Abstract
The dissolved labile and labile particulate fractions (LPF) of Cu and Zn were analyzed during different seasons and salinity conditions in estuarine waters of marina, port, and shipyard areas in the southern region of the Patos Lagoon (RS, Brazil). The dissolved labile concentration was determined using the diffusive gradients in thin films technique (DGT). DGT devices were deployed in seven locations of the estuary for 72 h and the physicochemical parameters were also measured. The LPF of Cu and Zn was determined by daily filtering of water samples. Seasonal variation of DGT–Cu concentrations was only significant (p < 0.05) at one shipyard area, while DGT–Zn was significant (p < 0.05) in every locations. The LPF of Cu and Zn concentrations demonstrated seasonal and spatial variability in all locations, mainly at shipyard areas during high salinity conditions. In general, except the control location, the sampling locations showed mean variations of 0.11–0.45 μg L−1 for DGT–Cu, 0.89–9.96 μg L−1 for DGT–Zn, 0.65–3.69 μg g−1 for LPF–Cu, and 1.35–10.87 μg g−1 for LPF–Zn. Shipyard areas demonstrated the most expressive values of labile Cu and Zn in both fractions. Strong relationship between DGT–Zn and LPF–Zn was found suggesting that the DGT–Zn fraction originates from the suspended particulate matter. Water salinity and suspended particulate matter content indicated their importance for the control of the labile concentrations of Cu and Zn in the water column. These parameters must be taken into consideration for comparison among labile metals in estuaries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are affected by physical and biogeochemical processes and human activity (Salomons and Föstner 1984). These areas are also associated with boat and shipping activities, which contribute to environmental metal loadings, in particular, antifouling paints (Schiff et al. 2004).
Antifouling paints prevent biofouling, which is a problem for shipping industries (Dafforn et al. 2011). The growth of organisms on the hulls of vessels increases the frictional drag, which reduces speed and increases fuel consumption (Abbot et al. 2000).
Antifouling paints have a combination of biocides and solvents (Okamura and Mieno 2006). In 1970, paints were formulated containing organic tin, such as tributyltin (TBT) and triphenyltin (Konstantinou and Albanis 2006). However, these compounds are highly toxic to nontarget species, such as bivalves and gastropods (Alzieu 2000). After the ban on TBT in 2008 (IMO 2001), Cu became the predominant biocide in antifouling paints (Readman 2006).
Studies have shown the release of Cu and Zn into seawater from the hulls of vessels (Valkirs et al. 2003; Schiff et al. 2007; Turner et al. 2008; Brooks and Waldock 2009), i.e., stemming from waste paint at shipyards (Singh and Turner 2009). Cu and Zn mobilized from fine antifouling particulates may represent an important local source of contamination of ground water, runoff, soil, and marine systems (Turner 2010; Jessop and Turner 2011).
Researchers have also reported levels of labile Cu in water at marinas and port areas (Webb and Keough 2002; Warnken et al. 2004; Dunn et al. 2007; Jones and Bolam 2007) but only one study which was carried out near of shipyard areas has been published (Montero et al. 2012).
Areas with boat traffic and maintenance of boats have been important sources of copper and zinc release. Despite being essential for many organisms, these elements may become toxic when their concentrations are above the necessary values for their physiology (Matthiessen et al. 1999).
Besides the normal sediment remobilization and redistribution in estuarine areas, this process is increased during boating activity and dreading to maintain the channel. As a result, the suspended particulate matter may enable good approach of exchange of constituents between sediment and water, and may carry large amount of pollutants. The dynamic changes in pH and salinity in overlying water can also dramatically influence the release of labile trace elements from estuarine sediments (Hong et al. 2011). Thus, the estimation of the labile fraction in water and in suspended particulate matter is more relevant because these fractions can be exchanged or incorporated by organisms (Leeuwen et al. 2005).
Potentially bioavailable dissolved labile metals can be determined using the technique of diffusive gradients in thin films (DGT), which integrates the metal concentration of the medium over time (Davison and Zhang 1994). The DGT system is designed according to Fick’s first law, with a filter of 0.45 μm in porosity through which metal ions are transported by molecular diffusion to a polyacrylamide hydrogel and are accumulated in the ion exchange resin of a DGT (Zhang and Davison 1995).
Studies carried out in estuarine areas using the DGT technique (Dunn et al. 2003; 2007; Wallner-Kersanach et al. 2009; Montero et al. 2012) have demonstrated the sensitivity of the system to accumulate labile metals. However, nothing has been demonstrated about seasonal variation in one location.
In the Patos lagoon estuary (RS, Brazil), there have been studies of total trace metal concentrations in water and suspended particulate matter (Niencheski et al. 1994; Windom et al. 1999; Niencheski and Baumgarten 2000), in bottom sediments (Baisch and Wasserman 1998; Niencheski et al. 2002), and in the biota (Baraj et al. 2003). All of them indicated low levels of metals in accordance with the national recommendations issued by Conselho Nacional do Meio Ambiente (CONAMA) 357 (2005) and CONAMA 344 (2004). However, the labile fraction analyzed in the water column is not well known. In two estuarine locations adjacent to the Rio Grande city, the labile fractions of metals were preliminarily analyzed using the DGT technique, and the results indicated an increase of the concentrations of metals during high-salinity periods (Wallner-Kersanach et al. 2009).
In Rio Grande city, there are three port areas for vessel mooring: the Porto Velho, for leisure boats as well as fishing and passenger vessels; the Porto Novo; and the Super Porto, for large vessels (30–40 m). There are also two shipyards for boat maintenance, and during the study, the Rio Grande Shipyard was built, the largest dry dock in Latin America, with 440,000 m2 of built area, for oil platform construction and maintenance. The construction of two more dry docks in the estuarine area near the city has been planned.
Because of the impact of antifouling paints and their gradual release into the water (Srinivasan and Swain 2007; Turner et al. 2008), the paints used in the shipyards of Rio Grande were analyzed. The dominance of Cu and Zn was confirmed based on percentages, while the other elements were found in parts per million concentrations. The data agreed with those provided for the paint brands studied by Valkirs et al. (2003), Paradas and Amado Filho (2007), Karlsson et al. (2010), and Parks et al. (2010).
The study of labile fractions of Cu and Zn in marinas and shipping activity areas in Rio Grande is important to determine the actual concentrations of those elements in the region and to support data comparison after the operation of the shipyards. The aim of the present study was to determine the DGT–labile and labile particulate concentrations of Cu and Zn in waters of marina, port, and shipyard areas under the influence of the antifouling paints of vessels. The results also aid to understand the dynamics of labile metals during seasonal periods and changes in salinity in such estuarine environment. This study is the first one to investigate the variations of labile metals during seasonal and intense El Niño effect periods in estuarine areas.
Materials and methods
Study area
The Patos Lagoon (10,360 km2), in Southern Brazil, is the largest choked coastal lagoon in the world (Kjerfve 1986) and has a narrow channel that restricts the tidal influence (Möller et al. 1996). The lagoon has the geomorphologic features of an estuary with a low tidal range (0.47 m), an average depth of 6 m, and a complex hydrodynamics due to winds and river discharge (Möller et al. 1996). The only contact with the Atlantic Ocean is through a narrow channel at the southern end of the lagoon.
Freshwater has a long residence time in the lagoon during winter (June–August) and during summer (January to March) the salt–water intrusion can reach up to 200 km. The residence time depends more on weather conditions than on the tide (Niencheski et al. 1994). At times, under the effects of the El Niño or La Niña phenomenon, there is a period of intense rainfall or prolonged drought, respectively, in the region. As a result, there is a prolonged permanence of fresh or saltwater within the estuary (Garcia 1998).
The main forcing is the NE–SW wind regime, which controls the salinity, circulation, and sea level (Garcia 1998). However, because of its shallow depth and low tidal range, the water of the Patos Lagoon is vertically well mixed (Niencheski et al. 1994).
Rio Grande city (32°02′06″ S and 52°05′55″ W) has an industrial park containing fertilizers, vegetable oil, and petrochemical products (Niencheski and Windom 1994). There has been an expansion of the port area, especially with the installation of a shipbuilding center, which is the largest in Latin America.
DGT system and in situ deployment
The plastic holders of the DGT devices were obtained from DGT Research Ltd, Lancaster (UK) and mounted with diffusive (0.8 mm thick) and resin hydrogel (0.4 mm thick). The polyacrylamide hydrogels were prepared according to Zhang and Davison (1995, 2004). After preparation, the DGT devices were tested in the laboratory and Cd concentrations were measured according to the DGT Research (2004) protocol. The results obtained in the experimental waters (duplicate) with Cd were 10.47 ± 0.23 and 9.84 ± 1.02 μg L−1. The recovery in the DGT resin for Cd was 90 and 103 %.
The sampling stations, shipyard areas, port, and mooring sites of recreational vessels were chosen based on the number of moored boats and maintenance and repair facilities.
The two oldest and more active shipyards in the region, the Gustavo Fernandes Filho LTDA Shipyard and the Santos Shipyard, receive boats during all of the year, especially between summer (December) and autumn (May), with sizes ranging from 10 to 27 m.
The Rio Grande Yacht Club has a mooring area for leisure boats, whose number usually keeps constant during the year. Boat maintenance happens mostly from early spring (September) to early summer (December), although it is also carried out along all year. No control of the disposal of antifouling paint particles generated during the maintenance has been carried out in both shipyards and in the yacht club.
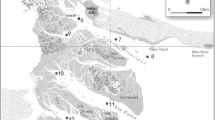
The DGT devices were deployed in situ at seven selected locations in the estuary, where there is not any input of wastewater sources. The yacht club, Gustavo Shipyard, and Porto Novo are located about 0.5 km from urban wastewater outflows, whereas the Porto Novo and Santos Shipyard are located about 9–12 km from the industrial area, which, in general, does not generate wastewater discharge into the estuary (Fig. 1, Table 1).
The DGT devices were deployed in situ in January, April, and July 2010 on the same day at all of the locations. Four devices were fixed to the pier of each location at a 0.5 m depth over the total deployment time of 72 h.
Water samples were collected daily at each location using a Niskin bottle, at the same depth as the DGT devices, for the determination of physicochemical parameters, including pH (pH meter by Toledo, Model DM), temperature, salinity (conductivity with WTW brand, Model 315i), and suspended particulate matter (SPM). Water samples were taken for an analysis of the labile particulate fraction (LPF) of metals (Cu and Zn).
During each deployment period, two DGT devices were randomly selected as a control, i.e., not deployed in the water column. The DGT average of the method detection limits for January, April, and July were 0.041 μg L−1 for Cu and 0.12 μg L−1 for Zn.
Laboratory procedure
In the laboratory, resin gels (Chelex-100) for each of the DGT devices were eluted in 1 ml of nitric acid Suprapur ® (Merck), as described by Zhang and Davison (1995) and kept under refrigeration (4 °C) until analysis.
The labile particulate fraction of Cu and Zn (LPF–Cu and LPF–Cu) were determined by daily filtering duplicate water samples with cellulose nitrate filters (porosity of 0.45 μm with 47 mm diameter; Sartorius, Germany). The filters with the particulate material were washed with ultrapure water (Milli-Q) for the reduction of chlorides (Kremling et al. 1996), dried in an oven at 60 °C, and treated by partial acid digestion with 0.1 M HCl Suprapur ® (Merck; adapted from Li et al. 2009). The 0.1 M HCl leachate, which affects the silicate matrix minimally, is a best approach to obtain the “easy exchangeable” metal fraction (Fiszman et al 1984; Nolting et al. 1996), because it releases nonresidual trace elements, i.e., those associated with hydrous Fe–Mn oxides, adsorbed on clays, besides amorphous oxides and carbonates, and organic matter, all closely associated with the summed concentrations released in three steps in a sequential extraction procedure (Sutherland 2002). Thus, this leach technique applied to the suspended particulate matter in water is the best approach to obtain easy labile metals, which may also be presumed available to the DGT system.
In this procedure, the three filters of each duplicate of water samples collected during the 72 h were combined to be representative for the DGT sampler. The analysis of dissolved organic carbon (DOC) followed the method described by Spyres et al. (2000). Samples were acidified with HCl which converted all inorganic carbon species to carbon dioxide and analyzed using an Elementar total organic carbon (TOC) analyzer (Shimatzu Brand, Model TOC V-Series).
An analysis of particulate organic carbon (POC) was performed according to Ehrhardt and Koeve (1999). Samples were previously fumed with HCl to remove the carbonate fraction and analyzed using an Elementar CHNS/O analyzer (Series 2400; Perkin Elmer).
Each Chelex-100 resin gel, i.e., the DGT-labile fraction (DGT), was analyzed for Cu and Zn using graphite furnace atomic absorption spectrometry (Zeiss, Model 5EA). The calculations of the DGT concentrations followed Zhang and Davison (1995). The quality control for the analysis of the DGT was conducted using river water from the National Research Council of Canada, a SLRS-4 certified reference material. The recovery was expressed as percentages of the certified values. The mean values for the in situ deployment period in January, April, and July 2010 were 96 % for Cu and 102 % for Zn. The metal detection limits (3σ) were 0.03 and 0.06 μg L−1 for Cu and Zn, respectively.
The accuracy of the method for the LPF was conducted spiking different aliquots of samples (n = 3) with three known concentrations of copper and zinc. The recovery variation of the applied method was between ±10 % for both metals (Harris 2007). The detection limits (3σ) analyzed with an inductively coupled plasma optical emission spectrometer (Perkin Elmer, Model Optima 2100 DV) were 0.05 and 0.13 μg g−1 for Cu and Zn, respectively. All of the material used for the analysis of trace metals and TOC was previously washed with a 20 % solution (v/v) of HNO3 and HCl pa ® (Merck), respectively.
For statistical analysis, the assumptions of an analysis of variance, such as normality and homogeneity, were verified. To meet the assumptions, the results of the DGT–Zn were transformed into log (Zn + 1). A two-factor analysis of variance was used to identify significant differences between the DGT–Cu and DGT–Zn among the different seasons at a significance level of 5 % (Zar 2010). After the establishment of a difference, a post hoc Tukey test was performed. The data of LPF–Cu and LPF–Zn met the assumptions and no transformation was necessary. To verify the relation of DGT–Cu and DGT–Zn and LPF–Cu and LPF–Zn between different physicochemical parameters analyzed at the end of the DGT device deployments of the DGT devices, a Pearson correlation test was applied for the different seasons, using a significance level of 5 %. A linear regression was applied in order to verify possible relationship between DGT–Cu and DGT–Zn with LPF–Cu and LPF–Zn at a 5 % significance level.
Results and discussion
Physicochemical parameters of estuarine water
The physicochemical parameters did not show variations during the DGT deployment period; thus, results were only presented at the end of the DGT device deployments at different locations (Table 2). The parameters only indicated variations among stations and seasons during the DGT device deployment. The influence of freshwater was dominant in January 2010 at all of the sampling stations in the estuary. The pH values, even at low salinities, were relatively high due to phytoplankton blooms, which are common in summer. The pH values in this period were higher at marinas and at the control station Justino. The same pattern of salinity and pH was found by Jones and Bolam (2007) in marinas, ports, and estuaries in the UK. The highest value of pH at Justino was due to phytoplankton blooms, which were denser than in other places, because this location is a more sheltered area. This difference was evidenced by the POC and DOC concentrations at Justino.
The SPM loads were generally higher in January than in other periods. The highest SPM content at the Santos Shipyard location in January was because that site’s location on the shore of the estuary channel and with high freshwater discharge influenced by an intense El Niño effect. This phenomenon ended in late January 2010 but was continuous during the previous 6 months.
At Porto Novo in April, the SPM was also higher because of sediment dredging in the area during the water sampling. However, the SPM concentrations showed an average below 50 mg L−1 (Table 2), which is within the concentration range considered normal for the Patos Lagoon estuary (Niencheski and Windom 1994).
In April, with a low salinity and an ebb tide regime in the estuary, the pH remained similar at all of the sampling stations, except for Justino, because of the high decomposition of organic matter resulting from the increased levels of POC and DOC in the water and intensified by the lack of rain. Low concentrations of DOC and high concentrations of POC were found at Porto Novo where sediment dredging also occurred and thus increased the SPM concentrations.
In July, the salinity in the estuary was high, except at Justino, because slow water renewal occurred, there were low SPM and high DOC concentrations in the water. These results are comparable with those from a river estuary in Japan, Arakawa, where high concentrations of POC and SPM were found close to the estuary mouth and where particulate organic matter was coagulated from dissolved organic matter with the mixture of fresh- and saltwater (Suzumura et al. 2004). The concentrations were more affected by the sampling location and its features than by salinity. In the Patos Lagoon estuary, the salinity effect is influenced not only by the wind but also by the rainfall intensity, especially under the influence of major events, such as El Niño.
DGT-labile concentrations of Cu and Zn
The DGT-labile concentrations of Cu and Zn (DGT–Cu and DGT–Zn) in water, analyzed at the seven sampling stations indicated significant (p < 0.05) variations among locations. No significant seasonal changes (p > 0.05) of DGT–Cu were observed in every location under study, except the Santos Shipyard site (Fig. 2). However, seasonal variations of DGT–Zn levels in water were significant (p < 0.05) in every location.
DGT-labile concentrations (in microgram per liter) of copper and zinc in January (black bar), April (light gray bar), and July (dark gray bar) 2010 after 72 h of deployment at Justino (JT control station), the yacht club (Y1, Y2, and Y3), Gustavo Shipyard (GS), Porto Novo of Rio Grande city (PN), and Santos Shipyard (SS). DGT data are represented by the mean ± SE, n = 3–4
January was an atypical period because the estuary was under the El Niño effect. The Gustavo Shipyard locations presented the highest DGT–Cu by comparison with other locations. DGT–Cu was higher at the Gustavo Shipyard site, probably reflecting the high presence of boats during the summer because this area has a boat fueling station. However, the shipyard operation in boat maintenance at the Gustavo Shipyard site was scarce during all the study period.
The Santos Shipyard sampling station also had high concentrations of DGT–Cu in January, but the DGT–Zn content in this location was almost as low as the one at the Justino site. Low salinity, high freshwater discharge, and high SPM (134 mg L−1) in water at the Santos Shipyard location during this period favored the decrease in DGT–Zn levels, but led to an increase in Zn particulate fraction in water (Fig. 3), because the El Niño effect changes the water characteristics in the Patos Lagoon estuary.
Labile particulate concentrations (in microgram per liter) of copper and zinc in January (black bar), April (light gray bar), and July (dark gray bar) of 2010 at Justino (JT control station), the yacht club (Y1, Y2, and Y3), Gustavo Shipyard (GS), Porto Novo of Rio Grande city (PN), and Santos Shipyard (SS). Data are represented by the mean values
The DGT–Cu and DGT–Zn were also significantly lower (p < 0.05) at the marina areas when compared to the Santos Shipyard site (Fig. 2). Yacht club-Y1 is inside the inlet and close to the boat maintenance facilities, and there is no mooring dock (Fig. 1). The DGT–Cu level showed a high variability in January because boat repair activity was more frequent.
The location Porto Novo showed DGT–Cu similar to the Justino in all seasonal periods, probably because ship normally have a short stay in the port and taking also account the higher water hydrodynamic of the port channel.
The low salinity during April did not change the concentrations of DGT–Cu in water but DGT–Zn indicated more differences at the mooring docks of the yacht club. The concentrations at Y1 showed significantly (p < 0.05) different DGT–Zn compared with the Justino in January and April. In April, the conditions of ebb tide and low salinity and the high number of boats moored to the dock may have favored the increased availability of DGT–Zn in the water, with significant differences (p < 0.05) from the Justino. The sources of Zn in the water inside the marina inlet can be associated with antifouling paints, oil waste from recreational boats, and quantity of boats. Zn sacrificial anodes are used on the hulls of vessels to prevent corrosion of the engine and rudder. Warnken et al. (2004) also mentioned the use of Zn in sacrificial anodes. However, there are no studies indicating the release of Zn in water from this source, and the release may be more localized. On the contrary, Cu release from antifouling paints directly from the hulls of vessels has been already observed (Valkirs et al. 2003).
In July, because the high water salinity, more significant environmental changes in the labile metal fraction were observed. The DGT–Cu and DGT–Zn in water was significant higher (p < 0.05) at the Santos Shipyard (0.45 and 9.96 μg L−1, respectively). The DGT–Zn at Justino during all of the seasonal periods was differed significantly from the Santos Shipyard location in April and July. The location Santos Shipyard had a great number of boats and they were moored and repaired during July, even having its largest repair activities in summer and autumn and presumably, remnants of paint waste from boat scraping and washing came into contact with water. Analyses of paint fragments revealed concentrations of Cu and Zn above 35 and 15 %, respectively, equivalent to Cu2O and ZnO of approximately 40 and 20 %, respectively (Turner 2010).
The high concentration of both metals at Santos Shipyard was related not only to the shipyard activity but primarily to the hydrological condition of the periods. Compared to the other locations, the salinity remained high in July at Santos Shipyard, as did the concentrations of POC and SPM (Table 2), showing a moderate correlations only with the DGT–Cu (Table 3). During periods of high salinity, the DOC values decreased, indicating a strong negative correlation (r = −0.80) with salinity. Correlations of salinity with pH (r = 0.98) and SPM (r = 0.75) were very significant (p < 0.05), demonstrating the importance of the entrance of salt water into the estuary and the remobilization of bottom sediment from the shallow areas of the estuary, as observed by Niencheski and Baumgarten (2000). This process normally increases labile metals in water in shallow areas (Wallner-Kersanach et al. 2009).
The sediment remobilization certainly contributed to an increase of the DGT–Cu and DGT–Zn available in the area near the estuary edge of the shipyards (Fig. 2) because of the contaminated sediment (Costa, unpublished data). The study area is a lagoon, in which the salinity is governed by the wind direction and intensity. Thus, sediments can be remobilized and promote the availability of metals affected by changes in pH and salinity (Hong et al. 2011). In estuarine environments, salinity is one of the major factors controlling the distribution of metals between dissolved and particulate phases (Hatje et al. 2003) and the metal speciation; as Dunn et al. (2007) suggested that changes in DGT–Cu are strongly influenced by the tidal changes, i.e., Cu, Zn, and Ni increase during the flood tidal phases.
In July, since boat maintenance activity decreased, the DGT–Cu also decreased at the Y1 location. As the locations Y2 and Y3 have mooring docks, DGT–Cu was higher in July because the presence of boats in the area. A higher number of boats often increase the dissolved total and labile fractions of Cu in water in confined places (Jones and Bolam 2007).
No significant correlation (p > 0.05) between the DGT–Cu and DGT–Zn during the different sampling periods was observed, except in July (r = 0.56) indicating that the metals might have the same anthropogenic source (Table 3).
In general, except the Justino site, the sampling locations indicated concentration variations of 0.11–0.45 μg L−1 for Cu and 0.89–9.96 μg L−1 for DGT–Zn. The results suggest that Cu and Zn are likely to be important contaminants, at least in marinas and shipyards, since they are not only used in antifouling paint formulation but Zn is also used for controlling the rate of coating erosion in many formulations and is often a booster in the form of zinc pyrithone (Turner et al. 2008).
Comparing values of labile metals regarding water salinity is an ideal but difficult task, because of the lack of data in few studies which have already been published. The DGT–Cu found by Wallner-Kersanach et al. (2009) during high salinity, at two separate locations in the Patos Lagoon estuary, were lower than the values observed at most locations in this study. Likewise, Warnken et al. (2004) have investigated DGT–Cu at marinas in Moreton Bay and Gold Coast Broadwater (Australia), a subtropical lagoon, with greater numbers and size of leisure boats. The authors found DGT–Cu values comparable to those at the Gustavo Shipyard location in January (0.41 μg L−1) and Santos Shipyard location in July (0.45 μg L−1).
The DGT–Cu and DGT–Zn observed by Dunn et al. (2007) in the same subtropical lagoon during flood periods with moored leisure boats were comparable to the levels found in July (high salinity) at Y2 for Cu (0.22 μg L−1) and Y1 for Zn (3.84 μg L−1) in this study. The highest concentrations of DGT–Zn found at the Santos Shipyard location (9.96 and 5.35 μg L−1, respectively) was similar to results found in some marinas with large numbers of boats in Australia (Warnken et al. 2004). Although the number of boats in the yacht club was lower than other major areas of leisure boats, the importance of Cu and Zn inputs from boating sources, such as through leaching of these metals used in fouling paints, cannot be dismissed.
The DGT–Cu at the Gustavo Shipyard site in the period of freshwater in the estuary (January) showed comparable value found in shipyard areas with freshwater in Spain (Montero et al. 2012). The shipyards of Patos Lagoon estuary most likely had a localized impact. However, the impact is minimized, since the shipyards are located in areas of complex hydrodynamics, which promote a dispersion of contamination, especially during extreme water changes in the estuary.
Understanding the causes of seasonal variability in trace metal concentrations in estuaries can be complicated by a potential controlling factor (Hatje et al. 2003). However, in this study, the use of the DGT technique to quantify labile Cu and Zn clearly indicated the capacity of the device for detecting changes in metal speciation due to changes in the salinity and environmental parameters in each season period at the study locations.
Labile particulate concentrations of Cu and Zn
The mean labile particulate fraction of Cu and Zn (LPF–Cu and LPF–Zn) in the water showed differences in seasonal and spatial variability in seven locations of the estuary (Fig. 3). The study areas had LPF–Cu and LPF–Zn higher concentrations than the ones found at Justino in all periods under study; they were also higher in January at all sampling stations than in April and July, except at the shipyard sites.
In January, there were phytoplankton blooms around the banks of the estuary, which are common during this time of the year, indicated by increased levels of DOC and POC in the studied periods, especially at the Justino (Table 2). High variations in the concentrations of SPM between the locations were observed, resulting from the high freshwater discharge caused by El Niño effects.
The LPF–Cu and LPF–Zn in January was higher inside of the yacht club marina (Y1, Y2, and Y3) and Santos Shipyard. The activity of recreational boats is intense in this period, promoting the remobilization of bottom sediment, mainly inside the inlet (Y1), indicated by the SPM value of 73 mg L−1 (Table 2).
The high SPM (134 mg L−1) in January at Santos Shipyard, situated in the narrow channel of the estuary, may have contributed to the increased LPF–Cu and LPF–Zn. Strong currents of freshwater outflow in this period may have contributed to the increase of the SPM in water, indicating a strong correlation (p < 0.05) between both two forms of carbon, DOC and POC (r = 0.77; Table 4). Not only phytoplankton blooms but also organic-rich fluvial material contributed to this increase.
In April, the mean LPF–Cu and LPF–Zn were low at all of the sampling stations. This period was characterized by a lack of rain, low salinity in the estuary. There were no differences in concentrations of both metals at the marina of the yacht club, although many boats were moored in this period. The highest concentrations of both metals were found at the Gustavo Shipyard and Santos Shipyard locations. In general, a trend can be evidenced under low salinity condition in April: there is a moderate correlation between the LPF–Cu and the pH, and between LPF–Cu and LPF–Zn and the salinity and the SPM (Table 4). Salinity and pH were highly correlated (r = 0.91, p < 0.05) because of the influence of saltwater in the estuary.
According to Windom et al. (1999) in salinity ranges from zero to c. 5–7 particle removal and high primary production, flocculation and particle scavenging is likely to occur at the Patos Lagoon estuary. Thus, the low LPF–Cu and LPF–Zn concentrations in April do appear to be influenced by desorption and removal processes at the low salinity. On the contrary, an increase of the labile metals were found in this period and more evident for zinc concentrations (Fig. 4).
DGT-labile (black bar) and labile particulate (hatched bar) concentrations of copper and zinc (Zn/10) in January, April, and July at Justino (JT control station), the yacht club (Y1, Y2, and Y3), Gustavo Shipyard (GS), Porto Novo of Rio Grande city (PN), and Santos Shipyard (SS). Data are represented by mean values (in microgram per liter) for both fractions
In July, the LPF–Cu showed many variations at all of locations, except between the yacht club Y1 and Y3 (Fig. 3). As the estuary had a flood tide and high salinity, there may have been greater availability of particulate labile Cu and Zn in water, mainly at both shipyards. The Gustavo Shipyard and Santos Shipyard of boat maintenance areas showed the highest mean LPF–Cu, with levels of 3.69 and 3.36 μg g−1, respectively. The same contributions at the shipyards was verified with high LPF–Zn at the Santos and Gustavo Shipyard sites (10.87 and 7.43 μg g−1, respectively), followed by the levels at the Porto Novo site (4.80 μg g−1; Fig. 3) and the SPM content was higher during this period at these sites compared to most of the sites studied (Table 2).
All seasonal periods showed strong correlation between the LPF–Cu and LPF–Zn (Table 4), indicating strong similarities in the chemical behavior of these metal phases. However, the saltwater in the estuary indicated a different pattern of the LPF–Cu and LPF–Zn correlation with the physicochemical parameters by comparison with the freshwater in the estuary, and very common for the SPM concentrations. While freshwater influenced in the estuary, the LPF–Cu and LPF–Zn were moderately correlated with the SPM concentration in water, but in low and high salinity, the correlation became stronger (Table 4), confirming the release of metals in conjunction with the increase of salinity and SPM in water. The higher the salinity, more SPM will be found (Niencheski et al. 1994; Turner 1996; Lores and Pennock 1998), since tidal induce resuspension processes cause temporary changes in the particulate trace metal concentrations (Millward et al. 1998).
In general, except at the control location, the sampling locations indicated concentration variations of 0.65–3.69 μg g−1 for LPF–Cu and 1.35–10.87 μg g−1 for LPF–Zn.
A study conducted by Kremling et al. (1996) and Sokolowski et al. (2001) in the Baltic Sea, indicated LPF–Cu and LPF–Zn higher than those found in the Patos Lagoon estuary. The environmental characteristics and anthropogenic input type at each location in addition to the distinct concentration of acid leaching used for the samples and the scarcity of studies of labile particulate Cu and Zn in estuaries makes it difficult to compare data.
At the Santos Shipyard site, due to its continued boat repair activity, there were high labile concentrations of Cu and Zn for both of the fractions studied. The Gustavo Shipyard site, although an older shipyard with reduced activity but an active boat fueling station, also showed high levels of LPF–Cu and LPF–Zn, most likely because of bottom sediment resuspension.
The process of sediment resuspension is dependent on the hydrodynamics of the Patos Lagoon estuary, given the input of saltwater and pH changes, providing the water with metal particles. The distribution of total particulate metal (total acid digestion) verified by Niencheski and Baumgarten (2000) in the Patos Lagoon, resulted in minimum concentrations of Cu and Zn in the range of labile particulates found at the marinas, ports, and shipyards investigated in this study. This result indicates that these anthropogenic input activities enrich the SPM with metals, such as Cu and Zn.
The normalization of the results by the concentration of SPM resulted in no significant relationship between DGT–Cu and LPF–Cu, but DGT–Zn concentrations indicated a strong relationship (R 2 = 0.733) with LPF–Zn, considering all sampling period. In the case of periods with saltwater in the estuary (April and July), a very strong relationship (R 2 = 0.940) was achieved for DGT–Zn and LPF–Zn; it shows that the DGT–Zn fraction originates from the SPM (Fig. 5). It occurs because Zn has more affinity for the particulate phase in water that Cu, as reported by some authors (Chiffoleau et al. 1994; Sokolowski et al. 2001) and may be released to the water column with changes in pH and salinity.
The use of the DGT technique and LPF analysis of both metals in water indicated that they make a useful method to evaluate the contribution and the degree of activities under the influence of antifouling paints. The evaluation showed that the estuary behaves differently across all study periods, depending on the location and the SPM content in the water, but the available Cu and Zn suspended particulate matter and even in the water column is evident; it confirms that the metals have a common anthropogenic input source, the antifouling paints. These results are consistent with the levels of the metals analyzed in the sediment of these areas (Costa, unpublished data).
Conclusion
This study investigated DGT-labile and the labile particulate concentrations of Cu and Zn in waters of marina, port, and shipyards areas under the influence of antifouling paints on vessels at the Patos Lagoon estuary. The dynamics of labile Cu and Zn concentrations during seasonal periods, changes in salinity and strong El Niňo effect was observed for the first time in estuarine areas. All study locations demonstrated contributions of antifouling paints to the water column, by comparison with an area without anthropogenic contributions and boat activity. Seasonal variations of Cu labile level of both DGT and suspended particulate matter were only apparent in areas with high amount of antifouling particles released in water such as shipyard areas. DGT-labile concentrations of Zn varied much more in different seasons and mainly in shipyards sites. A strong correlation between DGT-labile and labile particulate concentrations of Zn was found, suggesting that DGT-labile levels of Zn may originate from the suspended particulate matter. Aspects such as the salinity and SPM content of the estuarine water in this study indicated their importance for the control of the labile concentrations of Cu and Zn in the water column. Shipyard areas usually showed the highest concentrations of both fractions of labile Cu and Zn in water. The DGT-labile concentrations of Cu and Zn in these areas are comparable to levels found at shipyards and at marinas with a large number of recreational boats. The results presented herein for the yacht club marina indicated similarities with other marinas, although the marina is smaller and receives fewer boats. It is important to implement a greater control of areas regarding the disposal of antifouling paint waste, taking into account the future development of the region. The data from this study provide a basis for future studies that are needed because of the recent establishment of a large shipyard in the port area of Rio Grande city and the continuing development of another shipyard. Both shipyards have a dry dock for the construction and maintenance of oil platforms. These developments reinforce the need for a controlled final disposal of the waste generated by such activity. The DGT device is able to measure labile metal concentrations in a reproducible way, providing a representative average labile metal concentration in highly fluctuating system such as estuaries. Therefore, the use the DGT technique as routine monitoring networks in estuaries, or even data comparison among estuaries, should be accomplished with the use of parameters such as salinity and suspended particulate matter and knowledge of the estuarine hydrodynamics. In estuarine lagoons, the analysis of labile suspended particulate matter may provide useful data for more comprehensive understanding of the labile metal behavior in the water column.
References
Abbot, A., Abel, P. D., Arnold, D. W., & Milne, A. (2000). Cost-benefit analysis of the use of TBT: the case for a treatment approach. Science of the Total Environmental, 258, 5–19.
Alzieu, C. L. (2000). Environmental impact of TBT: the French experience. Science of the Total Environmental, 258, 99–102.
Baisch, P. R., & Wasserman, J. C. (1998). Chemistry and distribution of elements in the Patos Lagoon. In J. C. Wasserman, E. Silva-Filho, & R. Villa-Boas (Eds.), Environmental geochemistry in the tropics (pp. 97–126). New York: Springer.
Baraj, B., Niencheski, L. F., & Corradi, C. E. (2003). Trace metal content trends of mussel Perna perna (Linnaeus, 1758) from the Atlantic Coast of Southern Brazil. Water, Air, and Soil Pollution, 145, 205–214.
Brooks, S. J., & Waldock, M. (2009). Copper biocides in the marine environment. In T. Arai, H. Harino, M. Ohji & W. J. Langston (Eds.), Ecotoxicology of antifouling biocides. Springer: New York
Chiffoleau, J. F., Cossa, D., Auger, D., & Truquet, I. (1994). Trace metal distribution, partition and fluxes in the Seine estuary (France) in low discharge regime. Marine Chemistry, 47, 145–158.
CONAMA – @@Conselho Nacional do Meio Ambiente (2005). Resolução CONAMA 357. Brasília, DF.
CONAMA–Conselho Nacional do Meio Ambiente (2004). Resolução CONAMA 344. Brasília, DF.
Davison, W., & Zhang, H. (1994). In situ speciation measurement of trace components in natural waters using thin-film gels. Nature, 367, 546–548.
Dafforn, K. A., Lewis, J. A., & Johnston, E. L. (2011). Antifouling strategies: history and regulation, ecological impacts and mitigation. Marine Pollution Bulletin, 62, 453–465.
DGT Research. (2004). DGT- for measurements in waters, soils and sediment. http://dgtresearch.com/dgtreaserch.pdf.
Dunn, R. J. K., Teasdale, P. R., Warnken, J., & Schleich, R. R. (2003). Evaluation of the diffusive gradient in a thin film technique for monitoring trace metal concentrations in estuarine waters. Environmental Science and Technology, 37, 2794–2800.
Dunn, R. J. K., Teasdale, P. R., Warnken, J., Jordan, M. A., & Arthur, J. M. (2007). Evaluation of the in situ, time-integrated DGT technique by monitoring changes in heavy metal concentrations in estuarine waters. Environmental Pollution, 148, 213–220.
Ehrhardt, M., & Koeve, W. (1999). Determination of particulate organic carbon and nitrogen. In K. M. Grasshoff & K. Kremling (Eds.), Methods of seawater analysis (3rd ed., pp. 437–444). Weinhein: Wiley.
Fiszman, M., Pfeiffer, W. C., & Drude de Lacerda, L. (1984). Comparison of methods used for extraction and geochemical distribution of heavy metals in bottom sediments from Sepetiba Bay, R. J. (Brazil). Environmental Technology Letters, 5, 567–575.
Garcia, C. A. E. (1998). Características hidrológicas. In U. Seeliger, C. Odebrecht, & J. P. Castello (Eds.), Os Ecossistemas Costeiro e Marinho do Extremo Sul do Brasil (pp. 18–21). Rio Grande: Editora Ecoscientia.
Harris, D. C. (2007). Quantitative chemical analysis (7th ed.). China Lake: W.H. Freeman.
Hatje, V., Apte, S. C., Hales, L. T., & Birch, G. F. (2003). Dissolved trace metal distributions in Port Jackson estuary (Sydney Harbour), Australia. Marine Pollution Bulletin, 46, 719–730.
IMO, I.M.O. (2001). International conference on the control of harmful anti-fouling systems on ships. Adoption of the final act of the conference and any instruments, recommendations and resolutions resulting from the work of the conference. London: IMO Headquarters.
Jones, B., & Bolam, T. (2007). Copper speciation survey from UK marinas, harbours and estuaries. Marine Pollution Bulletin, 54, 1127–1138.
Jessop, A., & Turner, A. (2011). Leaching of Cu and Zn from discarded boat paint particules into tap water and rain water. Chemosphere, 83, 1575–1580.
Leeuwen, H. P. V., Town, R. M., Buffle, J., Cleven, R. F. M. J., Davison, W., Puy, J., et al. (2005). Dynamic speciation analysis and biovailability of metals in aquatic system. Environmental Science and Technology, 22, 8545–8556.
Li, L. Y., Hall, K., Yuan, Y., Mattu, G., McCallum, D., & Chen, M. (2009). Mobility and bioavailability of trace metals in the water-sediment system of the highly Urbanized Brunette Watershed. Water, Air, and Soil Pollution, 197, 249–266.
Lores, E. M., & Pennock, J. R. (1998). The effect of salinity on binding Cd, Cr, Cu and Zn to dissolved organic matter. Chemosphere, 37, 861–874.
Karlsson, J., Ytreberg, E., & Eklund, B. (2010). Toxicity of anti-fouling paints for use on ships and leisure boats to non-target organisms representing three trophic levels. Environmental Pollution, 158, 681–687.
Kjerfve, B. (1986). Comparative oceanography of coastal lagoons. In D. A. Wolfe (Ed.), Estuarine variability (pp. 63–81). New York: Academic.
Konstantinou, I. K., & Albanis, T. A. (2006). Antifouling paint biocides. Berlin: Springer.
Kremling, K., Tokos, J. J. S., Brügmann, L., & Hansen, H.-P. (1996). Variability of dissolved and particulate trace metals in the Kiel and Mecklenburg Bights of the Baltic Sea, 1990–1992. Marine Pollution Bulletin, 34, 112–122.
Matthiessen, P., Reed, J., & Johnson, M. (1999). Sources and potential effects of copper and zinc concentrations in the estuarine waters of Essex and Suffolk, United Kingdom. Marine Pollution Bulletin, 38, 908–920.
Millward, G. E., Morris, A. W., & Tappin, A. D. (1998). Trace metals at two sites in the southern North Sea: results from a sediment resuspension study. Continental Shelf Research, 18, 1381–1400.
Möller, O. O., Jr., Lorenzetti, J. A., Stech, J. L., & Mata, M. M. (1996). Patos Lagoon summertime circulation and dynamics. Continental Shelf Research, 16, 335–351.
Montero, N., Belzunce-Segarra, M. J., Gonzales, J.-L., Larreta, J., & Franco, J. (2012). Evaluation of diffusive gradients in the thin-film (DGT) as a monitoring tool for the assessment of the chemical status of transitional water with the water Framework Directive. Marine Pollution Bulletin, 64, 31–39.
Niencheski, L. F. H., Baraj, B., Franca, R. G., & Mirlean, N. (2002). Lithium as a normalizer for the assessment of anthropogenic metal contamination of sediments of the southern area of Pates Lagoon. Aquatic Ecosystem Health and Management, 5(4), 473–483.
Niencheski, L. F., & Baumgarten, M. G. Z. (2000). Distribution of particulate trace metal in the Southern Part of the Patos Lagoon Estuary. Aquatic Ecosystem Health and Management, 3(4), 515–520.
Niencheski, L. F., & Windom, H. L. (1994). Nutrient flux and budget in Patos Lagoon estuary. Science of the Total Environmental, 149, 53–60.
Niencheski, L. F., Windom, H. L., & Smith, R. (1994). Distribution of particulate trace metal in Patos Lagoon estuary (Brasil). Marine Pollution Bulletin, 28, 96–102.
Nolting, R. F., Dalen, M., & Helder, W. (1996). Distribution of trace and major elements in sediment and pore waters of the Lena Delta and Laptev Sea. Marine Chemistry, 53, 285–299.
Okamura, H., & Mieno, H. (2006). Present status of antifouling systems in Japan: Tributyltin substitutes in Japan. In O. Hutzinger (Ed.), The handbook of environmental chemistry (pp. 201–212). Berlin: Springer.
Paradas, W., & Amado Filho, G. M. (2007). Are metals of antifouling paints transferred to marine biota? Brazilian Journal of Oceanography, 55, 51–56.
Parks, R., Donnier-Marechal, M., Frickers, P. E., Turner, A., & Readman, J. W. (2010). Antifouling biocides in discarded marine paint particles. Marine Pollution Bulletin, 60, 1226–1230.
Readman, J. W. (2006). Development, occurrence and regulation of antifouling paint biocides: historical review and future trends. In O. Hutzinger (Ed.), The handbook of environmental chemistry (pp. 1–15). Berlin: Springer.
Salomons, W., & Föstner, U. (1984). Metals in the hydrocycle. Berlin: Springer.
Schiff, K., Diehl, D., & Valkirs, A. (2004). Copper emissions from antifouling paint on recreational vessels. Marine Pollution Bulletin, 48, 371–377.
Schiff, K., Brown, J., Diehl, D., & Greenstein, D. (2007). Extent and magnitude of copper contamination in marinas of the San Diego region, California, USA. Marine Pollution Bulletin, 54, 322–328.
Singh, N., & Turner, A. (2009). Leaching of copper and zinc from spent antifouling paint particles. Environmental Pollution, 157, 371–376.
Sokolowski, A., Wolowicz, M., & Hummel, H. (2001). Distribution of dissolved and labile particulate trace metals in the overlying bottom water in the vistula river plume (Southern Baltic Sea). Marine Pollution Bulletin, 42, 967–980.
Spyres, G., Nimmo, M., Worsfold, P. J., Achterberg, E. P., & Miller, A. E. J. (2000). Determination of dissolved organic carbon in seawater using high temperature catalytic oxidation techniques. Trends in Analytical Chemistry, 19, 498–506.
Srinivasan, M., & Swain, G. W. (2007). Managing the use of copper-based antifouling paints. Environmental Management, 39, 423–444.
Sutherland, R. A. (2002). Comparison between non-residual Al, Co, Cu, Fe, Mn, NI, Pb and Zn, released by three-step sequential extraction procedure and a dilute hydrochloric acid leach for soil and road deposited sediment. Applied Geochemistry, 17, 353–365.
Suzumura, M., Kokubun, H., & Arata, N. (2004). Distribution and characteristics of suspended particulate matter in a heavily eutrophic estuary, Tokyo Bay, Japan. Marine Pollution Bulletin, 49, 496–503.
Turner, A. (1996). Trace-metal partitioning in estuaries: importance of salinity and particle concentration. Marine Chemistry, 54, 27–39.
Turner, A., Fitzer, S., & Glegg, G. A. (2008). Impacts of boat paint chips on the distribution and availability of copper in an English ria. Environmental Pollution, 151, 176–181.
Turner, A. (2010). Marine pollution from antifouling paint particles. Marine Pollution Bulletin, 60, 159–171.
Valkirs, A. O., Seligman, P. F., Haslbeck, E., & Caso, J. S. (2003). Measurement of copper release rates from antifouling paint under laboratory and in situ conditions: implications for loading estimation to marine water bodies. Marine Pollution Bulletin, 46, 763–779.
Wallner-Kersanach, M., Andrade, C. F. F., Milani, M. R., & Niencheski, L. F. (2009). In situ measurement of trace metals in estuarine waters of the Patos Lagoon using the diffusive gradient in thin film (DGT). Journal of the Brazilian Chemical Society, 20, 333–340.
Warnken, J., Dunn, R. J. K., & Teasdale, P. R. (2004). Investigation of recreational boats as a source of copper at anchorage sites using time-integrated diffusive gradients in thin film and sediment measurements. Marine Pollution Bulletin, 49, 833–843.
Webb, J. A., & Keough, M. J. (2002). Quantification of copper doses to settlement plates in the field using diffusive gradients in thin films. Science of the Total Environmental, 298, 207–217.
Windom, H. L., Niencheski, L. F., & Smith, J. R. G. (1999). Biogeochemistry of nutrients and trace metals in the estuarine region of the patos lagoon (Brazil). Estuarine, Coastal and Shelf Science, 48(1), 113–123.
Hong, Y. S., Kinney, K. A., & Reible, D. D. (2011). Effects of cyclic changes in pH and salinity on metals release from sediment. Environmental Toxicology and Chemistry, 30(8), 1775–1784.
Zar, J. H. (2010). Biostatistical analysis (5th ed.). Upper Saddle River: Prentice Hall.
Zhang, H., & Davison, W. (1995). Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Analytical Chemistry, 67, 3391–3400.
Zhang, H., & Davison, W. (2004). Practical guide for making diffusive gels and chelex gel. Skelmorlie, Quernmore, Lancaster LA2 0QJ, UK: DGT Research Ltd.
Acknowledgments
The authors would like to thank the National Council for Scientific and Technological Development (CNPq; project no. 4824851/2009) for providing financial support and Coordination of Personnel’s of Superior Level Improvement (CAPES) for the research grants of Luiza Dy. F. Costa. Thanks for Rodrigo R. Pereira of the Federal University of Rio Grande (FURG) for the linguistic support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, L.D.F., Wallner-Kersanach, M. Assessment of the labile fractions of copper and zinc in marinas and port areas in Southern Brazil. Environ Monit Assess 185, 6767–6781 (2013). https://doi.org/10.1007/s10661-013-3063-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3063-0