Abstract
Increased atmospheric [CO2] is likely to affect photosynthesis, plant growth, and yield potential of plants. Mustard (Brassica juncea L.) is an important oil seed crop that is widely grown in India. Therefore, the impact of elevated [CO2] (585 μmol mol−1) on pigment and protein content, chlorophyll a fluorescence, photosynthetic electron transport reactions, CO2 assimilation, biomass production, and seed yield potential was measured in B. juncea cv Pusa Bold, grown inside free air carbon dioxide enrichment (FACE) rings installed on the campus of Jawaharlal Nehru University, New Delhi, India. Plants were grown for three consecutive winter seasons (2010—2013), in ambient (385 μmol mol−1) or elevated [CO2], in field conditions. Elevated [CO2] had no significant effect on the minimal chlorophyll fluorescence (F 0), while the quantum efficiency of Photosystem II, measured as variable fluorescence (F v = F m–F 0) to maximum fluoresence (F m), increased by 3 %. Electron transport rate, photosystem I, photosystem II, and whole chain electron transport rates increased by 8 % in elevated [CO2]. However, the net photosynthesis rate increased by ≈50 % in three growing seasons under elevated [CO2] condition. The stomatal conductance and transpiration rate decreased resulting in higher photosynthetic water use efficiency. The photosynthesizing surface, i.e., leaf area index substantially increased leading to higher biomass and seed yield under elevated [CO2] condition. Acclimatory downregulation of photosynthesis and plant productivity was not observed in three consecutive growing years suggesting that in the absence of nutrient limitation, B. juncea is highly responsive to elevated CO2 whose yield potential shall increase in changing climatic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concentration of carbon dioxide in the post-industrial era has tremendously risen due to high anthropogenic activities and is expected to reach up to 550 μmol mol−1 by the next 50 years. Plant metabolism is directly affected due to elevated concentrations of CO2. Our knowledge of plant responses to elevated CO2 concentrations mostly stems from studies in plant growth chambers or open top chambers (OTC) under controlled conditions with adequate water and nutrient available to plants and in the absence of weeds, diseases, and interaction with insects. Responses of plants to elevated [CO2], known as the CO2 fertilization effect (Dhakhwa et al. 1997), have been studied in a few crop species (for reviews, see Kimball et al. 2002; Bowes 1993; Long et al. 2004; Ainsworth and Long 2005; Reddy et al. 2010). Currently, ambient CO2 concentration is a limiting factor for C3 photosynthesis, and elevated atmospheric [CO2] is known to increase CO2 fixation because of acceleration of carboxylation over oxygenation mediated by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). The enhanced carboxylation results in a reduced photorespiration (for a review, see Leegood 2007).
Plants with C3 photosynthetic pathway are known to respond better to CO2 enrichment than those with C4 pathway (Amthor 1995; Bowes 1993; Poorter and Roumet 1996; Rogers et al. 1997). Exposure of C3 plants to elevated CO2 generally results in enhanced photosynthesis and carbon assimilation. Higher carbon uptake causes increased relative growth rate (RGR) (Lambers et al. 1998), greater number of mesophyll cells and chloroplasts, enhanced rooting (Chaudhuri et al. 1986; 1990; Rogers et al. 1992), and increase in biomass and yield (Kimball 1983). It also alters plant structure (Pritchard et al. 1999), timing of developmental stages (Bowes 1993), and growth rate (Heineke et al. 1999). Photosynthesis, its quantum yield, and net CO2 fixation rates in plants are increased during short-term exposures (second to hours) to elevated CO2 (Makino and Mae 1999). However, this effect is not sustained for long, and acclimatization takes place with lowered rate of photosynthesis and decline in growth, after days and weeks of CO2 fertilization (Moore et al. 1999; Stitt and Krapp 1999). Growth and above-ground biomass production generally increase, while the response varies with species, growing seasons, and experimental conditions (Jablonski et al. 2002). Elevated CO2 results in taller plants with larger stem diameter, increased branching and leaf number, and increased leaf area and leaf area index (LAI), which is related partly to the accumulation of non-structural carbohydrates (Lambers et al. 1998), Moreover, elevated [CO2] slows transpiration by partial closure of stomata (Jones and Mansfield 1970), and water use efficiency (WUE) is expected to rise due to reduced transpiration (Prior et al. 1991; Allen 1994; Dugas et al. 1997) with increased photosynthesis (Sionit et al. 1981; Morison 1985; Baker et al. 1990). Stomatal conductance decreases in angiosperms, including C4 plants, but not in the conifers (Saxe et al. 1998). Decrease in nitrogen per unit leaf area and increase in the number of flowers, fruits, and seeds lead to a greater individual seed mass and total seed mass but a lower seed nitrogen concentration (Jablonski et al. 2002) under elevated CO2 condition.
Results obtained from free air CO2 enrichment (FACE) rings and open-top chambers are often contradictory, although both are located in the field environment. The open-top chambers are covered from the sides and only partially open from the top. Due to changes in microclimatic conditions, data obtained from these chambers often do not match with the actual open field conditions. However, the FACE ring system is reliable for growing plants at elevated CO2 in the field. The FACE eliminates the limitations of chamber studies in terms of size limitation and artificial microclimatic condition (Long et al. 2004). FACE studies provide the most natural environment to obtain accurate responses of plants to increased [CO2]. Therefore, it is essential to evaluate the impact of elevated CO2 in the field environment by increasing the [CO2] in the plantation zone in the FACE facility. Mostly, 550–600 μmol mol−1 of [CO2] has been used in the FACE system to evaluate plant responses to elevated CO2 (Bernacchi et al. 2003; Leakey et al. 2009; Rogers et al. 2009).
Brassica juncea (mustard) is an important oil producing crop and a number of studies are available on responses of this plant to elevated concentrations of CO2 (Frick et al. 1994; Reekie et al. 1998; Mishra et al. 1999; Uprety and Mahalaxmi 2000; Uprety et al. 2001; Johannessen et al. 2002; Qaderi and Reid 2005; Qaderi et al. 2006). However, all these studies are done in plant growth chambers or open top chambers but not in FACE experiments. Therefore, this study was undertaken on mustard (B. juncea cv Pusa Bold) plants in the FACE system, which provides the most natural environment to ascertain accurate responses of plants to increased CO2. Plants were grown for three consecutive years in ambient and elevated CO2 (585 μmol mol−1) in the FACE facility on the Jawaharlal Nehru University (JNU) campus (see Fig. 1), and their chlorophyll a fluorescence, electron transport rate, photosynthetic CO2 assimilation, growth parameters, and seed yield were monitored. It is shown that although there was only a small increase in Photosystem II (PS II)-dependent electron transport rate, CO2 assimilation rate, above-ground plant dry matter, and seed yield increased substantially in response to high [CO2] mostly due to increased leaf area and carboxylation capacity of Rubisco in high CO2 environment.
Materials and methods
Plant material and growth conditions
Plant material
B. juncea (L.) cv. Pusa Bold, an amphidiploid (genome ab, n = 18) derivative of diploid species Brassica campestris (genome a, n = 10) and Brassica nigra (genome b, n = 8), was used in this work. Its seeds were obtained from the Indian Agricultural Research Institute (IARI), New Delhi, India.
Treatment and sampling
Plants were grown in free air CO2 enrichment facility at Jawaharlal Nehru University, New Delhi (28° 32′ 24″ N, 77° 10′ 2″ E), India, during three growing seasons: 2010–2011, 2011–2012, and 2012–2013 (see Fig. 1). Green manure was added to the soil during preparation of the field for sowing. Nitrogen (N) was provided at the rate of 100 kg ha−1 in the form of urea to the standing crop during vegetative phase. The crop was kept free from weeds by regular weeding operations. The FACE rings were maintained at 585 μmol mol−1 of [CO2]. The FACE ring was surrounded by a ring of eight pipes that released air enriched with CO2. Wind direction, wind velocity, and [CO2] were measured at the center of plot, and this information was used by a computer-controlled system to adjust CO2 flow rate, controlled by a mass-flow control valve, to maintain the target elevated [CO2] at 585 μmol mol−1. The fast feedback proportional integral differential (PID) algorithms were used in response to fluctuations in [CO2] which provided a stable [CO2] elevation. Clear and windless days were chosen for the experiments. All plants received the same agricultural management. Plant material harvested for analysis was either immediately analyzed or stored at −80 °C. In all the experiments dealing with chlorophyll (Chl), protein, fluorescence, and photosynthesis, the 3rd leaf from the top of the shoot was harvested from different plants for analysis.
Pigment and protein estimation
Chl a and Chl b content was estimated, after extraction from leaves, in 80 % acetone, as described by Porra et al. (1989). Protein content of the leaves was measured according to Bradford (1976). Six biological replicates were prepared for analysis for each season.
Morphological observations
The following measurements were made: (i) Plant height (cm) was recorded using a measuring tape; (ii) Number of leaves were evaluated visually; (iii) Leaf area (m2) was measured using Leaf Area Meter (Model LI COR 3000, Li-COR, Lincoln, NE, USA); (v) For fresh weight measurements, each plant was placed in polythene bags and was weighed immediately. The weight of polythene bag was deducted from the total weight to obtain exact weight of the plant. (vi) For dry weight measurement, whole plants, including the leaves, were cut into small pieces, dried in an oven at 80 °C for 72 h and then weighed. (vii) For seed weight, the harvested pods were sun-dried for 3 days, and grains were separated manually. Clean seeds were weighed on a precision balance. (viii) The 1000 seed weight (g) was determined by the mean over 100 grain weight samples, multiplied by 10; and (ix) harvest index (%) was calculated as the ratio of grain weight to total dry matter weight, expressed in percentage.
Chlorophyll a fluorescence measurements
Chl a fluorescence from the ventral side of the third attached leaves of different plants was measured with a PAM-2001 Chl fluorometer (Walz, Germany) at the FACE facility, as described by Dutta et al. (2009). Before each measurement, the sample leaf was dark-adapted for 20 min (Demmig et al. 1987). Optimum quantum efficiency of Photosystem II (PS II) was calculated as F v/F m = (F m − F 0)/F m (Schreiber and Armond 1978), where F 0 is the minimum fluorescence, F m is the maximum fluorescence, and F v is the variable fluorescence. Electron transport rate (ETR) was calculated by the formula described by Schreiber et al. (1994): ETR = Yield (φPS II) × PAR × 0.5 × 0.84, where the yield is the overall photochemical quantum yield (estimated from F v′/F m′, where F v′ and F m′ are variable and maximum Chl fluorescence under light); PAR is flux density of incident photochemically active radiation (μmol photons m−2 s−1); the 0.5 factor is used because transport of one electron requires absorption of two quanta by the two photosystems, i.e., it assumes that PS II:PS I ratio is 1:1; the use of 0.84 assumes that 84 % of the incident quanta are absorbed by the leaf. Non-photochemical quenching was calculated from the formula NPQ = F m − F m′/F m′ (see Schreiber 2004).
Isolation of thylakoid membranes
Thylakoid membranes were isolated from leaves of ambient and elevated [CO2]-grown plants, in ice-cold 0.4 M sucrose, 10 mM NaCl, and 50 mM Hepes/KOH buffer pH 7.6, as described by Tripathy and Mohanty (1980).
Electron transport assay
Assays of electron transport activity of the whole chain, PS II and PS I were carried out using a glass cuvette fitted within a Clark-type oxygen electrode (Hansatech, UK), as described by Tripathy and Chakraborty (1991). The reaction was maintained at 25 °C by using a temperature controlled water bath, and the samples were illuminated for 20 s using a tungsten light source at a photon flux rate of 1500 μmol photons m−2 s−1. The whole chain electron transport from H2O to methylviologen (MV) (1 mM) was monitored as O2 uptake. Assay mixture (3 mL) consisted of 50 mM Hepes (pH 7.5), 10 mM NaCl, 1 mM NH4Cl, 3 mM MgCl2, 1.0 mM NaN3, and 0.5 mM MV. Chloroplasts were added to the above reaction mixture to a total concentration of 50 μg Chl. PS II activity was monitored as O2 evolution from H2O to p-phenylenediamine (PD). The 3 mL reaction mixture for PD (p-phenylene diamine)-supported O2 evolution assay consisted of 50 mM Hepes buffer (at pH 7.5), 3 mM MgCl2, 10 mM NaCl, and freshly prepared PD (0.5 mM). The partial electron transport chain through PS I was measured as oxygen consumption, where ascorbate (1 mM)/dichlorophenol indophenol (DCIP) (0.1 mM) couple was used as electron donor to PS I and MV (1 mM) as electron acceptor; in this case, electron flow from PS II to PS I was blocked by 3-(3,4-dichlorophenyl)-1,1-dimethyl urea (DCMU) (20 μM).
Photosynthesis rate measurements
Photosynthesis rate, stomatal conductance, and transpiration rate measurements were made in the FACE facility using infrared gas analyzer (LICOR 6400 XT portable photosynthetic system). CO2 concentration was maintained at 385 μmol mol−1 for ambient CO2-grown plants and 585 μmol mol−1 for elevated CO2-grown plants. Leaves were pre-exposed to 6400-02B LED light source for 15 min at 1000 μmol photons m−2 s−1 before CO2 assimilation was monitored. The 6400-02B provided an actinic light source to drive photosynthesis and a blue component to control stomata (Zeiger, et al. 1987). Red LED’s provided radiant output at 665 nm ± 10 nm at 25 °C while blue LED’s had an output at 470 nm ± 10 nm at 25 °C. Leaf temperature was maintained at 25 °C. Water use efficiency was calculated as net photosynthetic assimilation rate/transpiration rate. Six replications were made in different location in the field for calculation of all photosynthetic parameters in every growing season.
Statistical analyses
Excel was used for the statistical analyses. After the calculation of averages, standard deviations and standard errors for each of the growth parameters were determined at each of the three growing seasons, a t test was used to assess the difference between ambient and elevated CO2-grown plants in each parameter (Biswal et al. 2012).
Results
Photosynthetic pigments
The Chl content of Brassica plants exposed to higher [CO2] (585 μmol mol−1) was marginally downregulated. Our measurements of Chl content for three consecutive years revealed that it declined by 3.3, 4.5, and 4.8 % in 2010–2011, 2011–2012, and 2012–2013, respectively (Fig. 2a). Further, on an average, carotenoid content decreased by 3, 2.3, and 2.0 % in plants grown at elevated CO2 (Fig. 2b). However, no significant change in Chl a/b ratio was observed (Fig. 2c).
Chlorophyll content (a), carotenoid content (b), Chl a/b ratio (c), and total protein content (d) of Pusa Bold leaves; plants were grown in ambient and elevated CO2 (585 μmol mol−1) in three different growing seasons. Each data point is the average of six replicates and the error bars represent standard errors (SE). Asterisks indicate significant differences determined by t test (*P < 0.05, **P < 0.001)
Protein
The protein content of leaves for three consecutive years revealed that on an average, it declined by 1.58, 1.8, and 3 % in mustard plants grown in elevated CO2 .in 2010–2011, 2011–2012, and 2012–2013, respectively (Fig. 2d).
Chlorophyll fluorescence
Chl a fluorescence measurement is used as a nondestructive and non-invasive signature of photosynthesis (for reviews, see Krause and Weiss 1991; Govindjee 1995, 2004; Baker 2008). Chl a fluorescence transient of dark-adapted (20 min) leaves was measured in all the growing seasons, using a plant efficiency analyzer (Hansatech, UK). The minimal Chl fluorescence (F 0) was almost similar in plants grown in ambient and elevated CO2 (Fig. 3a). The maximum primary photochemical efficiency of PS II, which was measured as F v/F m, where F v = F m–F 0, was slightly higher (3 %) in plants grown in elevated CO2 (Fig. 3c). Pulse amplitude-modulated (Walz, Germany) Chl a fluorescence measurements revealed that the electron transport rate (ETR) (μmole electrons m−2 s−1) of PS II increased in response to photosynthetic active radiation (PAR). The light response curves demonstrate that ETR in limiting (10–80 μmol photons m−2 s−1), as well as in saturating light intensities (1200 μmol photons m−2 s−1), was marginally higher (8 %) in plants grown in elevated [CO2] (Fig. 3d). Non-photochemical quenching (NPQ) of excited state of Chl increased in response to light intensity. The NPQ was slightly reduced (6–7 %) in plants grown in elevated [CO2] at all light intensities measured (Fig. 3e).
Chlorophyll fluorescence measurements on Brassica juncea L. cv Pusa Bold leaves; plants were grown in ambient and elevated CO2 (585 μmol mol−1) showing minimal fluorescence (F 0) (a), maximal fluorescence (F m) (b), and F v/F m ratio, where F v = F m−F 0 (c), Electron transport rate, as calculated from formula: ETR = Yield × PAR × 0.5 × 0.84 (d), Non photochemical quenching (NPQ) of excited state of chlorophyll, as calculated by NPQ = F m−F m′/F m′ where F m is maximal fluorescence yield of a dark-adapted sample and F m′ is maximal fluorescence yield reached during last saturation pulse (e). Each data point is the average of twenty five replicates for a, b, c and six replicates for d and e. Error bars represent SE. Asterisks indicate significant differences determined by t test (*P < 0.05, **P < 0.001)
Photochemical reactions
Light-saturated whole chain electron transport (H2O → methylviologen (MV)) rate, the partial reaction of PS II (H2O → phenylenediamine (PD)) and that of PS I (ascorbate/dichlorophenol indophenol (DCIP) → MV) were monitored polarographically in the thylakoid membrane suspensions, obtained from leaves of plants grown in ambient and elevated [CO2] conditions (Tripathy et al. 2007 and cited literature therein). Electron transfer rates of PS I, measured as oxygen uptake, in thylakoid membranes isolated from leaves of plants grown in elevated [CO2] were slightly higher (6–7 %) (Fig. 4a). As compared to plants grown in ambient [CO2], the partial reaction of PS II, measured as O2 evolution, was 6–8 % higher in thylakoid membranes isolated from high-[CO2]-grown plants (Fig. 4b). The whole chain electron transport rate, measured as O2 uptake, was also 7–8 % higher in elevated [CO2] (Fig. 4c).
Electron transport through Photosystem I (PS I) (ascorbate to methylviologen; O2 uptake) (a), Photosystem II (PS II) (O2 evolution; water to phenylene diamine) (b), and the whole chain (water to methylviologen; O2 uptake) (c), as measured polarographically in Brassica juncea L. cv Pusa Bold plants, grown in ambient and elevated CO2 (585 μmol mol−1). Each data point is the average of six replicates and the error bars represent SE. Asterisks indicate significant differences determined by t test (*P < 0.05, **P < 0.001)
Photosynthetic CO2 assimilation
Photosynthetic CO2 assimilation of attached leaves of plants grown in ambient and elevated CO2 was monitored using an infrared gas analyzer using red and blue 6400-02B LED light source at a light intensity of 1000 μmol photons m−2 s−1. The photosynthetic CO2 assimilation rates were measured at 400 μmoles mole−1 of [CO2] in plants grown in ambient and 585 μmol mole−1 of [CO2] in elevated CO2. The rate of photosynthesis (μmole CO2 assimilation m−2 of leaf area s−1) increased by 42, 48, and 54 % in 2010–2011, 2011–2012, and 2012–2013 growing seasons, respectively, in high-CO2-grown plants (Fig. 5a).
Net CO2 assimilation rate (a), stomatal conductance (b), transpiration rate (c), and water-use efficiency (d) of attached leaves of Brassica juncea L. cv Pusa Bold plants, monitored by infrared gas analyzer, IRGA (Licor 6400-XT portable photosynthetic system) in ambient CO2 and elevated CO2 (585 μmol mol−1) in three different growing seasons at 1000 μmols of photons m−2 s−1 at 20 °C. Each data point is the average of six replicates and the error bars represent SE. Asterisks indicate significant differences determined by t test (*P < 0.05, **P < 0.001)
Stomatal conductance (gs) (mol H2O m−2 s−1) decreased by 24, 23, and 22 % during 2010–2011, 2011–2012, and 2012–2013 growing seasons, respectively, in plants grown in elevated [CO2] (Fig. 5b). The decreased stomatal conductance resulted in reduced transpiration rate (mol H2O m−2 s−1) by 37, 40, and 41 % in the above-mentioned years in elevated [CO2] (Fig. 5c). Therefore, the photosynthetic water-use efficiency (μmol CO2 m−2 s−1/mol H2O m−2 s−1) (Fig. 5d) increased due to increased photosynthesis and decreased transpiration rates. The water-use efficiency increased in elevated CO2 by 126, 145, and 165 % in 2010–2011, 2011–2012, and 2012–2013, respectively.
Leaf number and area
The number of leaves per plant increased by 51, 57, and 63 % in three different growing seasons in high CO2 (Fig. 6a). Similarly, in plants grown in elevated CO2, the average increase of total leaf area per plant (m2 plant−1) was 36, 43, and 45 % in 2010–2011, 2011–2012, and 2012–2013, respectively (Fig. 6b). Further, the leaf area index (LAI, leaf area m−2 of land area) increased by 22, 31, and 34 % in plants grown under elevated CO2 (Fig. 6c), and these plants were significantly taller than those grown in ambient CO2.
Number of leaves (a), leaf area (b), and leaf area index (c) of Brassica juncea L. cv Pusa Bold leaves grown in ambient and elevated CO2 (585 μmol mol−1) in three different growing seasons. Each data point is the average of six replicates and the error bars represent SE. Asterisks indicate significant differences determined by t test (*P < 0.05, **P < 0.001)
Fresh weight and dry weight
Growth under elevated CO2 conditions resulted in an increase in height by 12, 14, and 17 % in 2010–2011, 2011–2012, and 2012–2013, respectively (Fig. 7a, b) Measurements of fresh weight of plants grown in ambient and elevated CO2 for consecutive 3 years revealed that on average plants had greater fresh weight due to enhanced growth of all plant parts. Shoot fresh weight of high-CO2-grown plants increased by 81, 94, and 103 % in 2010–2011, 2011–2012, and 2012–2013 (Fig. 7c). Similarly, these plants produced significantly higher shoot dry matter in elevated CO2 and had 93, 99, and 112 % increase in plant dry weight (Fig. 7d).
Plant height (a, b), fresh weight per plant (c), and dry weight per plant (d) of Brassica juncea L. cv Pusa Bold leaves grown in ambient and elevated CO2 (585 μmol mol−1) in three different growing seasons. Each data point is the average of six replicates and the error bars represent SE. Asterisks indicate significant differences determined by t test (*P < 0.05, **P < 0.001)
Seed dry weight and harvest index
Measurement of seed yield revealed that in high CO2, the average seed production per plant increased by 21, 24, and 26 % (Fig. 8a). The 1000-seed weight increased by 35, 37, and 40 % in 2010–2011, 2011–2012, and 2012–2013, respectively, as compared to those from plants grown in ambient CO2 (Fig. 8b, c). However, harvest index (HI) of these plants had a smaller increase, i.e., 7, 8, and 12 %, respectively, during the three growing seasons (Fig. 8d).
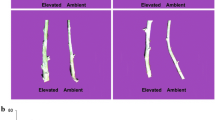
Seed weight per plant (a), 1000 seed weight (b), seed morphology (c), and harvest index (%) (d) of Brassica juncea L. cv Pusa Bold leaves grown in ambient and elevated CO2 (585 μmol mol−1) in three different growing seasons. Each data point is the average of 50 replicates and the error bars represent SE. Asterisks indicate significant differences determined by t test (*P < 0.05, **P < 0.001)
Discussion
Adjustment of photosynthetic apparatus and sustainability of photosynthesis to elevated CO2 are critical for plants for their growth and development in changing climatic conditions. The climate of Delhi, India, is favorable for the growth of Brassica. In this paper, we were able to assess the direct effect of elevated CO2 on photosynthesis and plant productivity under field conditions in the absence of nutrient deficiency and water stress.
Chl is the central part of the energy manifestation of each and every green plant. Therefore, any significant alteration in its levels is likely to cause a marked effect on plant metabolism and growth (Pattanayak and Tripathy 2011; Biswal et al. 2012). Elevated [CO2] tends to alter the foliar chemistry of plants (Lindroth et al. 2001). The Chl and leaf protein contents were lower by 3–5 and 1.5–3 %, respectively, in B. juncea plants grown in CO2-enriched environment than the ambient ones. Previous reports also mention a decrease in Chl and carotenoid content in elevated [CO2] in soybean grown in FACE facility and barley and wheat in open-top chamber (Sicher and Bunce 1997). On the other hand, Nie et al. (1995) found no change in the amount of Chl in wheat grown under elevated [CO2] in FACE experiments. In a comparative study employing five different plant species, Sage et al. (1989) observed no consistent response of leaf Chl or leaf N to high [CO2] in greenhouse environment. Chl content declined in Chenopodium album, and there was no change in Phaseolus vulgaris, Solanum melongena, and Solanum tuberosum. However, species that exhibited decreased leaf Rubisco content during growth in elevated [CO2] also exhibited a low Chl content (Sicher et al. 1994). In contrast, high [CO2] was reported to increase the total chlorophyll content in Quercus suber seedlings under greenhouse conditions (Faria et al. 1996). In our experiments, elevated CO2 concentration led to partial downregulation of Chl and total protein content. However, the ratio of Chl a to Chl b remained the same with an increase in CO2 concentration. Our results demonstrate that the growth of plants increases in high [CO2]. Therefore, Chl content and growth responses are not necessarily positively correlated under high [CO2] conditions. High photon flux density decreases chlorophyll content and increases Chl a to Chl b ratio (Biswal et al. 2012). Optimum light absorption is needed to utilize the increased supply of CO2 in the FACE ring. Changes in the leaf Chl content in response to elevated CO2 are not due to the increase in light intensity as both ambient and elevated FACE rings received almost equal solar light intensity. Growth in elevated CO2 had affected the N status of mustard leaves. Marginal downregulation of protein content in successive growing seasons could be due to slightly limited nutrient availability to plants because of increased soil carbon sequestration and soil acidification. Collectively, these results suggest that plant growth in elevated [CO2] may have a broad impact on the Chl and protein contents of plants.
Elevated [CO2] has different effects on photochemistry. In the present experiment, the ETR (see “Materials and methods”) and F v/F m (a measure of maximum quantum efficiency of PS II) of leaves increased by 8 and 3 %, respectively, in high [CO2] which suggests that the PS II was modulated marginally. Further, Murray et al. (2000) observed that under conditions of high nitrogen (N) supply, the maximum rate of electron transport (J max) did not differ in response to elevated [CO2] in Sitka spruce (Picea sitchensis). In our experiments, the NPQ of Chl fluorescence (a measure of heat dissipation, see Demmig-Adams et al. 2014) decreased by 6–7 % in the elevated CO2-grown B. juncea plants (Fig. 3e). Our measurements (Figs. 3 and 4) revealed only a small (3–5 %) increase in PS I, PS II, and whole chain electron transport in high-CO2-grown plants. This could be attributed partly to a small decline in Chl content of plants in high [CO2]. However, the increased PS II photochemical activity corroborates well with slightly higher ETR which is a function of φPS II (yield), among other parameters. In elevated [CO2] in a FACE facility, φPS II was shown to either increase or decrease under different developmental conditions, as observed in Loblolly Pine (Hymus et al. 1999). In the carbon reduction cycle, to reduce phosphoglyceric acid to phosphoglyceraldehyde, higher NADPH is required and RuBP regeneration needs high ATP to run carbon reduction cycle efficiently at the elevated [CO2]. However, we did not observe any substantial increase in the electron transport rate or PS II, PS I, or whole chain activities that could have provided ATP and NADPH to sustain increased demand of carbon reduction. This suggests that with appropriate nitrogen supply, electron transport rate is not a limitation for increased carbon reduction at elevated CO2 in our measurements on mustard plants.
Our study reveals that photosynthetic rate significantly increased in mustard plants in response to elevated [CO2] repeatedly for three consecutive seasons. Similarly, the rate of CO2 assimilation also increased in soybean and poplar grown in elevated [CO2] inside FACE rings (Bernacchi et al. 2003; Ainsworth and Rogers 2007). Our results did not show downregulation of photosynthesis. Similarly, Garcia et al. (1998) found little evidence of a decline in photosynthetic capacity of spring wheat under field conditions, using FACE. In their study, photosynthesis increased substantially for the entire life of the crop. In our study, stomatal conductance decreased in mustard plants with elevated [CO2] (Fig. 5b) that might have helped leaves to prioritize water for leaf expansion over transpiration. The decreased stomatal conductance resulted in reduced transpiration rate. Increased photosynthesis and decreased transpiration rate per unit leaf area led to increased photosynthetic water-use efficiency of mustard plants grown under elevated [CO2] (Fig. 5d). Water-use efficiency is strongly affected by stomatal density (Woodward and Kelly 1995). Both stomatal density and stomatal index of leaves, which are negatively correlated with elevated [CO2], have decreased over the past 100 years (Woodward 1987). Improved water status of plants, due to partial closure of stomata, causes a higher turgor pressure, which could stimulate leaf expansion (Lenssen and Rozema 1990). Our results reveal that not only photosynthesis rate but photosynthesizing surface, i.e., leaf area per plant and leaf area index increase 30–40 % and 25–34 %, respectively, with high [CO2] indicating a strong morphogenic effect of CO2 on leaf initiation. Elevated [CO2] has varied effects on leaf area index from very little or no significant increase (Pinter et al. 1996; Weerakoon et al. 2000; Baker et al. 1990; Ziska et al. 1997) to a significant increase (Wilson et al. 1999; Bunce 2001) in different plant species. The rate of transpiration of plant increases with leaf area of the plant. The increased leaf area per plant (Fig. 6b) is likely to offset effects of reduced stomatal conductance on transpiration. Some studies have shown that increased leaf area can more than compensate for reductions in stomatal conductance and can actually increase water use per plant at elevated [CO2] (Samarakoon and Gifford 1995).
In the present study, the increased photosynthesis rate coupled with a higher leaf area per plant led to increased biomass and yield under elevated [CO2]. We did not observe any downregulation of photosynthesis per unit leaf area in B. juncea and the acclimatory loss of photosynthesis, if any, in other species could be offset by morphological characteristics, such as greater leaf area leading to increased biomass and yield. Elevated [CO2] is known to increase photosynthesis during different phenological phases resulting in increased dry matter production (Mitchell et al. 1999; Lawlor and Mitchell 2000; Ziska et al. 2004). On average across several species and under unstressed conditions, recent data analyses show that, compared to current atmospheric CO2 concentrations, crop yield increases at 550 μmol mol−1 [CO2] are in the range of 10–20 % for C3 crops and 0–10 % for C4 crops (Ainsworth et al. 2004; Gifford 2004; Long et al. 2004). Increases in economic yield, i.e., seed production were 21–26 % in B. juncea at elevated CO2 (Fig. 8a). Furthermore, 1000-seed weight increased by 35–40 % (Fig. 8b) demonstrating that the higher seed yield was mostly due to increased grain filling from long-lasting leaves whose senescence was substantially delayed by 10 days.
In conclusion, percent increase in seed yield was lower than the increase in total biomass in elevated CO2. If most of the additional photosynthate produced in elevated CO2 would have been used for economic yield, i.e., increased seed production, a much higher grain output should have been possible in changing climatic conditions. Further studies should be directed towards augmenting the economic yield from the available increased photosynthate (increase in harvest index) produced in high [CO2] environment. We did not observe acclimatory downregulation of photosynthesis and plant productivity in high [CO2] for three consecutive growing years. These clearly suggest that in the absence of any kind of nutrient limitation, B. juncea is highly responsive to elevated CO2 whose yield potential shall increase in changing climatic conditions. However, the increases in overall biomass are important towards the goal of obtaining bioenergy for other purposes.
Abbreviations
- Chl:
-
Chlorophyll
- DCIP:
-
2,6-dichloroindophenol
- DCMU:
-
3-(3,4-dichlorophenyl) 1,1-dimethyl urea
- FACE:
-
Free air carbon dioxide enrichment
- F 0 :
-
Minimal chlorophyll fluorescence
- F m :
-
Maximal chlorophyll fluoresecence
- F v/F m :
-
Optimum quantum efficiency of PS II
- MV:
-
Methylviologen
- NPQ:
-
Non-photochemical quenching of fluorescence
- OTC:
-
Open top chamber
- PD:
-
p-phenylenediamine
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- PAM:
-
Pulse amplitude modulation
- PAR:
-
Photosynthetically active radiation
- Rubisco:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- WUE:
-
Water-use efficiency
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Ainsworth EA, Rogers A, Nelson R, Long SP (2004) Testing the ‘source–sink’ hypothesis of downregulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122:85–94
Allen LH (1994) Carbon dioxide increase: direct impact on crops and indirect effects mediated through anticipated climatic changes. In: Boote KJ, Sinclair TR, Bennett JM (eds) Physiology and determination of crop yield. ASA, SSSA, Madison, pp 425–459
Amthor JS (1995) Terrestrial higher-plant response to increasing atmospheric CO2 in relation to the global carbon cycle. Glob Chang Biol 1:243–274
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baker JY, Allen LH Jr, Boote KJ (1990) Growth and yield responses of rice to carbon dioxide concentration. J Agric Sci 115:313–320
Bernacchi CJ, Calfapietra C, Davey PA, Wittig VE, Scarascia-Mugnozza GE, Raines CA, Long SP (2003) Photosynthesis and stomatal conductance responses of poplars to free-air CO2 enrichment (PopFACE) during the first growth cycle and immediately following coppice. New Phytol 159:609–621
Biswal AK, Pattanayak GK, Pandey SS, Leelavathi S, Reddy VS, Govindjee TBC (2012) Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase (CAO) in tobacco. Plant Physiol 159:433–459
Bowes G (1993) Facing the inevitable: plants and increasing atmospheric CO2. Annu Rev Plant Biol Plant Mol Biol 44:309–332
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bunce JA (2001) Seasonal patterns of photosynthetic response and acclimation to elevated carbon dioxide in field-grown strawberry. Photosynth Res 68:237–245
Chaudhuri UN, Burnett RB, Kirkham MB, Kanemasu ET (1986) Effect of carbon dioxide on sorghum yield, root growth, and water use. Agric For Meteorol 37:109–122
Chaudhuri UN, Kirkham MB, Kanemasu ET (1990) Root growth of winter wheat under elevated carbon dioxide and drought. Crop Sci 30:853–857
Demmig B, Winter K, Krüger A, Czygan FC (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84:218–224
Demmig-Adams B, Garab G, Adams WW III, Govindjee (eds) (2014) Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria. advances in photosynthesis and respiration, volume 40. Springer, Dordrecht
Dhakhwa GB, Campbell CL, LeDuc SK, Cooter EJ (1997) Maize growth: assessing the effects of global warming and CO2 fertilization with crop models. Agric For Meteorol 87:253–272
Dugas WA, Prior SA, Rogers HH (1997) Transpiration from sorghum and soybean growing under ambient and elevated CO2 concentrations. Agric For Meteorol 83:37–48
Dutta S, Mohanty S, Tripathy BC (2009) Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol 150:1050–1061
Faria T, Wilkins D, Besford RT, Vaz M, Pereira JS, Chaves MM (1996) Growth at elevated CO2 leads to down regulation of photosynthesis and altered response to high temperature in Quercus suber L. seedlings. J Exp Bot 47:1755–1761
Frick J, Nielsen S, Mitchell CA (1994) Yield and seed oil content response of dwarf, rapid-cycling Brassica to nitrogen treatments, planting density, and carbon dioxide enrichment. J Am Soc Hortic Sci 119:1137–1143
Garcia RL, Long SP, Wall GW, Osborne CP, Kimball BA, Nie GY, Pinter PJ Jr, LaMorte RL, Wechsung F (1998) Photosynthesis and conductance of spring-wheat leaves: field response to continuous free-air atmospheric CO2 enrichment. Plant Cell Environ 21:659–669
Gifford RM (2004) The CO2 fertilising effect—does it occur in the real world? New Phytol 163:221–225
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol (now Funct Plant Biol) 22:131–160
Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee (eds) Chlorophyll fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 1–42
Heineke D, Kauder F, Frommer W, Kuhn C, Gillissen B, Ludewig F, Sonnewald U (1999) Application of transgenic plants in understanding responses to atmospheric change. Plant Cell Environ 22:623–628
Hymus GJ, Ellsworth DS, Baker NR, Long SP (1999) Does free-air carbon dioxide enrichment affect photochemical energy use by evergreen trees in different seasons? A chlorophyll fluorescence study of mature loblolly pine. Plant Physiol 120:1183–1191
Jablonski LM, Wang X, Curtis PS (2002) Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol 156:9–26
Johannessen MM, Mikkelsen TN, Jorgensen RB (2002) CO2 exploitation and genetic diversity in winter varieties of oilseed rape (Brassica napus); varieties of tomorrow. Euphytica 128:75–86
Jones MB, Mansfield TA (1970) A circadian rhythm in the level of the carbon dioxide compensation point in Briophyllum and Coffea. J Exp Bot 21:159–163
Kimball BA (1983) Carbon dioxide and agricultural yields: an assemblage and analysis of 430 prior observations. Agron J 75:779–788
Kimball BA, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. Adv Agron 77:293–368
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, New York
Lawlor DW, Mitchell RAC (2000) Crop ecosystem responses to climatic change: wheat. In: Reddy KR, Hodges HF (eds) Climate change and global crop productivity. CABI Publishing, Wallingford, pp 57–80
Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60:2859–2876
Leegood RC (2007) A welcome diversion from photorespiration. Nat Biotechnol 25:539–540
Lenssen GM, Rozema J (1990) The effect of atmospheric CO2-enrichment and salinity on growth, photosynthesis and water relations of salt marsh species. In: Goudriaan J, Van Keulen H, Van Laar HH (eds) The greenhouse effect and primary productivity in european agroecosystems. Pudoc, Wageningen, pp 64–67
Lindroth RL, Kopper BJ, Parsons WF, Bockheim JG, Karnsosky DF, Hendry GR, Pregitzer KS, Isebrands JG (2001) Consequences of elevated carbon dioxide and ozone for foliar chemical composition and dynamics in trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera). Environ Pollut 115:395–404
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Makino A, Mae T (1999) Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol 40:999–1006
Mishra RS, Abdin MZ, Uprety DC (1999) Interactive effects of elevated CO2 and moisture stress on the photosynthesis, water relation and growth of Brassica species. J Agron Crop Sci 182:223–229
Mitchell RAC, Black CR, Burkart S, Burke JI, Donnelly A, de Temmmerman L, Fangmeier A, Mulholland BJ, Theobald JC, van Oijen M (1999) Photosynthetic responses in spring wheat grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment ‘ESPACE-wheat’. Eur J Agron 10:205–214
Moore BD, Cheng S-H, Sims D, Seemann JR (1999) The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ 22:567–582
Morison JIL (1985) Sensitivity of stomata and water use efficiency to high CO2. Plant Cell Environ 8:467–474
Murray MB, Smith RI, Friend A, Jarvis PG (2000) Effect of elevated [CO2] and varying nutrient application rates on physiology and biomass accumulation of Sitka spruce (Picea sitchensis). Tree Physiol 20:421–434
Nie GY, Long SP, Garcia RL, Kimball BA, Lamorte RL, Pinter PJ, Wall GW, Webber AN (1995) Effects of free-air co2 enrichment on the development of the photosynthetic apparatus in wheat, as indicated by changes in leaf proteins. Plant Cell Environ 8(18):855–864
Pattanayak GK, Tripathy BC (2011) Overexpression of protochlorophyllide oxidoreductase c regulates oxidative stress in arabidopsis. PLoS ONE 6(10):e26532
Pinter PJ Jr, Kimball BA, Garcia RL, Wall GA, Hunsaker DJ, LaMorte RL (1996) Free-air CO2 enrichment: responses of cotton and wheat crops. In: Koch GW, Mooney HA (eds) Carbon dioxide and terrestrial ecosystems. Academic, San Diego, pp 215–249
Poorter HC, Roumet CBD (1996) Interspecific variation in the growth response of plants to elevated CO2: a search for functional types. In: Körner C, Bazzaz FA (eds) Carbon dioxide, populations, communities. Physiological ecology series. Academic, San Diego, pp 375–412
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standard by atomic absorption spectrometry. Biochim Biophys Acta 975:384–394
Prior SA, Rogers HH, Sionit N, Patterson RP (1991) Effects of elevated atmospheric CO, on water relations of soya bean. Agric Ecosyst Environ 35:13–25
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Glob Chang Biol 5:807–837
Qaderi MM, Reid DM (2005) Growth and physiological responses of canola (Brassica napus) to UV-B and CO2 under controlled environment conditions. Physiol Plant 125:247–259
Qaderi MM, Kurepin LV, Reid DM (2006) Growth and physiological responses of canola (Brassica napus) to three components of global climate change: temperature, carbon dioxide and drought. Physiol Plant 128:710–721
Reddy AR, Rasineni GK, Raghavendra AS (2010) The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr Sci 99:48–57
Reekie EG, MacDougall G, Wong I, Hicklenton PR (1998) Effects of sink size on growth response to elevated atmospheric CO2 within the genus Brassica. Can J Bot 76:829–835
Rogers HH, Peterson CM, McCrimmon JM, Cure JD (1992) Response of soybean root to elevated atmospheric carbon dioxide. Plant Cell Environ 15:749–752
Rogers HH, Runion GB, Krupa SV, Prior SA (1997) Plant responses to atmospheric CO2 enrichment: implications in root-soilmicrobe interactions. In: Allen LH Jr, Kirkham MB, Olszyk DM, Whitman CE (eds) Advances in carbon dioxide effects research. ASA Special Publication No. 61. ASA, CSSA, and SSSA, Madison, pp 1–34
Rogers A, Ainsworth EA, Leakey DB (2009) Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol 151(3):1009–1016
Sage RF, Sharkey TD, Seeman JR (1989) Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiol 89:590–596
Samarakoon AB, Gifford RM (1995) Soil water content under plants at high CO2 concentration and interactions with the direct CO2 effects: a species comparison. J Biogeogr 22:193–202
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436
Schreiber U, Armond PA (1978) Heat-induced changes of chlorophyll fluorescence in isolated chloroplasts and related heat-damage at the pigment level. Biochim Biophys Acta 502:138–151
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Galdwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 49–70
Schreiber U, Papageorgiou GC, Govindjee (2004) Pulse amplitude modulation (PAM) fluorometry and saturation pulse method: an overview. In: Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration, volume 19. Springer, Dordrecht, pp 279–319
Sicher RC, Bunce JA (1997) Relationship of photosynthetic acclimation to changes of Rubisco activity in field-grown winter wheat and barley during growth in elevated carbon dioxide. Photosynth Res 52:27–38
Sicher RC, Kremer DF, Rodermel SR (1994) Photosynthetic acclimation to elevated CO2 occurs in transformed tobacco with decreased ribulose-1,5-bisphosphate carboxylase/oxygenase content. Plant Physiol 104:409–415
Sionit N, Strain BR, Hellmers H, Kramer PJ (1981) Effects of atmospheric CO2 concentrations and water stress on water relations of wheat. Bot Gaz 142:191–196
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22:583–621
Tripathy BC, Chakraborty N (1991) 5-Aminolevulinic acid induced photodynamic damage of the photosynthetic electron transport chain of cucumber (Cucumis sativus L.) cotyledons. Plant Physiol 96:761–767
Tripathy BC, Mohanty P (1980) Zinc-inhibited electron transport of photosynthesis in isolated barley chloroplasts. Plant Physiol 66:1174–1178
Tripathy BC, Mohapatra A, Gupta I (2007) Impairment of the photosynthetic apparatus by oxidative stress induced by photosensitization reaction of protoporphyrin IX. Biochim Biophys Acta 1767:860–868
Uprety DC, Mahalaxmi V (2000) Effect of elevated CO2 and nitrogen nutrition on photosynthesis, growth and carbon-nitrogen balance in Brassica juncea. J Agron Crop Sci 184:271–276
Uprety DC, Dwivedi N, Mohan R, Paswan G (2001) Effect of elevated CO2 concentration on leaf structure of Brassica juncea under water stress. Biol Plant 44:149–152
Weerakoon WMW, Ingram KT, Moss DN (2000) Atmospheric carbon dioxide and fertilizer nitrogen effects on radiation interception by rice. Plant Soil 220:99–106
Wilson KB, Carlson TN, Bunce JA (1999) Feedback significantly influences the simulated effect of CO2 on seasonal evapotranspiration from two agricultural species. Glob Chang Biol 5:903–917
Woodward FI (1987) Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327:617–618
Woodward FI, Kelly CK (1995) The influence of CO2 concentration on stomatal density. New Phytol 131:311–327
Zeiger E, Iino M, Shimazaki K, Ogawa T (1987) The blue-light response of stomata: mechanism and function. In: Zeiger E, Farquar DD, Cowan IR (eds) Stomatal function. Stanford University Press, Stanford, pp 209–227
Ziska LH, Namuco O, Moya T, Quilang J (1997) Growth and yield response of field-grown tropical rice to increasing carbon dioxide and air temperature. Agron J 89:45–53
Ziska LH, Morris CF, Goins EW (2004) Quantitative and qualitative evaluation of selected wheat varieties released since 1903 to increasing atmospheric carbon dioxide: can yield sensitivity to carbon dioxide be a factor in wheat performance? Glob Chang Biol 10:1810–1819
Acknowledgments
This work was supported by a grant from the Department of Biotechnology, Government of India (BT/PR14827/BCE/08/841/2010) to BCT.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Ruhil, K., Sheeba, Ahmad, A. et al. Photosynthesis and growth responses of mustard (Brassica juncea L. cv Pusa Bold) plants to free air carbon dioxide enrichment (FACE). Protoplasma 252, 935–946 (2015). https://doi.org/10.1007/s00709-014-0723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0723-z