Abstract
Tin, Cu, Zn, Cd, Pb, Ag and Hg concentrations were measured in waters, sediments and three ubiquitous sedentary molluscs: the oyster, Saccostrea glomerata, a rocky intertidal gastropod, Austrocochlea porcata, and a sediment-dwelling gastropod, Batillaria australis, at 12 locations along the far south coast of NSW, Australia, from Batemans Bay to Twofold Bay during 2009. Metal concentrations in water for Sn, Cd, Ag and Hg were below detection limits (< 0.005 μg/L). Measurable water metal concentrations were Cu: 0.01–0.08 μg/L, Zn: 0.005–0.11 μg/L and Pb: 0.005–0.06 μg/L. Mean metal concentration in sediments were Sn < 0.01–2 μg/g, Cu < 0.01–605 μg/g, Zn 23–765 μg/g, Cd < 0.01–0.5 μg/g, Pb < 0.01–0.3 μg/g, Ag < 0.01–0.9 μg/g and Hg < 0.01–2.3 μg/g. Several locations exceeded the Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand (Australian and New Zealand guidelines for fresh and marine water quality 2000) low and high interim sediment quality guidelines’ levels for Cu, Zn, Cd and Hg. Some sites had measurable Sn concentrations, but these were all well below the levels of tributyltin known to cause harm to marine animals. Elevated metal concentrations are likely to be from the use of antifoulants on boats, historical mining activities and agriculture in the catchments of estuaries. All molluscs had no measurable concentrations of Sn (< 0.01 μg/g) and low mean Ag (< 0.01–1.5 μg/g) and Hg (< 0.01–0.5 μg/g) concentrations. Mean Cu (24–1516 μg/g), Zn (45–4644 μg/g), Cd (0.05–5μg/g) and Pb (0.05–1.1 μg/g) in oysters were close to background concentrations. Oysters have Cd and Pb concentrations well below the Australian Food Standards Code (2002).] There were no significant correlations between metal concentrations in sediments and in organisms within locations, and no relationship with levels of boating activity and suspected antifouling contamination. Although not pristine, the low levels of metal contamination in sediments and molluscs in comparison with known metal-contaminated areas indicate that this region is not grossly contaminated with metals and suitable for the development of mariculture.]
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marinas and associated boating activities introduce contaminants into surrounding waters (McAllister et al. 1996; Bighiu et al. 2017). In well-flushed systems contaminants such as metals are dispersed, but when marinas are built, circulation is impaired and localised contamination can occur by metals being adsorbed to sediments and taken up by sessile plants and animals (Johnston et al. 2011). Antifouling agents are applied to suppress the growth of organisms on the hulls of marine vessels and structures. Copper has been used since the 1700s, but in the 1960s organotin compounds such as tributyltin (TBT) were incorporated into paints. TBT proved to be like DDT, in that substantial unintended effects on marine organisms, from algae to cetaceans, occurred (Huang et al. 2004; Tanabe 1999). The use of TBT in NSW as an antifouling agent was limited to vessels longer than 25 m in 1989 (Batley et al. 1992) and later totally banned internationally (IMO 2001). TBT is persistent in sediments and continues to effect ecosystems, affecting endocrine systems resulting in elevated testosterone levels giving rise to imposex (Matthiessen and Gibbs 1998) and causing shell deformity in bivalves (Gibson and Wilson 2003).
The banning of TBT has revived the use of Cu compounds such as cuprous oxide, cuprous thiocyanate and copper hydroxide as antifouling paints, and concern is rising over increased Cu concentrations in marine environments, particularly antifoulant paint particles (Turner 2010) and the resulting effects on resident organisms (Matthiessen et al. 1999; Jones and Turner 2010). Copper may induce immunomodulation and growth retardation (Pipe et al. 1999), while organisms resistant to Cu toxicity may become dominant in affected areas (Johnston and Roberts 2009). Some governments, such as Denmark, Sweden and the US State of California, are moving to ban or restrict the use of Cu-based antifouling paints (Showalter and Savarese 2004). Booster biocides, such as zinc pyrithione and zineb, are also being added to antifouling mixtures and may have chronic effects at low concentrations (Guardiola et al. 2012; Boxall et al. 2000). Copper and Zn are essential micronutrients for plants and animals, but are toxic in excessive concentrations (Bao et al. 2008; Koutsaftis and Aoyama 2006). The use of Zn-based biocides may be less environmentally damaging than other antifouling additives due to their lower toxicity (Voulvoulis et al. 2002). Zinc is also extensively used in sacrificial anodes fixed to the keels and propeller mechanisms of boats (Matthiessen et al. 1999). These metals will partition to sediments and be taken up and assimilated by biota at varying rates, based on environmental conditions (Comber et al. 2002).
Sediments accumulate metal concentrations that reflect inputs from both natural and anthropogenic sources as metals readily adsorb to fine silt–clay sediments (Tessier and Campbell 1987). Molluscs, both bivalves and gastropods concentrate metals from the water column and food (Edge et al. 2015). As such, they are employed as biomonitors (Phillips 1977). We have investigated the suitability of Australian molluscs for use as biomonitors and found their metal concentrations reflect environmental exposure (Robinson et al. 2005; Gay and Maher 2003; Maher et al. 2016; Ubrihien et al. 2016). Measurement of metals in sediments and molluscs allows time-integrated measurements of metal inputs allowing spatial assessment of metal contamination on a regional scale.
No systematic study of metal concentrations in estuarine waters and sediments and their resident organisms of the far south coast of NSW has been undertaken. Four published studies have measured metal concentrations in south coast estuarine sediments (Jones et al. 2003; Waring et al. 2006; Mikac et al. 2007; Sim et al. 2015) and reported very low concentrations of Cu: 3.1–80 μg/g, Zn: 5–160 μg/g, Cd: < 0.05–0.8 μg/g, Pb: 4–60 μg/g, Se: < 0.05–0.41 μg/g, Co: 0.7–6 μg/g dry mass, Ni: 10–16 μg/g and Hg: < 0.05 μg/g. Metal concentrations in oysters have been extensively measured (Robinson et al. 2005; Scanes and Roach 1999; Mackey et al. 1975) with concentrations of Cu: 15–140 μg/, Zn: 500–1547 μg/g, Cd: 0.5–5 μg/g and Pb: < 0.05–5.5μg/g reported. Metal concentrations for other organisms such the gastropods Bembicium nanum and Cellana tramoserica (Cu: 4–160 μg/g, Zn: 400–120 μg/g dry mass, Cd: 2–8 μg/g, Pb; 0.2–0.8 Cu: 15–140 μg/g, Zn: 500–1547 μg/g, Cd: 0.5–5 μg/g and Pb: < 0.05–5.5μg/g reported) have been sporadically reported (Gay and Maher 2003, Maher et al. 2016, Ubrihien et al. 2016).
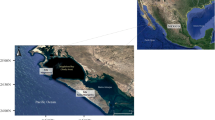
The far south coast of NSW is marketed as the ‘Nature Coast’ to promote tourism. The coastal areas primarily contain forests (> 85%) with some agriculture and urban development. As well, historically, mining for Sn, Cu and Zn has occurred in some watersheds (Supplementary Fig. 1, Geosciences Australia 2000). The region has numerous estuaries with varying levels of boating activity, from the irregularly used Mummuga Lake to the commercial port of Eden at Twofold Bay (Fig. 1). The estuaries of this area are used extensively for mariculture of the Sydney rock oyster Saccostrea glomerata. Along the NSW south coast there are around 138 lease holders that contribute 30% of NSW oysters with a value of $13,241,073 (DIST/DIRD 2017).
Sampling locations on the south coast, NSW, Australia. Sampling location details and organisms collected are given in Supplementary Table S1. a Batemans Bay, b Tomaga River, c Moruya River, d Tuross Lake, e Mummuga Lake, f Wagonga Inlet, g Corunna Lake, h Wallaga Lake, i Bermagui River, j Bega River, k Merimbula lake, l Twofold Bay
Metals can enter estuaries from coastal urban developments (Cu, Zn, Cr, Pb, Se and Ag), agriculture (Cu, Zn, Cd, Co and Mo), current and historical mining and boating activities (Cu, Zn and Sn). Five medium-sized coastal towns are present in watersheds (Fig. 1). These urban centres have small populations (Batemans Bay 11,334, Moruya 2531, Narooma 2409, Bega 16,866 and Eden/Merimbula 18,000) with only light industry and are likely sources of Cd, Pb, Ni and Cr in urban runoff (Liston and Maher 1986; McPherson et al. 2005) and Ag in sewage effluent (Sanudo-Wilhelmy and Flegalt 1992; Vogel et al. 2016). Agriculture on the south coast by land area is grazing (14%), cropping and horticulture (0.06%) and intensive animal production (0.01%) (DIST/DIRD 2017), and metals such as Cd can be in runoff from the use of fertilisers. In addition, Sn, Cu and Zn inputs may have occurred from historical mining operations (Supplementary Fig. 1, Geoscience Australia 2000).
In this study waters, sediments and three ubiquitous resident marine molluscs, the oyster Saccostrea glomerata, the rocky intertidal platform gastropod Austrocochlea porcata and the sediment-dwelling gastropod Batillaria australis, each utilising a different feeding strategy, i.e. filter feeders, algal grazers and deposit feeders, respectively (Underwood and Chapman 1995), from estuaries along the southern coast of NSW, Australia, were surveyed for metal concentrations and possible correlations of metal concentrations within and across locations determined. Results are compared with Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand (ANZECC/ARMCANZ 2000) interim sediment guidelines, Australian Food Standards Code (ANFA 2002) and contemporary studies of background and contaminant concentrations in estuarine waters, sediments and biota to assess the extent of metal contamination in the region. Tributyl Sn concentrations were not specifically measured due to cost of analyses and the need to use a different sampling design to obtain interpretable results. Total Sn concentrations were measured, however, to give preliminary data on tin contamination in sediments and biota.
These data will give a baseline to evaluate the effects of urban development expected from an increase in retirees relocating to the coast. According to the NSW Department of Planning and Environment, the population of the far south coast is expected to reach 177,650 by 2021 and 185,250 by 2031 (DIST/DIRD 2017). As well, the region is being evaluated for future mariculture ventures and results will be used to assess the suitability of estuaries for mariculture.
Methods
Sampling locations
Samples were collected from 12 estuaries along the southern coast of NSW, Australia, during August and September 2009 (Fig. 1). Samples were collected from sediment depositional areas and rocky platforms at the mouth of estuaries. Location coordinates, descriptions and sampled species are listed in “Supplementary Table S1”. Catchments feeding into each location reside over the Lachlan Fold Belt which extends along the southern coastal area of NSW (Supplementary Figure S1).
Study design
The study used a nested design with ten replicates (n = 10) of each sample collected from each location over the whole estuarine mouth. Organisms selected for the study were chosen based on being available at the majority of locations and having different feeding strategies: S. glomerata (filter feeder), A. porcata, previously known as A. constricta (algal grazer) and B. australis (deposit feeder). Oysters were less than 2 years old based on their mass size range (0.5–1 g dry mass), while A. porcata and B. australis had restricted mass ranges (0.1–0.3 and 0.2–0.4 dry mass, respectively) so that metal concentrations were independent of size and to minimise inherent metal concentration associated with age or other differences (Lobel and Wright 1982; Lobel et al. 1982; Taylor and Maher 2003; Robinson et al. 2005).
Sampling
All sampling containers were washed with 2% v/v nitric acid and rinsed three times with deionised water. Water (5 l) was collected in polyethylene carboys and 30 mL subsamples transferred to 50 mL polypropylene centrifuge tubes and double-bagged in polyethylene bags. Sediments were collected, using a Perspex (5 × 5 cm) tube and 100 g samples transferred into zip-lock plastic bags. Saccostrea glomerata were chiselled from rocks with an oyster knife, while other gastropods were collected by hand. All samples were stored on ice for transport and then frozen until analyses.
Sample preparation and analysis
Metal analyses
Water (n = 3), metals were pre-concentrated after filtration through 0.2-µm filters (Minisart RC 15, Sartorius, Germany) onto iminodiacetic acid resin at pH 5.5–8.3, using a flow injection interface leaving the major salts in solution (Maher et al. 2003). Detection limits were 0.005 µg/L using a 5-mL sample.
Sediment (n = 10 per site) was homogenised using a stainless-steel spatula and ground with an agate mortar and pestle. Subsamples (0.2 g dry mass) were weighed into a 50-mL polypropylene centrifuge tubes and 2 mL of Suprapur nitric acid (Merck, Australia) and 1 mL of Suprapur hydrochloric acid (Merck, Australia) added. Samples were digested using a MARS-5 microwave oven (CEM, USA) at 90 °C for 45 min and after cooling, diluted with deionised water (Milli-Q, Millipore, Australia) to 30 mL (Telford et al. 2008).
Molluscs (n = 10 per site) were depurated for ~ 6 h in aerated seawater from the collection site and soft tissues removed from shells. Oysters were shucked by cutting the abductor muscle and gastropods broken in a small vice and tissue severed from the operculum using a stainless-steel blade. Whole tissues were freeze-dried and ground to a powder using a spatula. All blades and spatulas were cleaned between uses with ethanol to remove organic material. Approximately 0.07 g dry mass was weighed into a 7-mL Teflon polypropylene digestion vessel and 1 mL of Aristar nitric acid added. Samples were digested at 600 W for 2 min, 0 W for 2 min, and 300 W for 45 min (Baldwin et al. 1994) and on cooling, transferred to plastic centrifuge tubes and diluted with deionised water to 10 mL.
Prior to analysis, all samples were centrifuged; biological extracts were diluted 1:10 v/v and sediments 1:100 v/v with internal standards Li, Sc, Y, Rh, In, Te and Ho (10 μg/L). Metal concentrations in extracts were determined using a PerkinElmer Elan 6000 inductively coupled plasma mass spectrometer (Telford et al. 2008; Maher et al. 2001). Accuracy of the procedure was assessed by the analysis of five reference materials. Measured metal concentrations indicate good agreement with certified values for water (NRCC CASS-1), biological (NRCC DORM 2 and NIST 1566b) and sediment (NRCC MESS 1 and NRCC PAC2) reference materials except Cr in sediments (Supplementary Table S2).
Physiochemical measurements
Particle size distributions of sediments were determined by dry sieving, 50 g of sediment through a series of sieve sizes to separate coarse sand (> 250 μm), fine sand–silt (250 μm–63 μm) and clay (< 63 μm). Sediment particles retained in each sieve were weighed and the composition of each fraction calculated by dividing the mean weight of each fraction by the total mean weight of the sample and multiplying by 100.
Organic carbon concentrations were determined by treating sediments (1 g) with 1 M hydrochloric acid until evolution of carbon dioxide ceased. Sediments were redried at 70 °C and heated in a muffle furnace at 550 °C for 12 h, and the % organic carbon determined by weight loss on ignition (Wang et al. 2012).
Statistical analysis
Analysis of results was undertaken using Microsoft Excel 2007 to determine means and standard errors and ANOVAs using Statistical Analysis System 9.1.3. Pearsons correlation and simple linear regressions were used to check relationships between metal concentrations and with grain size, organic carbon, iron and manganese concentrations. Procedures followed were as per Georges (2002). Data below detection limits were replaced by the value of half the detection limit (0.005 µg/g). All data were log-transformed to meet the requirements of normality and homogeneity of variances. Kruskal–Wallis test was used when data were not normally distributed. Cluster analysis and principal component analysis (PCA) were used to classify metal concentrations into groups (Primer 5; Primer-E, Plymouth, UK) (Clarke and Warwick 1994).
Results and Discussion
Water
Metal concentrations in water samples for Sn, Cd, Ag and Hg were below detection limits (< 0.005 μg/L). Measurable metal concentrations in water were Cu: 0.01–0.08 μg/L, Zn: 0.005–0.11 μg/L and Pb: 0.005–0.06 μg/L. Metal concentrations are well below those recommended for the protection of biological species at the 99% of Cu: 0.3 μg/L, Zn: 7μg/L, Cd: 0.7 μg/L, Pb: 2.2 μg/L, Ag: 0.8 μg/L and Hg: 0.1 μg/L (ANZECC/ARMCANZ 2000). These results agree with published studies that find very low concentrations of dissolved metals in waters from the NSW coastline (Hatje et al. 2003; Apte et al. 1998) and other parts of Australia (Munksgaard and Parry 2001; Fabris and Monahan 1995). Sewage does not enter most estuaries, while storm water drains primarily receive runoff from urban areas with little industry; thus, inputs of dissolved metals such as Ag are relatively low. As well, metal concentrations are probably reduced by adsorption to suspended matter and dilution with oceanic water (Hatje et al. 2003; Turner 1996). Metal concentrations are well below those reported for industrialised estuaries, e.g. Scheldt estuary, Netherlands, Weser estuary, Germany, and Humber and Mersey estuaries, the UK (Zwolsman et al. 1997; Comber et al. 1995; Turner et al. 1992).
Sediment
Grain size, organic carbon, Fe and Mn concentrations
Sediments had a large variation in silt–clay, sand and organic carbon (Table 1) because of deposition–resuspension processes during tidal movements (Fauser et al. 2013; Boyle et al. 1977). Organic carbon concentrations were not significantly correlated with clay content (< 63 μm), but had a trend of being higher as silt–clay content increased (Table 1). Sediments also exhibited considerable variation in iron (Fe) and manganese (Mn) concentrations again attributed to particle movement and flocculation–deflocculation because of variations in salinity and redox during tidal movement (Boyle et al. 1977). Iron and Mn concentrations were significantly correlated (r = 0.61 p = 0.000) as were organic carbon and Fe concentration (r = 0.8652, p = 0.0001), clay and Fe concentration (r = 0.7553, p = 0.001) and clay and Mn concentration (r = 0.2193, p = 0.01). Clay particles can be coated with organic matter, and both can complex with Fe and Mn (Douzva et al. 2011; Tombacz 2004; Chandrika et al. 1997).
Metal concentration patterns
Metal concentrations for sediments showed significant variation between locations (Table 2) with ranked metals concentrations also being different between locations (Table 3) with no suite of metals responsible for the differentiation of sites. Regression analyses showed that Sn–Cu, Sn–Zn, Sn–Ag, Cu–Zn, Cu–Cd, Cu–Ag, Cu–Hg, Zn–Ag and Ag–Hg concentrations were significantly correlated (Table 4A), indicating their common occurrence together.
Cadmium and Pb concentrations were below the ANZECC/ARMCANZ (2000) interim sediment quality (ISQ) low guideline levels (1.5 and 50 µg/g, respectively). Batemans Bay and Wagonga Inlet had mean copper and or zinc sediment concentrations above ISQ high guideline levels of 270 and 410 µg/g, respectively. Moruya River and Wallaga Lake have Cu and Zn concentrations above the ISQ low guideline levels of 65 and 200 µg/g, respectively. Silver concentrations were low, reflecting the lack of sewage inputs (Sanudo-Wilhelmy and Flegalt 1992) into these estuaries. Mercury concentrations at Tuross Lake, Wagonga Inlet, Bermagui River, Bega River and Merimbula Lake exceeded the ISQ high guideline level of 1 µg/g, while at Mummuga Lake, Corunna Lake and Twofold Bay exceeded the ISQ low guideline of 0.15 µg/g. Where sediment guidelines were exceeded, no consistent pattern of exceedance occurred across sites.
When sediment metal concentrations measured in this study were compared to other Australian estuarine locations (Supplementary Table S3), widespread metal contamination was not found. Most sites contained metal concentrations considered typical of non-contaminated Australian sites, Cu: 1–80 µg/g, Zn: 1–141 µg/g, Pb: 0.2–60 µg/g, Cd: 0.2–0.8 µg/g, while contaminated sites have Cu: 1–1400 µg/g, Zn: 1–3900 µg/g, Pb: 1–3900 µg/g, Cd: 0.05–270 µg/g (Supplementary Table S3).
Geochemical controls
The major adsorptive phases present in estuarine sediments are clay and silt particles coated with iron and manganese oxohydroxide coatings, iron and manganese oxohydroxy minerals, organic matter and iron sulphides (Tessier and Campbell 1987). All sediments were a mixture of oxic and anoxic phases caused by resuspension, inundation and bioturbation from feeding, burrowing, breathing and other activities of benthic organisms (Meadows and Tait 1989; McCall and Tevesz 1982; Rhoads and Boyer 1982).
There were no significant correlations between metal concentrations and silt–clay and organic carbon content. The presence of organic carbon can influence metal adsorption as organic carbon diminishes the number of active surface adsorption sites inhibiting complexation with metals (Oades 1988; Zhuang and Yu 2002).
Fe concentration was correlated with Sn (r = 0.38, p = 0.000), Cu (r = 0.54 p = 0.000) and Zn (r = 0.76, p = 0.000) concentration and Mn concentration with Sn (r = 0.22, p = 0.02), Cu (r = 021, p = 0.02) and Zn (r = 0.76, p = 0.0001) concentration. Many studies have reported significant correlations between metals and Fe concentrations in sediments (Tessier and Campbell 1987) attributed to iron oxyhydroxides coating particles (McCready et al. 2006). Similarly, Mn-coated sediments are very good scavengers of metals (Guo et al. 1997).
Tin
Mean Sn concentrations (Fig. 2) are less than those found in other Australian estuaries with marinas (Natural Heritage Trust 2004): Cairns, Queensland, 2.0–2.1 µg/g; Derwent River, Tasmania, 3.3–5.0 µg/g; Domain Slipway, Tasmania, 14–17 µg/g; St. Kilda, Victoria, 5.6–6.3 µg/g. Tin was probably present as inorganic Sn as there was no evidence that biota was being affected by TBT, primarily the lack of imposex (Smith and McVeagh 1991; Oberdorster and McClellan-Green 2002).
Metal concentrations in Saccostrea glomerata from estuaries of the south coast, NSW, Australia. Comparison with Robinson et al. (2005) approximation to Eastern Australian background concentrations: as noted on relevant graphs with green bands. Comparison with Scanes and Roach (1999) approximation to Eastern Australian background concentrations: as noted on relevant S. glomerata graphs red line. See Fig. 1 for sampling locations. Box: median, 25th and 75th percentiles; Whiskers: 5th and 95th percentiles; Black dots are outliers
Copper
Only Wagonga Inlet had Cu concentrations above the ISQ high level of 270 µg/g. This is a major harbour. Batemans Bay that also has a large marina did not have Cu concentrations above the ISQ low guideline level of 65 µg/g. These are comparable to those found in Northern Queensland marinas and harbours (9–113 µg/g, 45 ± 41, n = 8; Haynes and Loong 2002). Banning of TBT has revived the use of copper compounds as antifouling agents, and some of this Cu may be present due to its use as an antifouling agent (Warnken et al. 2004; Singh and Turner 2009), but widespread Cu contamination is not evident. The Moruya River Cu concentrations were well above the ISQ low guideline level (65 µg/g), but below the ISQ high guideline level (270 µg/g). The Moruya River catchment was extensively mined for gold (Au), Ag and associated Cu and Zn in the 1860s, resulting in considerable silt–sand inputs into the Moruya River. The higher Cu (and Zn) concentrations in Wallaga Lake are probably due to agricultural activities in the catchment. A high percentage (62%) of the lake catchment is under agricultural use, there have been large increases in sediment input since European settlement resulting in high nutrient and chlorophyll-a concentrations and low water clarity and the entrance is closed for long periods and hence no flushing occurs (Bega Valley and Eurobodalla Shire Council 2000).
Zinc
Batemans Bay and Wagonga Inlet have mean Zn sediment concentrations above the SQG high guideline level of 410 µg/g. Again, some Zn may be present from its use as a substitute for Sn antifouling agents or from historical mining activities in their watersheds (Supplementary S1). Moruya River and Wallaga Lake Zn concentrations were above the ISQ low guideline of 200 µg/g and as mentioned above, probably the result of mining activities and agriculture, respectively. All other locations had Zn concentrations below the guideline ISQ low guideline level of 200 µg/g.
Mercury
Mercury was found in all sediments except at the Tomaga and Moruya Rivers, with many Hg concentrations exceeding the ISQ low and high guideline levels of 0.15 and 1 µg/g, respectively. Gold was discovered in the Moruya District in 1851, and Araluen proved to be one of the three principal goldfields in Australia producing more than 26 tonnes of pure gold. In 1857–1861 Au was found near Broulee, Mogo, Mt Dromedary, Gulph Creek and Nerrigundah, Nelligen, Merricumbene and Deua. Mercury was used to scavenge the Au using Hg sluices with few precautions taken to prevent Hg contamination of waterways. This has resulted in extensive low-level Hg contamination of catchments in the Eurobodalla and Bega shires. Some Hg may also come from natural hydrothermal fissures, biotite-rich granite formations and cinnabar deposits known to contain Hg (Jardin and Bunn 2010). As well, bushfires that regularly occur in the adjacent coastal catchments can release Hg into the environment (Packham et al. 2009).
Molluscs
Non-detectable Sn concentrations (< 0.01 µg/g) and non-detectable or very low mean Ag (< 0.01–0.9 µg/g) and Hg concentrations (< 0.01–0.5 µg/g) were found in molluscs. Metal concentrations measured in mollusc species showed no widespread contamination of Cu and Zn (Figs. 2, 3, 4) when compared to published literature on metal concentrations in S. glomerata (Supplementary Table S4) and A. porcata (Supplementary Table S5). Any effect of organism size and mass can be discounted as previous studies have indicated that for oysters (0.5–1 g dry mass; less than 2 years old) and marine gastropods (0.1–0.4 g dry mass), metal concentrations are independent of size/mass in the ranges collected for this study (Robinson et al. 2005; Taylor and Maher 2003). Significant variation between locations for metal concentrations occurs (Figs. 2, 3, 4) with ranked metals concentrations being different between locations, and organisms at different locations have different ranked metal concentrations (Table 3).
Metal concentrations in Austrocochlea porcata from estuaries of the south coast, NSW, Australia. See Fig. 1 for sampling locations. Box: median, 25th and 75th percentiles; Whiskers: 5th and 95th percentiles; Black dots are outliers
Metal concentrations in Batillaria australis from estuaries of the south coast, NSW, Australia. See Fig. 1 for sampling locations. Box: median, 25th and 75th percentiles; Whiskers: 5th and 95th percentiles; Black dots are outliers
Saccostrea glomerata
Most S. glomerata had mean Cu concentrations within background concentrations of 20–90 µg/g (Fig. 2) as defined by Robinson et al. (2005). Oysters at Batemans Bay, Wagonga Inlet, Bermagui River and Twofold Bay had mean Cu concentrations greater than the 170 µg/g proposed by Scanes and Roach (1999). At Tuross Lake and Merimbula Lake, mean Cu concentrations (64 and 72 µg/g, respectively) were comparable to those found by Mackey et al. (1975); however, at Batemans Bay and Wagonga Inlet, mean Cu concentrations (236 and 552 µg/g, respectively) were more than double those reported by Mackey et al. (1975). Differences between studies at the same location can be attributed to the increase in urbanisation and boating activities over the last 25 years.
For S. glomerata, all locations except Bermagui River and Twofold Bay had mean Zn concentrations (1259–2394 µg/g, Fig. 2) below the 2610 µg/g background level proposed by Scanes and Roach (1999). Only oysters at Bega River were within background levels of 792–1465 µg/g as defined by Robinson et al. (2005). Mean Zn concentrations in oysters at Batemans Bay and Wagonga Inlet were comparable; Tuross Lake was more than double, and Merimbula only 6% of Zn concentrations reported by Mackey et al. (1975) at the same locations. Oysters had Cd and Pb concentrations that are well below the Australian Food Standards Code (10 µg/g dry mass) (ANFA 2002).
Regression analyses showed that Cu–Zn, Cu–Pb, Cu–Hg, Zn–Cd, Zn–Pb and Zn–Hg concentrations were significantly correlated (Table 4B), indicating their common occurrence together.
Austrocochlea porcata
Copper, Zn and Cd concentrations measured in A. porcata (Fig. 3; 24–116 µg/g; 45–92 µg/g and < 0.01–0.9 µg/g, respectively) were lower than those measured in this gastropod in the contaminated Lake Macquarie, NSW (40–200 µg/g; 80–260 µg/g and 1–7 µg/g, respectively, Taylor and Maher 2003), and lower than those found in the relatively uncontaminated Jervis Bay (10–160; µg/g; 20–180 µg/g and 0–3 µg/g, respectively, Taylor and Maher 2003). Austrocochlea porcata are missing at the Bega River site probably because of the lack of suitable habitat. Copper and Zn concentrations are higher and Cd concentrations lower than concentrations reported for this gastropod (Cu 0.58–5.23 µg/g; Zn 4.46–47.57 40 µg/g; and Cd 0.44–5.53 µg/g, respectively) around Newcastle, NSW (Walsh et al. 1994), which is also contaminated with metals. The study of Walsh et al. (1994), however, combined soft tissues and shells which would have considerably diluted the soft tissue metal concentrations. Regression analyses showed that Cu–Zn and Cu–Cd concentrations were significantly correlated (Fig. 4c), indicating their common occurrence together.
Batillaria australis
Copper, Zn, Cd and Pb concentrations in B. australis, like those in A. porcata, indicated little metal contamination (Fig. 4). Batillaria australis were missing at several sites although suitable habitat (i.e. soft clay–silt–sand sediments) was present at these locations. Batillaria australis are generally found near mangrove forests, but these sites were not near mangroves probably explaining their absence. Regression analyses showed that like A. porcata, Cu–Zn and Cu–Cd concentrations were significantly correlated (Fig. 4d), indicating their common occurrence together.
Understanding contaminant sources
There were no significant correlations between metal concentrations in waters, sediments and organisms, or between organisms across locations. Metal concentrations in molluscs from within a location do not consistently reflect the concentrations of metals found in the location’s sediments or metal concentrations found in other species. Although the locations were different based on their sediment metal composition (Table 3), the metals causing the discrimination were different to those causing the site discrimination for organisms (Table 3). This further illustrates that the presence of metals in sediments is not a good predictor of metals in filter feeding and grazing organisms. Similar results have been found for polychaetes living in south coast sediments and rocky intertidal zones (Waring et al. 2006).
All three organisms had much lower Sn and Hg concentrations than the corresponding sediment Sn and Hg concentrations, indicating low uptake and/or regulation or low bioavailability of Sn and Hg directly from sediments.
Sampling of sediments and organisms to detect metals originating from specific metal sources such as antifouling agents is problematic. Confounding factors can be multiple sources of metals, factors affecting metal uptake such as temperature, salinity and dissolved organic carbon (Lobel and Wright 1982; Lobel et al. 1982; Wood 1983; Wang and Fisher 1999) and potential inherent biological variability affecting metal accumulation within species (Robinson et al. 2005; Taylor and Maher 2003). Commercial S. glomerata farming has been a key driver for the need to understand anthropogenic inputs, in terms of both oyster quality and negation of risks for human consumption. Maintaining healthy environmental conditions for oyster production will also support other resident organisms. While monitoring using molluscs may not reflect all impacts on ecosystems, the use of bivalves and gastropod molluscs has gained universal acceptance as an indicator of the biologically available fraction of metals (Phillips 1977). Oysters and gastropods have the ability to accumulate metals without lethal effects, are sedentary and easy to sample and provide sufficient tissue for analysis (Spooner et al. 2003; Taylor and Maher 2003; Robinson et al. 2005; Maher et al. 2016). Any substantial increase in the use of boats, with resulting contaminants, sharing locations with oyster farms may force farmers and supporting government agencies to undertake structured ongoing monitoring to detect possible increases in metal contamination and allow for mitigation through further antifouling agent regulation.
Summary
At present, although the region is not pristine, the low levels of metal contamination in sediments, oysters and gastropods indicate that this region is generally suitable for mariculture. Tin concentrations in sediments at several locations indicated that speciation of the Sn compounds present in sediment should be considered to confirm if organisms are exposed to geologically derived Sn or TBT. There is, however, no evidence indicating that biota is being affected by TBT to warrant large-scale measurements of TBT. Copper and Zn concentrations at some locations are higher than ANZECC/ARMCANZ (2000) ISQ low and high guidelines and expected background levels in S. glomerata. These locations warrant further investigation as to the source of the metals, possibly from natural mineral deposits, historical mining legacies, boating activities or urban centres within their watersheds. Metal concentrations in general do not, however, appear to be correlated with levels of boating activity and thus the use of antifouling agents.
References
ANFA. (2002). Australian food standards code. Melbourne: Australia New Zealand Food Authority.
ANZECC/ARMCANZ. (2000). Australian and New Zealand guidelines for fresh and marine water quality. Melbourne: Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand.
Apte, S. C., Batley, G. E., Szymczak, R., Rendell, P. S., Lee, R., & Waite, T. D. (1998). Baseline metal concentrations in New South Wales coastal waters. Marine & Freshwater Research, 49, 201–214.
Baldwin, S., Deaker, M., & Maher, W. (1994). Low-volume microwave digestion of marine biological tissues for the measurement of trace elements. Analyst, 119, 1701–1704.
Bao, V. W., Leung, K. M., Kwok, K. W., Zhang, A. Q., & Lui, G. C. (2008). Synergistic toxic effects of zinc pyrithione and copper to three marine species: Implications on setting appropriate water quality criteria. Marine Pollution Bulletin, 57, 616–623.
Batley, G. E., Brockbank, C. I., & Scammel, M. S. (1992). The impact of banning of tributyltin based anti-fouling paints on the Sydney rock oyster, Saccostrea commercialis. Science of the Total Environment, 122, 301–314.
Bega Valley and Eurobodalla Shire Council. (2000). Wallaga lake estuary management plan (no. 5, pp. 1–57).
Bighiu, M. A., Eriksson-Wiklundi, A.-K., & Eklundi, B. (2017). Biofouling of leisure boats as a source of metal pollution. Environmental Science and Pollution Research, 24, 997–1006.
Boxall, A. B. A., Comber, S. D., Conrad, A. U., Howcroft, J., & Zaman, N. (2000). Modelling of Antifouling Biocides in UK Estuaries. Marine Pollution Bulletin, 40, 898–905.
Boyle, E. A., Edmond, J. M., & Sholkovitz, E. R. (1977). The mechanism of iron removal in estuaries. Geochimica et Cosmochimica Acta, 41, 1313–1324.
Chandrika, V., Tarit, C., & Kunal, G. (1997). Complexation of humic substances with oxides of iron and aluminium. Soil Science, 162, 28–34.
Clarke, K. R., & Warwick, R. M. (1994). Changes in marine communities: An approach to statistical analysis and interpretation. Plymouth: Plymouth Marine Laboratory.
Comber, S. D. W., Franklin, G., Gardner, M. J., Watts, C. D., Boxall, A. B. A., & Howcroft, J. (2002). Partitioning of marine antifoulants in the marine Environment. The Science of the Total Environment, 286, 61–71.
Comber, S. D. W., Gunn, A. M., & Whalley, C. (1995). Comparison of the partitioning of trace metals in the Humber and Mersey estuaries. Marine Pollution Bulletin, 30, 851–860.
DIST/DIRD. (2017). Regional jobs and investment packages. South Coast region of New South Wales local Investment plan. Department of Industry, Innovation and Science-Department of Infrastructure and regional development, May 2017, pp. 1–36.
Douzva, B., Lhotka, M., Grygar, T., Machovic, V., & Herzogova, L. (2011). Insitu co-adsorption of arsenic and iron/manganese ions on raw clays. Applied Clay Science, 54, 166–171.
Edge, K. J., Daffon, K. A., Simpson, S. L., Ringwood, A. H., & Johnston, E. L. (2015). Resuspended contaminated sediments cause sub-lethal stress to oysters: a biomarker differentiates TSS and contaminant effects. Environmental Toxicology and Chemistry, 34, 1345–1353.
Fabris, G. J., & Monahan, C. A. (1995). Characterisation of toxicants in Port Phillip Bay: Metals. Technical Report No 18, Commonwealth Scientific and Industrial Research Organisation INRE Port Phillip Bay Environmental Study, Melbourne, p. 48.
Fauser, P., Sanderson, H., Hedegaard, R. V., Sloth, J. J., Larsen, M. M., Krongaard, T., et al. (2013). Occurrence and sorption properties of arsenicals in marine sediments. Environmental Monitoring and Assessment, 185, 4679–4691.
Gay, D., & Maher, W. A. (2003). Natural variation of copper, zinc, cadmium and selenium concentrations in Bembicium namum and their potential use as a biomonitor of trace metals. Water Research, 37, 2173–2185.
Georges, A., (2002). Biometry: Statistics for ecology and natural resource management. Workbook 1: Introduction to SAS for windows (version 8). Flexible Delivery Development Unit, Centre for the Enhancement of Learning, Teaching and Scholarship (CELTS), University of Canberra, ACT 2601, Australia (IBSN: 1 740880269).
Geosciences Australia. (2000). Lachlan fold belt project (1991–2000). Viewed 17 January, 2010. http://www.ga.gov.au/minerals/research/archive/lachlan_fold_belt.jsp#1-250000.
Gibson, C. P., & Wilson, S. P. (2003). ‘Imposex” still evident in eastern Australia 10 years after tributyltin restrictions. Marine Environmental Research, 55, 101–112.
Guardiola, F. A., Cuesta, A., Meseguer, J., & Esteban, M. A. (2012). Risks of using antifouling biocides in aquaculture. International Journal of Molecular Science, 13, 1541–1560.
Guo, T., DeLaune, R. D., & Patrick, W. H., Jr. (1997). The influence of sediment redox chemistry on chemically active forms of arsenic, cadmium, chromium, and zinc in estuarine sediment. Environment International, 23(3), 305–316.
Hatje, V., Apte, S. C., Hales, L. T., & Birch, G. F. (2003). Dissolved trace metal distributions in Port Jackson estuary (Sydney Harbour), Australia. Marine Pollution Bulletin, 46, 719–730.
Haynes, D., & Loong, D. (2002). Antifoulant (butyltin and copper) concentrations in sediments from the Great Barrier Reef world heritage area, Australia. Environmental Pollution, 120, 391–396.
Huang, G., Bai, Z., Dai, S., & Xie, Q. (2004). Accumulation and toxic effect of organometallic compounds on algae. Applied Organometallic Chemistry, 7, 373–380.
IMO (International Maritime Organization). (2001). International convention on the control of harmful anti-fouling systems on ships, 2001. In International conference on the control of harmful anti-fouling systems for ships.
Jardin, T., & Bunn, S. (2010). Northern Australia, whither the mercury? Marine & Freshwater Research, 61, 451–463.
Johnston, E. L., Marizinelli, E. M., Wood, C. A., Speranza, D., & Bishop, J. D. D. (2011). Bearing the burden of boat harbours: Heavy contaminant and fouling loads in a native habitat-forming alga. Marine Pollution Bulletin, 62, 2137–2144.
Johnston, E., & Roberts, D. (2009). Contaminants reduce the richness and evenness of marine communities: A review and meta-analysis. Environmental Pollution, 157, 1745–1752.
Jones, B. G., Killian, H. E., Chenhall, B. E., & Sloss, C. R. (2003). Anthropogenic effects in a coastal lagoon: Geochemical characterisation of Burrill Lake, NSW, Australia. Journal of Coastal Research, 19, 621–632.
Jones, D. E., & Turner, A. (2010). Bioaccessibility and mobilisation of copper and zinc in estuarine sediment contaminated by antifouling paint particles. Estuarine, Coastal and Shelf Science, 8, 399–404.
Koutsaftis, A., & Aoyama, I. (2006). The interactive effects of binary mixtures of three antifouling biocides and three heavy metals against the marine algae Chaetoceros gracilis. Environmental Toxicology, 21, 432–439.
Liston, P., & Maher, W. A. (1986). Trace metal export in urban runoff and its biological significance. Bulletin of Environmental Contamination and Toxicology, 36, 900–905.
Lobel, P. B., Mogie, P., Wright, D. A., & Wu, B. L. (1982). Gonadal and non-gonadal zinc concentrations in mussels. Marine Pollution Bulletin, 13, 320–332.
Lobel, P. B., & Wright, D. A. (1982). Metal accumulation in four molluscs. Marine Pollution Bulletin, 13, 170–174.
Mackey, N. J., Williams, R. J., Kacprzac, J. L., Kazacos, M. N., Collins, A. J., & Auty, E. H. (1975). Heavy metals in cultivated oysters (Crassostrea commercialis = Saccostrea cucullata) from the estuaries of New South Wales. Australian Journal of Marine and Freshwater Research, 26, 31–46.
Maher, W., Forstner, S., Krikowa, F., Snitch, P., Chapple, G., & Craig, P. (2001). Measurement of trace metals and phosphorus in marine animal and plant tissues by low volume microwave digestion and ICPMS. Journal of Analytical Atomic Spectrometry, 22, 361–369.
Maher, W., Krikowa, F., Kirby, J., Townsend, A. T., & Snitch, P. (2003). Measurement of trace elements in marine environmental samples using solution ICPMS. Current and future applications. Australian Journal of Chemistry, 56, 103–116.
Maher, W. A., Maher, N., Taylor, A., Krikowa, F., Ubrihien, R., & Milac, K. M. (2016). The use of the marine gastropod, Cellana tramoserica as a biomonitor of metal contamination in near shore Environments. Environmental Monitoring and Assessment, 188, 391–406.
Matthiessen, P., & Gibbs, P. E. (1998). Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environmental Toxicology and Chemistry, 17, 37–43.
Matthiessen, P., Reed, J., & Johnson, M. (1999). Sources and potential effects of copper and zinc concentrations in the estuarine waters of Essex and Suffolk, United Kingdom. Marine Pollution Bulletin, 38, 908–920.
McAllister, T. L., Overton, M. F., & Brill, E. D., Jr. (1996). Cumulative impact of marinas on estuarine water quality. Environmental Management, 20, 385–396.
McCall, P. L., & Tevesz, M. J. S. (Eds.). (1982). Chapter 3. The effects of Benthos on physical properties of freshwater sediments. In Animal-sediment relations—The biogenic alteration of sediments. Topics in Geobiology (Vol. 100, pp. 105–176). New York: Springer.
McCready, S., Birch, G. F., & Long, E. R. (2006). Metallic and organic contaminants in sediments of Sidney Harbour, Australia and vicinity: A chemical dataset for evaluating sediment quality guidelines. Environmental International, 32, 455–465.
McPherson, T. N., Burian, S. J., Stenstrom, M. K., Turin, H. J., Brown, M. J., & Suffet, I. H. (2005). Trace metal pollutant load in urban runoff from Southern California watershed. Journal of Environmental Engineering, 131, 1073–1080.
Meadows, P. S., & Tait, J. (1989). Modification of sediment permeability and shear strength by two burrowing invertebrates. Marine Biology, 101, 75–82.
Mikac, K. M., Maher, W. A., & Jones, A. R. (2007). Do physicochemical sediment variables and their soft sediment macrofauna differ among micro size coastal lagoons with forested and urban catchments? Estuarine and Coastal Shelf Science, 72, 308–318.
Munksgaard, N. C., & Parry, D. L. (2001). Trace metals, arsenic and lead isotopes in dissolved and particulate phases of North Australian coastal and estuarine seawater. Marine Chemistry, 75, 165–184.
Natural Heritage Trust. (2004). Tributyltin (TBT) analysis protocol development and current contamination assessment. A report from Natural Heritage Trust (Coasts and Clean Seas) Project No 25425 December 2004, Canberra.
Oades, J. M. (1988). The retention of organic matter in soils. Biogeochemistry, 5, 35–70.
Oberdorster, E., & McClellan-Green, P. (2002). Mechanisms of imposex induction in the mud snail, Ilyanassa obsoleta: TBT as a neurotoxin and aromatase inhibitor. Marine Environmental Research, 54, 715–718.
Packham, D., Tapper, N., Griepsma, D., Friedli, H., Hellings, J., & Harris, S. (2009). Release of mercury in the Australian environment by burning: A preliminary investigation of biomatter and soils. Air Quality and Climate Change, 43, 24–27.
Phillips, D. J. H. (1977). The use of biological indicator organisms to monitor metal pollution in marine and estuarine environments-a review. Environmental Pollution, 13, 281–311.
Pipe, R. K., Coles, A., Carissan, F. M. M., & Ramanathan, K. (1999). Copper induced immunomodulation in the marine mussel, Mytilus edulis. Aquatic Toxicology, 46, 43–54.
Rhoads, D. C., & Boyer, L. F. (1982). The effects of marine benthos on physical properties of sediments: A successional perspective. In P. L. McCall & M. J. S. Tevesz (Eds.), Animal-sediment relations. New York: Plenum Press.
Robinson, W. A., Maher, W. A., Krikowa, F., Nell, J. A., & Hand, R. (2005). The use of the oyster Saccostrea glomerata as a biomonitor of metal contamination: Intra-sample, local scale and temporal variability and its implications for biomonitoring. Journal of Environmental Monitoring, 7, 208–223.
Sanudo-Wilhelmy, S. A., & Flegalt, A. R. (1992). Anthropogenic silver in the Southern California Bight: A new tracer of sewage in coastal waters. Environmental Science and Technology, 26, 2147–2151.
Scanes, P. R., & Roach, A. C. (1999). Determining natural ‘background’ concentrations of metals in oysters from New South Wales, Australia. Environmental Pollution, 105, 437–446.
Showalter, S., & Savarese, J. (2004). Restrictions on the use of marine antifouling paints containing tributyltin and copper. CA: California Sea Grant Extension Program.
Sim, V. X. Y., Dafforn, K. A., Simpson, S. L., Kelaher, B. P., & Johnston, E. L. (2015). Sediment contaminants and infauna associated with recreational boating structures in a multi-use marine park. PLoS ONE, 10, 1–15.
Singh, N., & Turner, A. (2009). Metals in antifouling paint particles and their heterogeneous contamination of coastal sediments. Marine Pollution Bulletin, 58, 559–564.
Smith, P. J., & McVeagh, M. (1991). Widespread organotin pollution in New Zealand coastal waters as indicated by imposex in dogwhelks. Marine Pollution Bulletin, 22, 409–413.
Spooner, D., Maher, W., & Otway, N. (2003). Metal concentrations in sediments and oysters of Botany Bay, Australia. Archives of Environmental Contamination and Toxicology, 45, 92–101.
Tanabe, S. (1999). Butyltin contamination in marine mammals: A Review. Marine Pollution Bulletin, 39, 62–72.
Taylor, A., & Maher, W. (2003). The use of two marine gastropods, Austrocochlea constricta and Bembicium auratum as biomonitors of zinc, cadmium and copper exposure: Effects of mass, within and between site variability and net accumulation relative to environmental exposure. Journal of Coastal Research Progress Series, 19, 541–549.
Telford, K., Maher, W., Krikowa, F., & Foster, S. (2008). Measurement of total antimony and antimony species in mine contaminated soils by ICPMS and HPLC-ICPMS. Journal of Environmental Monitoring, 10, 136–140.
Tessier, A., & Campbell, P. G. C. (1987). Partitioning of trace metals in sediments: Relationships with bioavailability. In R. L. Thomas, R. Evans, A. L. Hamilton, M. Munawar, T. B. Reynoldson, & M. H. Sadar (Eds.), Ecological effects of in situ sediment contaminants. Developments in hydrobiology (Vol. 39). Dordrecht: Springer.
Tombacz, E. (2004). The role of reactive surface sites and complexation by humic acids in the interaction of clay minerals and iron oxide particles. Organic Geochemistry, 35, 257.
Turner, A. (1996). Trace-metal partitioning in estuaries: Importance of salinity and particle concentration. Marine Chemistry, 54, 27–39.
Turner, A. (2010). Marine pollution from antifouling paint particles. Marine Pollution Bulletin, 60, 159–171.
Turner, A., Millward, G. E., Schuchardt, B., Schirmer, M., & Prange, A. (1992). Trace metal distribution coefficients in the Weser estuary (Germany). Continental Shelf Research, 12, 1277–1292.
Ubrihien, R., Taylor, A. M., & Maher, W. A. (2016). Bioaccumulation, oxidative stress and cellular damage in the intertidal gastropod Bembicium namum exposed to a metal contamination gradient. Marine & Freshwater Research, 67, 1–9.
Underwood, A. C., & Chapman, M. G. (1995). Coastal marine ecology of temperate, Australia (pp. 1–341). Kensington: UNSW Press.
Vogel, C., Kruger, O., Herzel, H., & Adam, C. (2016). Chemical state of mercury and selenium in sewage sludge ash-based P fertilizers. Journal of Hazardous Materials, 313, 179–184.
Voulvoulis, N., Scrimshaw, N. D., & Lester, J. N. (2002). Comparative environmental assessment of biocides used in antifouling paints. Chemosphere, 47, 789–795.
Walsh, K., Dunstan, R. H., Murdoch, R. N., Conroy, B. A., Roberts, T. K., & Lake, P. (1994). Bioaccumulation of pollutants and changes in population parameters in the gastropod mollusk Austrocochlea constricta. Archives of Environmental Contamination and Toxicology, 26, 367–373.
Wang, W., & Fisher, N. S. (1999). Delineating metal accumulation pathways for marine invertebrates. The Science of the Total Environment, 237(238), 459–472.
Wang, X., Wang, J., & Zhang, J. (2012). Comparisons of three methods for organic and inorganic carbon in calcareous soils of Northwestern China. PLoS ONE, 7, e44334.
Waring, J., Maher, W. A., & Krikowa, F. (2006). Trace metal bioaccumulation in eight common Australian polychaeta. Journal of Environmental Monitoring, 8, 1149–1157.
Warnken, J., Dunn, R. J. K., & Teasdale, P. R. (2004). Investigation of recreational boats as a source of copper at anchorage sites using time-integrated diffusive gradients in thin film and sediment measurements. Marine Pollution Bulletin, 49, 833–843.
Wood, M. A. (1983). Available copper ligands and the apparent bioavailability of copper to natural phytoplankton assemblages. Science of the Total Environment, 28, 51–64.
Zhuang, J., & Yu, G.-R. (2002). Effects of surface coatings on electrochemical properties and contaminant sorption of clay minerals. Chemosphere, 49, 619–628.
Zwolsman, J. J. G., Eck, B. T. M., & Van der Weijden, C. H. (1997). Geochemistry of dissolved metals in the Scheldt estuary, southwest Netherlands: Impact of seasonal variability. Geochimica et Cosmochimica Acta, 61, 1635–1652.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McVay, I.R., Maher, W.A., Krikowa, F. et al. Metal concentrations in waters, sediments and biota of the far south-east coast of New South Wales, Australia, with an emphasis on Sn, Cu and Zn used as marine antifoulant agents. Environ Geochem Health 41, 1351–1367 (2019). https://doi.org/10.1007/s10653-018-0215-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0215-8