirak, A., & Tuna, A. L. (2007). Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chemistry, 100, 830–835.
Acknowledgements
Study carried out in the framework of FONDECYT Project 1080317 and 1110798. The authors are also grateful to the Environmental Chemistry Laboratory belonging to the Institute of Chemistry of Natural Resources, University of Talca, Chile.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tapia, J., Vargas-Chacoff, L., Bertrán, C. et al. Heavy metals in the liver and muscle of Micropogonias manni fish from Budi Lake, Araucania Region, Chile: potential risk for humans. Environ Monit Assess 184, 3141–3151 (2012). https://doi.org/10.1007/s10661-011-2178-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2178-4