Abstract
Field experiments were conducted in open top chamber during rabi seasons of 2009–10 and 2010–11 at the research farm of the Indian Agricultural Research Institute, New Delhi to study the effect of tropospheric ozone (O3) and carbon dioxide (CO2) interaction on yield and nutritional quality of Indian mustard (Brassica juncea (L.) Czern.). Mustard plants were grown from emergence to maturity under different treatments: charcoal-filtered air (CF, 80–85 % less O3 than ambient O3 and ambient CO2), nonfiltered air (NF, 5–10 % less O3 than ambient O3 and ambient CO2 ), nonfiltered air with elevated carbon dioxide (NF + CO2, NF air and 550 ± 50 ppm CO2), elevated ozone (EO, NF air and 25–35 ppb elevated O3), elevated ozone along with elevated carbon dioxide (EO + CO2, NF air, 25–35 ppb O3 and 550 ± 50 ppm CO2), and ambient chamber less control (AC, ambient O3 and CO2). Elevated O3 exposure led to reduced photosynthesis and leaf area index resulting in decreased seed yield of mustard. Elevated ozone significantly decreased the oil and micronutrient content in mustard. Thirteen to 17 ppm hour O3 exposure (accumulated over threshold of 40 ppm, AOT 40) reduced the oil content by 18–20 %. Elevated CO2 (500 ± 50 ppm) along with EO was able to counter the decline in oil content in the seed, and it increased by 11 to 13 % over EO alone. Elevated CO2, however, decreased protein, calcium, zinc, iron, magnesium, and sulfur content in seed as compared to the nonfiltered control, whereas removal of O3 from air in the charcoal-filtered treatment resulted in a significant increase in the same.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the industrial revolution, anthropogenic activity has increased the concentration of tropospheric ozone (O3), which is an air pollutant and a greenhouse gas. During the past three decades, the O3 concentration in the Northern hemisphere has increased by 0.5–2 % per year (Vingarzan 2004) and nearly one quarter of the Earth’s surface is currently at risk from mean tropospheric ozone in excess of 60 ppbV during midsummer with even greater local concentrations occurring (Morgan et al. 2006). The IPCC Fourth Assessment Report projects an increase in tropospheric O3 across the globe of 20–25 % by 2050 (Jaggard et al. 2010). Plants are often more sensitive to ambient air pollutants than other organisms as they are stationary and are always exposed to the natural environment (Wahid et al. 2012). Adverse impacts of air pollutants on plants around industrial sources have been reported from many parts in India, with concentrations between 10 to 273 ppb being recorded in Delhi (Varshney and Agrawal 1992). Elevated levels of tropospheric O3 may cause foliar injury in susceptible plants, accelerated leaf senescence, reduction in photosynthesis, and stomatal conductance in leaves, leading to reduced dry matter production and productivity in crops (Wahid 2006; Feng et al. 2010). The impact of O3 is known to vary between species and cultivars (Black et al. 2007; Biswas et al. 2008; Rai et al. 2010) and to be influenced by both climatic factors (Heath et al. 2009) and cultural practices (Singh et al. 2009).
Estimated reductions of global yields ranged from 2.2–5.5 % for maize to 3.9–15 and 8.5–14 % for wheat and soybean, respectively (Avnery et al. 2011). Ambient O3 concentrations (varying between 35 and 75 ppb growing season mean) have been reported to result in yield losses of 5–48 % in wheat, 3–47 % in rice, and 10–65 % in legumes respectively in the Asian region (Emberson et al. 2009). Wahid et al. (2012) have reported yield losses up to 45 % in sesame (Sesamum indicum L.) due to air pollutants. In a recent meta-analysis of 30 experiments representing nine countries in North America, Europe, and Asia, and spanning 18 wheat genotypes, average wheat yield improvement by removing ozone from the air entering open top chambers in the field was 9 % (Pleijel and Uddling 2011). According to Singh et al. (2010), there may also be tremendous crop yields losses in India due to rising O3 concentration in the troposphere.
Ozone enters the plants through their stomata, which is also the site of carbon dioxide (CO2) uptake. Both elevated CO2 and O3 alter plant function but in opposite ways. In general, photosynthetic capacity, growth, and yield are positively affected by elevated CO2 but negatively affected by elevated O3 (Eastburn et al. 2010). The atmospheric carbon dioxide (CO2) concentrations (385 ppm) have increased by at least 35 %, since the start of the industrial revolution (IPCC 2007) and are predicted to reach 540–970 ppm by the year 2100 (IPCC 2007). Elevated CO2 concentrations tend to counteract O3 effects on plant growth, biomass, and yield (Booker et al. 2009; Ainsworth and McGrath 2010). This protective effect of CO2 is due to increased photosynthetic rate, dry matter production, and more allocation of carbohydrate to the grain (Pleijel and Uddling 2011). The nature of the interaction depends on the sensitivity of the crop, the gas concentrations, and the influences of other biotic and environmental factors.
India is the second largest rapeseed-mustard growing country after China in the world. Rape seed-mustard (Brassica species) group of crop is the third most important oil seed crop contributing nearly 20–25 % of the total oilseed production in India (Hegde 2005). Elevated CO2 affected the Brassica species and increased its oil content (Uprety et al. 2010). However, higher ozone concentrations had an opposite impact on its growth and yield. Singh et al.(2009) in a study growing Brassica campesteris L. var. Kranti under ambient O3 levels ranging from 42 to 54 ppb at a rural site in Varanasi, India observed reductions in photosynthetic rate, yield, and protein, oil, and nutrient contents of mustard seeds over charcoal-filtered air. Singh et al. (2012) also studied the possibility of alleviating the ozone-induced losses in yield by increasing the nutrient levels in two mustard (B. campesteris L. var. Vardan and Aashirwad) varieties. Earlier, Black et al. (2007) observed that O3 exposure affected gas exchange, growth, and reproductive development of B. campesteris cv. Wisconsin Fast Plants upon 10-day exposure to 70 ppb O3 during vegetative phase. De Bock et al. (2011) reported significant reduction in seed yield and oil percentage in Brassica napus cv. Ability in response to high ozone concentration (ambient + 20–40 ppb O3). However, there are no many studies on how the presence of elevated levels of ozone alone and in combination with CO2 will affect the yield and nutritional quality of crops in the tropical region. Since mustard is one of the most important tropical oilseed crops, the present study was conducted with the objective to assess the effect of different O3 treatments alone and in combination with elevated CO2 concentration on the yield and nutritional quality of Indian mustard seed.
Materials and methods
Location, climate, and soil characteristics
Field experiments were conducted during rabi (winter) season of 2009–10 and 2010–11 at research farm of Indian Agricultural Research Institute, New Delhi, situated at a latitude of 28°40′ N and longitude of 77°12′ E, altitude of 228.6 m above the mean sea level (Arabian sea). The mean annual rainfall of Delhi is 650 mm, and more than 80 % generally occurs during the south-west monsoon season (July–September) with mean annual evaporation 850 mm. The climate of Delhi is continental, sub-tropical, and semi-arid type with hot, dry summer, and cold winter and falls under the agro-climatic zone “Trans-Gangatic plains.” January is the coldest month of the year with a minimum temperature ranging from 5 to 7 °C. The soils of experimental field was sandy clay loam and slightly alkaline (pH 8.26) with low electrical conductivity values (0.29–0.31 dS m−1). Soil was medium in organic carbon (0.46–0.53 %) content and low in available nitrogen (172–260 kg ha−1), medium in available potassium (189–198 kg ha−1), and available phosphorous (15.23–18.78 kg ha−1).
Treatments and crop management
Mustard crop was grown in open–top chambers (OTCs) of 3 m in diameter and 2.5 m in height consisting of a circular aluminum frame covered with transparent film. The experiment was carried out with six treatments arranged in completely randomized design with three replications. The treatments were nonfiltered air (NF: 5–10 % less than ambient O3), charcoal-filtered air (CF: 80–85 % less than ambient O3), elevated ozone (EO: NF + 25–35 ppb O3), elevated ozone with elevated carbon dioxide (EO + CO2: NF + (25–35 ppb O3 + 500 ± 50 ppm CO2), nonfiltered air with elevated carbon dioxide (NF + CO2: NF + ( 500 ± 50 ppm CO2), and chamber less ambient air control (AC) and are listed in Table 1. The OTCs were fitted with an inert PVC pipe of 10 cm in diameter (adjustable height) with many small holes which released either charcoal-filtered air (CF), nonfiltered air (NF), or elevated ozone along with nonfiltered air (EO) at the crop canopy level. Air was blown into the OTCs through a fan that provided uniform air speeds. The ventilation rates were kept at three air changes per minute to keep the leaf boundary layer resistances down and the chamber temperature close to ambient. O3 and CO2 were applied for 7 h day−1 (09.30–16.30 h) in the elevated O3 and elevated CO2 chambers. Additional O3 was generated from oxygen with the help of reaction with UV radiation < 200 nm using ozone generators (Systocom, Varanasi, India). Air was sampled from the middle of each OTC at the crop canopy level and fed to an O3 analyzer (model APOA-370, Horiba, Germany) for measuring the ozone concentrations daily from 9.30 to 16.30 h. Charcoal filters adsorbed ozone from ambient air blown inside the OTCs and lowered the ozone concentrations by 80–85 % of the ambient air. The nonfiltered (NF) treatment was the open top chamber control treatment, and a 5–10 % decrease in concentration than the ambient ozone levels was observed in this treatment.
Pusa Tarak (EJ-13) variety of Indian mustard (Brassica juncea) was sown at 4 kg ha−1 in rows at 40 cm distance, keeping 15 cm plant to plant spacing in open top chambers. All the cultural practices like sowing, thinning, weed management, irrigation, crop pest control, and harvesting were carried out as per standard procedures in both the year of experimentation. Inorganic fertilizers were applied at 60:40:40, N/P2O5/K2O kg ha−1 through urea and di-ammonaical phosphate and muriate of potash, respectively. Nitrogen was applied in two splits—one half as basal before sowing with full dose of P and K, the rest at 40 days after sowing. Sulfur at 20 kg ha−1 was applied through gypsum.
Carbon dioxide generation and monitoring
The enhanced CO2 concentrations were maintained inside the OTCs using high pressurized cylinders containing CO2 with the help of dual stage regulators and gas flow meter. CO2 was supplied from the cylinders to the OTCs through 6-mm polyurethane tubing and mixed with the ambient air at the outlet of the air blowers and subsequently distributed evenly inside the OTCs. CO2 levels inside the chambers were monitored using a CO2 gas monitor, Industrial Scientific, USA (CDU-440).
Measurement of photosynthetic rate
Single-leaf net photosynthetic rates were measured with portable photosynthesis systems (LI-6400-40 Portable Photosynthesis System) at 45, 75, and 105 days after sowing (DAS) during crop growth period. The measurements were made on the third fully expanded mature leaf from the top of each plant on cloud free days between 10:00 and 11:00 A.M. local time on three randomly selected plants in each chamber.
Measurement of yield parameters
After harvesting, threshing, cleaning, and drying, the seed yield was recorded at 14 % moisture. Data on yield attributes, viz., number of siliqua per plant, number of seeds per siliqua, 1,000-seed weight, seed yield, biological yield, and harvest index, were recorded. The value of harvest index (HI) was calculated by using the following relationship. Harvest index (%) = Economic yield (g m−2)/Biological yield (g m−2) × 100, where economic yield = seed yield.
Measurement of nutritional quality parameters
The oil content in mustard seed was estimated by pulse nuclear magnetic resonance (PNMR) technique (Tiwari et al. 1974), the weighing and drying method. The PNMR (Oxford Instruments, MQC, UK) using liquid signal for seed oil determination is a rapid and nondestructive method for determining the oil content in oilseeds using the principle of free induction decay (FID) of low-field NMR. Seeds were oven dried for overnight at 105 °C to remove moisture completely. Thereafter, the dried seeds were allowed to cool inside desiccators. The weight of the seed up to the 2.5 cm in height of the sample NMR tube was measured. The NMR signal of the sample was recorded. Measurements of the FID amplitude following a 90° pulse were made at a resonant frequency of 23 MHz, a pulse length of 2.5 μs, and a pulse repetition rate of 1 s. The decay of oil signal after 90° pulse is independent of sample to sample variation in spin–spin relaxation time (T2). FID signal was sampled at a delay of 250 μs. The FID amplitude, following a 90″ pulse, was proportional to the total number of resonant nuclei in the seed sample, and the FID amplitude was proportional to the hydrogen content of the oil. The oil content was determined from calibration curve constructed by recording the NMR signal of known increasing quantity of pure oil seeds of mustard. The quantity of oil in an unknown seed sample was read against its NMR signal from the calibration curve.
Protein content (percent) in seed was calculated by multiplying the nitrogen percent in seed by the factor 6.25 (AOAC 1960). For estimation of nitrogen concentration, 0.5 g sample was taken and N concentration was determined by macro Kjeldahl method (Jackson 1973).
For nutrient other than N, 0.5 g of seed was digested in a diacid by using a 9:4 mixture of HNO3/ HClO4. After the digestion, sulfur concentration in the digested sample was determined by turbidimetric method (Chesnin and Yien 1950) with Spectrophotometer (GS-5702, Electronics Corporation of India, Ltd.). Other macro- (Ca, Mg, and S) and micronutrients (Fe and Zn) were determined with the help of atomic absorption spectrophotometer (Jackson 1973).
Statistical analysis
Analysis of variance and significance of a completely randomized design was tested by “F” test (Gomez and Gomez 1984). Standard error of means (SEM) and critical difference (CD) at 5 % level of significance were worked out for each parameter.
Results and discussion
Ozone levels in different treatments
The daily average ozone concentrations measured during the experiment period, i.e., from the month of October to March, are shown in Fig. 1. O3 exposure began in November when the mustard was in the three-leaf stage and ended in March when it was ripe. The average ozone concentration during the entire crop growth period in ambient air was 28 ppb in 2009–10 and 33 ppb in 2010–11. Critical levels for ozone were calculated as a cumulative exposure over a threshold of 40 ppbv; the resulting index of AOT40 (accumulated exposure over a threshold of 40 ppbv during the crop growth season) were 12.74 and 16.96 ppm hour in 2009–10 and 2010–11, respectively, in the elevated ozone treatment. Higher concentration of ambient ozone was observed during February to March in 2010–11. Ozone concentration ranged from 31 to 92 ppb inside the OTC in elevated ozone treatment.
Impact of different treatments on photosynthetic rate
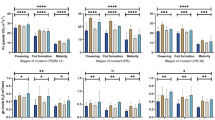
As photosynthesis is the primary physiological process by which sensitive plants respond to changes in environment, photosynthetic rates were measured at three growth stages of vegetative, flowering, and grain filling at 45, 75, and 105, DAS, respectively, in both the years. A significant increase in single-leaf net photosynthetic rate was observed in the CF treatment, whereas a significant decrease was observed under EO levels over NF control in both the years. Photosynthetic rate gradually increased till 75 DAS and thereafter it decreased at 105 DAS (Fig 2). The photosynthetic rate was highest in CF treatment at 29.8 and 31.4 μmol CO2 m−2 s−1 at 75 DAS in first and second year, respectively. There was a reduction observed in 2009–10 in photosynthetic rate by 19, 25.4, and 27.8 % in EO over NF, and it decreased by 17.6, 23.6, and 28 % in 2010–11 at 45, 75, and 105 DAS, respectively. Singh et al. (2009) reported a decline in photosynthetic rate under higher ozone levels in nonfiltered air in Brassica campestris L. var. Kranti at a rural site in India. Feng et al. (2008) observed that high concentrations of ozone caused damage to leaves and decreased plant growth and caused a decline in photosynthesis. The decrease in stomatal conductance (result not shown here) might have led to lower internal leaf CO2 levels, thus reducing the photosynthetic rates under higher ozone levels. However the presence of elevated CO2 significantly increased the photosynthetic rate. The photosynthetic rate increased in EO + CO2 as compared to EO alone by 10–12 %, whereas it increased by 16–21 % in NF + CO2 over NF alone. In a meta-analysis carried out by Feng et al. (2008), elevated CO2 substantially decreased the negative effects of high ozone on photosynthesis, growth, and seed yield in both soybeans and rice. When elevated CO2 and elevated O3 were combined, the decrease attributed to O3 was smaller than in elevated O3 at ambient CO2. These results confirm that elevated CO2 provides some protection to a crop against the damaging effects of O3 on photosynthetic activity (Ainsworth and Rogers 2007).
Wahid (2006) observed a significant reduction in photosynthetic rate (20–22 %) in three wheat cultivars under higher ozone concentration. Cao et al. (2009) also observed a significant decrease of photosynthetic rate in wheat cultivars under O3 treatment. Ariyaphanphitak et al. (2005) reported that decline of photosynthesis was a result of the direct effect of O3 on stomata and also due to the oxygen radicals generated by O3 altered the photosynthetic electron transport and enzymatic activities. Vandermeiren et al. (2005) reported a significant increase (40 %) in photosynthetic rate under elevated CO2 concentration and 16 % reduction under elevated O3 in potato plant.
Impact of different treatments on yield attributes
The seed yield parameters were recorded after final harvest of the plant. The plants under EO treatment took significantly more time to attain 50 % flowering (4 more days) and for siliqua formation (5 more days) as compared to others treatments. The crop matured 7 to 8 days earlier under EO treatment as compared to NF and CF treatments. The seed yield of Indian mustard was significantly affected by the different treatments (Table 2). The CF, NF + CO2, EO, and EO + CO2 treatments were significantly different from NF in both the years. The highest number of seeds per siliqua was obtained in CF followed by NF + CO2, NF, EO + CO2, and EO; however, there were significant differences only among CF and EO over the NF treatment. Among all the treatments, the number of siliqua per plant was the lowest under the EO treatment. It decreased by 8 % under EO as compared to NF and increased by 4 % in EO + CO2 treatment over EO alone. In a meta-analysis of 128 studies, Leisner and Ainsworth (2012) observed that elevated O3 had a negative effect on most reproductive yield parameters when compared to plants grown in charcoal-filtered air. Relative to ambient air, fumigation with O3 between 70 and 100 ppb decreased yield by 27 % and individual seed weight by 18 %. The number of siliqua per plant increased by 7 % in charcoal-filtered treatment as compared to nonfiltered control. Singh et al. (2010) observed a 19.4 and 29.5 %, increase in number of seeds per plant and per pod, respectively, in B. campesteris L. var. Kranti under CF air as compared to ambient O3 levels of 42 to 54 ppb. The presence of higher ozone levels also affected the 1,000-seed weight, which was the lowest in EO treatment and the highest in CF air.
The presence of CO2 in the NF + CO2 treatment increased the number of siliqua per plant by 4.4 % over the NF treatment. A significant decrease in 1,000-seed weight was observed under EO as compared to NF. Similar decrease in 1,000-seed weight was observed by Vandermeiren et al. (2012) in B. napus L. Significant increase in 1,000-seed weight was observed due to elevated CO2 in both EO + CO2 and NF + CO2 over EO and NF alone, respectively. No significant difference was observed in the ambient air control (AC) and NF treatments with respect to any of these parameters.
Seed yield of mustard was significantly affected by elevated ozone concentration in both years. Minimum seed yield was observed in EO (139.39 and 148.82 g m−2) treatment followed by EO + CO2 (157.67 and 168.10 g m−2) in 2009–10 and 2010–11, respectively. There was a 22.7 to 26.2 % decrease in seed yield of EO treatment over the NF. Black et al. (2007) observed that O3 exposure reduced the yield of B. campesteris cv. Wisconsin Fast Plants after 10-day exposure of 70 ppb O3 by reducing bud formation and flowering. Wang et al. (2008) observed a reduction in yield of B. napus cv. Hoyou 19 due to reduction in siliqua number. Seed yield was negatively correlated with ozone dose in B. napus cv. Ability (De Bock et al. 2011). Maximum seed yield in our experiment was observed in CF (218.79 and 229.49 g m−2) followed by NF + CO2 (209.71 and 216.34 g m−2) in 2009–10 and 2010–11, respectively, which had the highest number of siliqua per plant. Elevated CO2 (ambient + 173 μmol mol−1) increased yield parameters by 7 to 17 % in peanut (Arachis hypogaea L.) grown in CF air and restored yield in NF air and elevated O3 treatments to control levels. Bhatia et al. (2011) observed the presence of higher ozone led to 11 to 12 % decrease in rice yield and charcoal filtration of air increased rice yield by 17 to 22 %. According to Leisner and Ainsworth (2012), ambient O3 significantly decreased seed number by (16 %), as compared to charcoal-filtered air. Chronic exposure to O3 leads to reduced biomass production and reproductive output in crops. Ozone-induced yield losses have often been attributed to reductions in photosynthetic activity and assimilate supply to support reproductive development and seed growth (Feng et al. 2010). In our study, elevated ozone levels averaging 58 ppb led to a 26 % decrease in seed yield of mustard as compared to nonfiltered control, whereas a 15 % increase in seed yield was observed in CF treatment as compared to NF. Singh et al. (2012) observed that pod weight per plant and number of seeds per pod decreased by 6.1 and 30.6 %, respectively, in B. campesteris L. var Aashirwad in NF air as compared to CF leading to a decline in seed yield and its nutritional quality. In other crops, too similar reductions in yields have been observed under elevated levels of ozone in ambient air. Reductions in grain yields ranging from 5–42 % (Wahid 2006; Feng et al. 2010; Zhu et al. 2011; Avnery et al. 2011) have been observed due to higher ozone concentrations in rice and wheat cereal crops grown in open top chamber and fully open air O3-enriched conditions.
The average concentrations of ozone in ambient air were higher during the cropping period in 2010–11 as compared to 2009–10. In 2009–10, the O3 concentrations were higher during the vegetative phase of the crop, whereas higher concentrations were observed during the reproductive phase of the crop in 2010–11. Ambient ozone averaging more than 45 ppb was observed during the month of February 2011. The seed yields were marginally higher during the second year as compared to first year of the study. Thus, higher ozone levels during the vegetative phase of the crop in 2009 might have affected the carbon assimilation (Leisner and Ainsworth 2012), leading to a lower seed yields under elevated ozone levels in the first year of the study. De Bock et al. (2011) also observed highest correlation between the reduction of oilseed rape yield and ozone uptake during the vegetative growth stage. There was an increase in all the yield parameters under EO + CO2 treatment as compared to elevated ozone treatment. In EO + CO2 treatment, seed yield increased by 13 % over EO alone. There was an increase in the photosynthetic activity under EO + CO2 treatment which led to an increase in photosynthates. The protective effect of CO2 was due to increased photosynthetic rate, dry matter production, and more allocation of carbohydrate to the seed. The presence of elevated CO2 along with EO thus countered the negative effect of O3 to a certain extent, thereby increasing the yield and yield parameters. Piikki et al. (2008) also observed an increase in grain yield due to increasing grain number per unit ground area due to the presence of elevated CO2 along with elevated O3 in an open top chamber experiments study, conducted in three European countries with four cultivars of wheat. The yield of wheat, rice, and soybean decreased under elevated ozone and increased under elevated CO2 (Ainsworth et al. 2008; Feng et al. 2008; Long et al. 2006).
The biological yield (seed + straw) was significantly impacted under different ozone concentrations, but no significant difference was observed between the ambient air control (AC) and NF treatments. It was the highest in CF treatment (864 and 873 g m−2) in 2009–10 and 2010–11, respectively, and increased by 12–13.5 % in CF over NF. In CF treatment, there was appreciable improvement in vegetative growth as was evidenced by significantly taller plants and more number of branches which ultimately resulted in higher dry matter production. Higher production due to favorable condition at the different growth stages increased the photosynthetic assimilation resulting in enhanced biological yield. In EO treatment, biological yield was the lowest (620 and 654 g m−2) and decreased by 15.1–19.2 % over the NF control treatment. The biological yield in EO + CO2 treatment increased by 8.17–11.4 % over the EO alone treatment. Piikki et al. (2008) observed that elevated O3 decreased biological yield by decreasing grain and straw yield, while elevated CO2 increased both the parameters. There was a 9 % increase in biological yield in NF + CO2 over the NF air. This protective effect of CO2 was due to increased photosynthetic rate, dry matter production, and more allocation of carbohydrate to the grain (Pleijel and Uddling 2011). Bhatia et al. (2011) observed highest biological yield under charcoal filtration and lowest under EO concentrations in rice. As HI is dependent on grain yield and biological yield, it was the lowest in EO and highest in CF air. It was the highest in CF treatment (25.33 and 26.36) and lowest in EO (22.49 and 22.79) in both the years. There was 9 % decrease in harvest index in EO over NF (24.59 and 25.01), and the presence of elevated CO2 increased the HI to 22.81 and 22.78 (EO + CO2) and to 25.18 and 25.83 (NF + CO2) in 2009 and 2010, respectively. Kharel and Amgain (2010) reported that ambient ozone (29.3 to 39.1 ppb) reduced harvest index (6.25 %) in mung bean over ethylenediurea (ozone protectant) treated plants at Rampur in Nepal.
Impact of different treatments on oil content of mustard seed
The oil contents in seeds of B. juncea were influenced by O3 and CO2 levels and are presented in (Table 3). In addition to reductions in seed yield, our study indicated that there was economically important impact of elevated O3 on nutritional quality of mustard. The oil content ranged from 31.98 to 43.01 % in 2009–10 and 33.19 to 44.40 % in 2010–11 in different treatments in mustard seed. During both the years, highest oil content was observed in CF treatment, whereas it decreased under EO. The sub-ambient ozone levels in CF air significantly increased oil content, in both years. There was 8.7–9 % increase in seed oil content in CF over the NF control in both years.
We observed minimum oil content in EO (31.98 and 33.19 %) treatment followed by EO + CO2 (35.39 and 37.33 %) in 2009–10 and 2010–11, respectively. There was 18–20 % decrease in oil content under EO over the NF control. Enhanced senescence and lower uptake of nutrients may have led to a reduction in oil content in EO treatment. Oil content decreased by 5 to 6 % under elevated O3 in B. napus (Kollner and Krause 2003). De Bock et al. (2011) observed a 30 % decrease in oil content of B. napus L. upon exposure to ambient + 40 ppb O3 for the entire growing season. In peanut, elevated O3 (1.5 times ambient O3) decreased oil concentrations by about 10 % as compared to the nonfiltered air in open top chambers (Burkey et al. 2007).
In EO + CO2 treatment, oil content increased by 10–12 % over EO alone, but it was 8.5–10 % lower than NF control. These results confirm that elevated CO2 was able to counter the negative effect of EO on oil content to a certain extent. The presence of elevated CO2 in NF + CO2 treatment increased the oil content by 6 % over the NF alone. Under elevated CO2, more oil content was probably due to more accumulation of carbohydrate in the seed (Uprety et al. 2010). However, carbohydrate requirement for the conversion to oil was greater as compared to that for protein. Heagle et al. (1998) also observed a positive, significant effect of CO2 enrichment on soybean seed oil content, and seeds exposed to elevated ozone (1.5 times ambient O3) concentrations showed decline in oil content with lowering in the oleic acid content of the seed. Elevated CO2 affected the oil content in Brassica species due to greater acetyl CoA enzyme activity (Uprety et al. 2010). Acetyl CoA is an important enzyme for fatty acid biosynthesis (De Bock et al. 2011). In our study, the oil content increased under elevated CO2, probably due to larger accumulation of carbohydrates.
Impact of different treatments on protein content of mustard seed
The protein content ranged from 18.62 to 25.52 % in 2009–10 and 20.53 to 27.07 % in 2010–11 (Table 3) in mustard seed. We observed minimum protein content in EO + CO2 treatment (18.62 and 20.53 %) followed by EO (20.70 and 21.80 %) in 2009–10 and 2010–11, respectively. In EO treatment, protein content decreased by 7–8 % over the NF control. Elevated ozone causes advanced senescence in leaves, and accelerated catalysis leads to the rapid loss of protein (Grandjean and Fuhrer 1989). Our results however differ from Vandermeiren et al. (2012) who reported a significant increase in protein concentration of B. napus cv. Ability seeds in response to elevated O3 levels of 20 and 40 ppb during the entire growing season. Agrawal et al. (2006) also observed significant reduction in protein content in seeds of mung bean plants exposed to high O3 concentration due to lesser translocation of assimilates to the growing seed. There was 13 to 15 % increase in seed protein content in CF over the NF control in our study. In CF treatment, favorable growth conditions led to a higher uptake of nitrogen, which was utilized for carbon assimilation, and at the stage of seed filling, this nitrogen was redistributed to seed (Pleijel et al. 1999), thereby increasing the protein content.
Protein content decreased by 5.5 % in NF + CO2 treatment as compared to NF alone. The presence of elevated CO2 along with EO further decreased the protein content of seed. There was 12–17 % decrease in protein content in EO + CO2 treatment over the NF control. Reduction in plant nitrogen under elevated CO2 was probably due to increase in concentration of carbohydrates and changes in distribution of protein or other nitrogen-containing compounds due to higher photosynthetic rates and probably caused by a dilution effect induced by great increment of carbohydrate in seed (Uprety et al. 2010; Pleijel and Uddling 2011). Uptake of nitrogen may also be reduced at high CO2 due to lower transpiration rates, resulting in lower percentage of protein under elevated CO2. Leaf nitrogen concentrations in plant tissues typically decrease under elevated CO2, with nitrogen per unit leaf mass decreasing on average by 13 % (Ainsworth and Long 2005) due to dilution of nitrogen from increased carbohydrate concentrations.
Impact of different treatments on macro- and micronutrients in mustard seed
Seed of Indian mustard (B. juncea) is known to contain 2.6–5.2 mg g−1 calcium, 2.9–3.7 mg g−1 magnesium, 3.5–5.5 mg g−1 sulfur, 48–60 mg kg−1 zinc, and 79–99 mg kg−1 iron. The macro- and micronutrient content of mustard seed was significantly affected by the different ozone and carbon dioxide treatments. There was reduction in the calcium (Ca), zinc (Zn), iron (Fe), magnesium (Mg), and sulfur (S) content under EO treatment (Table 4). However, the presence of elevated CO2 led to a further decline in the nutritional quality of the mustard seed.
We observed minimum Ca content in EO + CO2 treatment (2.9 and 3.1 mg g−1) followed by EO treatment (3.2 and 3.4 mg g−1). In EO treatment, Ca content decreased by 16–21 % over the NF control. High ozone has been shown to affect the rate and duration of photosynthesis and the translocation of assimilates from the source to the sink; ozone exposure probably caused a reduced assimilate supply to the growing seed and thus reduced the nutrient levels in seed (Agrawal et al. 2006). There was 24–28 % decrease in Ca content of EO + CO2 treatment over the NF control. CO2 enrichment caused alterations in the concentrations of macro- and microelements decreasing N, P, K, Ca, Zn, Fe, Mg, and S in mature grains (Uprety et al. 2010) probably due to the dilution effect, induced by increased concentration of carbohydrates in the seed. In NF + CO2 treatment, Ca content decreased by 12 % over the NF alone.
Similar results were obtained for Zn, Fe, Mg, and S under the different treatments of the experiment in both the years. Significantly higher Zn concentration in seed, viz., 54.2 and 55.1 mg kg−1, was observed under CF treatment (Table 4) in 2009 and 2010, respectively. There was 11 to 13 % increase in Zn content in CF over the NF control in both the years. In EO treatment, Zn content decreased by 11.5–13.5 % over the NF control. There was 19–21 % decrease in Zn content under EO + CO2 treatment over the NF control. The filtration of air (CF) significantly increased the Fe content of mustard seed. There was 13 to 14.5 % increase in seed Fe content in CF over the NF control. In EO treatment, Fe content decreased by 8 % over the NF control. There was 17–19 % decrease in Fe content under EO + CO2 treatment over the NF control in both seasons. Fe content decreased by 7 % in NF + CO2 treatment as compared to NF alone. There was 13 % increase in seed Mg content in CF over the NF control. Maximum S content in seed was observed in the CF treatment. We observed minimum S content in EO + CO2 treatment (3.7 and 3.9 mg g−1) followed by EO (4.1 and 4.2 mg g−1) in 2009–10 and 2010–11, respectively. There was 15–17 % decrease in S content under EO + CO2 treatment over the NF control in both years. Most of the nutrients in seed originate from redistribution from vegetative pools during seed filling. Piikki et al. (2008) observed reduction in macroelements (Ca, Mg, and S) and microelements (Fe and Zn) under elevated CO2 and ozone. High ozone concentration in the atmosphere reduced the carbohydrate formation and uptake of important minerals, e.g., nitrogen, phosphorus, potassium, and sulfur (Karberg et al. 2005), which led to a decrease in the green leaf area and net CO2 assimilation resulting in a lower production of carbohydrates and reduced source strength. Uprety et al. (2010) observed that elevated CO2 significantly decrease the concentration of macro- and microelements in grain of wheat and rice. Hogy and Fangmeier (2008) observed that elevated CO2 significantly reduced the concentration of macroelements ranging from 7–19.5 % and microelements, and Fe and Zn by 18.3 and 13.1 %, respectively, in wheat grains due to the dilution effect, induced by increased concentration of carbohydrates in grains.
Conclusions
Comparison of responses of mustard plants grown in environments with filtered and nonfiltered air showed that the current ambient levels of ozone may be significantly affecting the oil content and nutritional quality of Indian mustard. The present study clearly suggests that elevated O3 levels (ambient + 25–30 ppb O3) are potentially capable of significantly decreasing the seed yield of mustard and also cause a decline in its oil and protein content. Reduction in the protein, calcium, zinc, iron, magnesium, and sulfur content under elevated ozone indeed may degrade the nutritional quality of mustard seed. The presence of elevated levels of CO2 may, however, overcome some of the yield losses (up to 12 %) due to elevated ozone, but a reduction in protein and micro- and macronutrient content of mustard suggest a greater threat to quality maintenance which cannot even be countered by the presence of elevated CO2 in the atmosphere.
References
Agrawal, M., Singh, V., Agrawal, S. B., Bell, J. N. B., & Marshall, F. (2006). The effect of air pollution on yield and quality of Mung bean grown in peri-urban areas of Varanasi. Water, Air, and Soil Pollution, 169, 239–254.
Ainsworth, E. A., & Long, S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytologist, 165, 351–371.
Ainsworth, E. A., & McGrath, J. M. (2010). Direct effects of rising atmospheric carbon dioxide and ozone on crop yields. Climate Change and Food Security Advances in Global Change Research, 37(II), 109–130.
Ainsworth, E. A., & Rogers, A. (2007). The response of photosynthesis and stomatal conductance to rising (CO2): mechanisms and environmental interactions. Plant, Cell & Environment, 30, 258–270.
Ainsworth, E. A., Rogers, A., & Leakey, A. D. B. (2008). Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiology, 147(1), 13–9.
AOAC (1960). Official method of analysis of the Association of Official Agricultural Chemists, Washington D.C. 9th ed. pp. 15–16.
Ariyaphanphitak, W., Chidthaisong, A., Sarobol, E., Bashkin, V. N., & Towprayoon, S. (2005). Effects of elevated ozone concentrations on Thai jasmine rice cultivars (Oryza sativa L.). Water, Air, and Soil Pollution, 167, 179–200.
Avnery, S., Mauzerall, D. L., Liu, J., & Horowitz, L. W. (2011). Global crop yield reductions due to surface ozone exposure: 2. Year 2030 potential crop production losses and economic damage under two scenarios of O3 pollution. Atmospheric Environment, 45, 2297–2309.
Bhatia, A., Ghosh, A., Kumar, V., Tomer, R., Singh, S. D., & Pathak, H. (2011). Effect of elevated tropospheric ozone on methane and nitrous oxide emission from rice soil in north India. Agriculture Ecosystem Environment, 144, 21–28.
Biswas, D. K., Xu, H., Li, Y. G., Sun, J. Z., Wang, X. Z., Han, X. G., & Jiang, G. M. (2008). Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Global Change Biology, 14, 46–59.
Black, V. J., Stewart, C. A., Roberts, J. A., & Black, C. R. (2007). Ozone affects gas exchange, growth and reproductive development in Brassica campestris (Wisconsin Fast Plants). New Phytologist, 176, 150–163.
Booker, F., Muntifering, R., McGrath, M., Burkey, K., Decoteau, D., Fiscus, E., Manning, W. J., Krupa, S., Chappelka, A., & Grantz, D. (2009). The ozone component of global change: potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. Journal of Integrative Plant Biology, 51, 337–351.
Burkey, K. O., Booker, F. L., Pursley, W. A., & Heagle, A. S. (2007). Elevated carbon dioxide and ozone effects on peanut. II. Seed yield and quality. Crop Science, 47, 1488–1497.
Cao, J. L., Wang, L., Zeng, Q., Liang, J., Tang, H. Y., Xie, Z. B., Gaang, L., Zhu, J. G., & Kazhuhiko, K. (2009). Characteristics of photosynthesis in wheat cultivars with different sensitivities to ozone under O3-free air concentration enrichment conditions. Acta Agronomica Sinica, 35, 1500–1507.
Chesnin, L., & Yien, C. H. (1950). Turbidimetric determination of available sulphate. Proceeding of the Soil Science Society of America, 15, 149–151.
De Bock, M., Op de Beeck, M., De Ludwig, T., Yves, G., Reinhart, C., & Karine, V. (2011). Ozone dose–response relationships for spring oilseed rape and broccoli. Atmospheric Environment, 45(9), 1759–1765.
Eastburn, D., Degennarow, M. S. S. A. M., Deluciaz, E. H., Dermody, O., & Mcelrone, A. J. (2010). Elevated atmospheric carbon dioxide and ozone alter soybean diseases at SoyFACE. Global Change Biology, 16, 320–330.
Emberson, L. D., Buker, P., Ashmore, M. R., Mills, G., Jackson, L. S., Agrawal, M., & Wahid, A. (2009). A comparison of North American and Asian exposure–response data for ozone effects on crop yields. Atmospheric Environment, 43, 1945–1953.
Feng, Z., Kobayashi, K., & Ainsworth, E. A. (2008). Impact of elevated ozone concentration on growth, physiology and yield of wheat (Triticum aestivum L.): a meta-analysis. Global Change Biology, 14, 2696–2708.
Feng, Z., Wang, S., Szantoi, Z., Chen, S., & Wang, X. (2010). Protection of plants from ambient ozone by applications of ethylenediurea (EDU): a meta-analytic review. Environmental Pollution, 158, 3236–3242.
Gomez, K. A., & Gomez, A. A. (1984). Statistical procedures for agricultural research (2nd ed.). New York: Wiley. An International Rice Research Institute Book. A Wiley-Inter-science Publication.
Grandjean, A., & Fuhrer, J. (1989). Growth and leaf senescence in spring wheat (Triticum aestivum) grown at different ozone concentrations in open-top field chambers. Physiologia Plantarum, 77, 389–394.
Heagle, A. S., Miller, J. E., & Pursley, W. A. (1998). Influence of ozone stress on soybean response to carbon dioxide enrichment. III. Yield and seed quality. Crop Science, 38, 128–134.
Heath, R. L., Lefohn, A. S., & Musselman, R. C. (2009). Temporal processes that contribute to nonlinearity in vegetation responses to ozone exposure and dose. Atmospheric Environment, 43, 2919–2928.
Hedge, D.M. (2005). Oilseed scenario in India—past, present and future with special reference to rapeseed-mustard. In: Winter School on Advances in Rapeseed-Mustard Research Technology for Sustainable Production of Oilseeds, National Centre on Rapeseed-Mustard, Sewar, Bharatpur, Dec 15 to Jan 04, 2005, 1–15.
Hogy, P., & Fangmeier, A. (2008). Effects of elevated atmospheric CO2 on grain quality of wheat. Journal of Cereal Science, 48, 580–591.
IPCC. (2007). In M. L. Parry, O. F. Canziani, J. P. Paultikof, P. J. van der Linden, & C. E. Hanon (Eds.), Climate change—impacts, adaptation and vulnerability. Technical summary of Working group II. Fourth Assessment Report Inter-governmental Panel on Climate Change (pp. 23–78). Cambridge: Cambridge University press.
Jackson, M. L. (1973). Soil Chemical Analysis. New Delhi: Prentice Hall of India Pvt.Ltd.
Jaggard, K. W., Qi, A., & Ober, E. S. (2010). Possible changes to arable crop yields by 2050. Philosophical Transactions of the Royal Society of London Series B, Biological, 365, 2835–2851.
Karberg, N. J., Pregitzer, E. K. S., King, E. J. S., Friend, A. L., & Wood, E. J. R. (2005). Soil carbon dioxide partial pressure and dissolved inorganic carbonate chemistry under elevated carbon dioxide and ozone. Oecologia, 142, 296–306.
Kharel, K., & Amgain, L. P. (2010). Assessing the impact of ambient ozone on growth and yield of crop at Rampur, Chitwan. The Journal of Agriculture and Environment, 11, 40–45.
Kollner, B., & Krause, G. H. M. (2003). Effects of two different ozone exposure regimes on chlorophyll and sucrose content of leaves and yield parameters of sugar beet (Beta vulgaris) and rape (Brassica napus). Wate, Air and Soil Pollution, 144, 317–332.
Leisner, C. P., & Ainsworth, E. A. (2012). Quantifying the effects of ozone on plant reproductive growth and development. Global Change Biology, 18(2), 606–616.
Long, S. P., Ainsworth, E. A., Leakey, A. D. B., Nosberger, J., & Ort, D. R. (2006). Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science, 312, 1918–1921.
Morgan, P. B., Mies, T. A., Bollero, A., Nelson, R. L., & Long, S. P. (2006). Season-long elevation of ozone concentration to projected 2050 levels under fully open-air conditions substantially decreases the growth and production of soybean. New Phytologist, 170, 333–343.
Piikki, K., De Temmerman, L., Ojanpera, K., Danielsson, H., & Pleijel, H. (2008). The grain quality of spring wheat (Triticum aestivum L.) in relation to elevated ozone uptake and carbon dioxide exposure. European Journal of Agronomy, 28, 245–254.
Pleijel, H., & Uddling, J. (2011). Yield vs. quality trade-offs for wheat in response to carbon dioxide and ozone. Global change Biology. doi: 10.1111/j.1365-2486.2011.02489.x
Pleijel, H., Mortensen, L., Fuhrer, J., Ojanpera, K., & Danielsson, H. (1999). Grain protein accumulation in relation to grain yield of spring wheat (Triticum aestivum L.) grown in open-top chambers with different concentrations of ozone, carbon dioxide and water availability. Agriculture, Ecosystems and Environment, 72, 265–270.
Rai, R., Agrawal, M., & Agrawal, S. B. (2010). Threat to food security under current levels of ground level ozone: a case study for Indian cultivars of rice. Atmospheric Environment, 44, 4272–4282.
Singh, P., Agrawal, M., & Agrawal, S. B. (2009). Evaluation of physiological, growth and yield responses of a tropical oil crop (Brassica campestris L. var. Kranti) under ambient ozone pollution at varying NPK levels. Environmental Pollution, 157, 871–880.
Singh, S., Kaur, D., Agrawal, S. B., & Agrawal, M. (2010). Responses of two cultivars of Trifolium repens L. to ethylene diurea in relation to ambient ozone. Journal of Environment. Science (China), 22, 1096–103.
Singh, P., Singh, S., Agrawal, S. B., & Agrawal, M. (2012). Assessment of the interactive effects of ambient O3 and NPK levels on two tropical mustard varieties (Brassica campestris L.) using open-top chambers. Environ Monitoring and Assessment, 184, 5863–5874.
Tiwari, P. N., Gambhir, P. N., & Rajan, T. S. (1974). Rapid and non-destructive determination of seed oil by pulsed NMR technique. Journal of American Oil Chemist Society, 51, 104–109.
Uprety, D. C., Sangita, S., & Neeta, D. (2010). Rising atmospheric carbon dioxide on grain quality in crop plants. Physiology and Molecular Biology of Plants, 16(3), 215–227.
Vandermeiren, K., Black, C., Pleijel, H., & Temmerman, L. D. (2005). Impact of rising tropospheric ozone on potato: effects on photosynthesis, growth, productivity and yield quality. Plant, Cell & Environment, 28, 982–996.
Vandermeiren, K., De Bock, M., Horemans, N., Guisez, Y., Ceulemans, R., & De Temmerman, L. (2012). Ozone effects on yield quality of spring oilseed rape and broccoli. Atmospheric Environment, 47, 76–83.
Varshney, C. K., & Aggarwal, M. (1992). Ozone pollution in the urban atmosphere of Delhi. Atmospheric Environment, 26, 291–294.
Vingarzan, R. (2004). A review of surface ozone background levels and trends. Atmospheric Environment, 33, 3431–3442.
Wahid, A. (2006). Influence of atmospheric pollutants on agriculture in developing countries: a case study with three new wheat varieties in Pakistan. Science of the Total Environment, 371, 304–313.
Wahid, A., Ahmad, S. S., Zhao, Y., & Bell, J. N. B. (2012). Evaluation of ambient air pollution effects on three cultivars of Sesame (Sesamum indicum L.) by using ethylenediurea. Pakistan Journal of Botany, 44, 99–110.
Wang, X., Zheng, Q., Feng, Z., Xie, J., Feng, Z., Ouyang, Z., & Manning, W. J. (2008). Comparison of a diurnal vs steady-state ozone exposure profile on growth and yield of oilseed rape (Brassica napus L.) in open-top chambers in the Yangtze Delta, China. Environmental Pollution, 156, 449–453.
Zhu, X., Feng, Z., Sun, T., Liu, X., Tang, H., Zhu, J., Guo, W., & Kobayashi, K. (2011). Effects of elevated ozone concentration on yield of four Chinese cultivars of winter wheat under fully open-air field conditions. Global Change Biology, 17, 2697–2706.
Acknowledgments
The financial assistance provided by Indian Agricultural Research Institute, New Delhi-110 012, India in the form of fellowship during the Ph.D. research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Bhatia, A., Tomer, R. et al. Synergistic action of tropospheric ozone and carbon dioxide on yield and nutritional quality of Indian mustard (Brassica juncea (L.) Czern.). Environ Monit Assess 185, 6517–6529 (2013). https://doi.org/10.1007/s10661-012-3043-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-3043-9