Abstract
A study was performed in Del Rey Lagoon, City of Los Angeles, to determine if the lagoon was as a source or sink for fecal indicator bacteria (FIB: total coliforms, Escherichia coli, enterococci) and to screen for the presence of other potentially pathogenic bacteria. The lagoon receives tidal flows from the adjacent Ballona Estuary whose water usually is contaminated with FIB originating from the highly urbanized Ballona Creek Watershed. During 16 sampling events from February 2008 through March 2009, replicate water samples (n = 3) were collected 1 h prior to the high tide and 1 h prior to the following low tide. FIB concentrations were measured by the defined substrate method (IDEXX, Westbrook, Me) followed by culturing of bacterial isolates sampled from positive IDEXX Quanti-Tray wells and were identified using the Vitek 2 Compact (bioMérieux, Durham, NC). Mean concentrations of FIB often differed by an order of magnitude from flood to ebb flow conditions. The lagoon tended to act as a sink for total coliforms based on the ratio of mean flood to ebb densities (R F/E) >1.0 during 56 % of the sampling events and during ebb flows, as a source for E. coli and enterococci (R F/E <1.69 % of events). Approximately 54 species were identified from 277 isolates cultured from the IDEXX Quanti-Trays. Of these, 54 % were species known to include pathogenic strains that can be naturally occurring, introduced in runoff, or originated from other sources. Diversity and cluster analyses indicated a dynamic assemblage that changes in species composition with day-to-day fluctuations as well as tidal action. The concept of monitoring the lagoon and estuary as a sentinel habitat for pathogenic assemblages is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
California's coastline is a major resource for state fisheries, recreation, tourism, biodiversity, and esthetics, so achieving and maintaining good water quality is of prime concern for beach and resource managers. This objective has been a challenge at many of the state's urban coastal beaches and embayments having poor water quality due to microbial pollution from fecal indicator bacteria (FIB) and associated pathogenic organisms. Although FIB only indicate the possible presence of pathogens, epidemiological studies have linked elevated FIB concentrations to swimmer illness in waters contaminated by human sewage (Cabelli et al. 1982; Dufour 1984; Haile et al. 1999; Wade et al. 2003) from exposure to a variety of waterborne pathogens (Maier et al. 2000; Hlavsa et al. 2011). However, FIB are poorly linked to human health effects in waters impacted by contaminated runoff but no obvious sewage inputs (Calderon et al. 1991; Colford et al. 2007). These microorganisms can come from a variety of sources including contaminated runoff from storm drains and freshwater inputs (Jiang et al. 2001; Reeves et al. 2004; Stein and Tiefenthaler 2004; Ackerman et al. 2005), feces from birds and other wildlife (Ricca and Cooney 1998; Alderisio and DeLuca 1999; Ferguson et al. 2003; Grant et al. 2001; Surbeck et al. 2006), outflows from coastal wetlands or other embayments (Steets and Holden 2003; Surbeck et al. 2006), and reservoirs of microorganisms residing within beach sands and other sediments (Davies et al. 1995; Ferguson et al. 2005; Lee et al. 2006; Yamahara et al. 2009). Chief among these in California would be contaminated runoff impacting the surf zone water of beaches (Noble et al. 2000), especially during wet weather (Noble et al. 2003).
Consequences of poor water quality are significant. Swimmers, surfers, and other people exposed to beach waters contaminated by pathogenic microorganisms have a greater risk of illness (Stewart et al. 2008), while frequent beach closures due to poor water quality can result in great economic losses for a region. For example, during the dry and wet seasons of 2000, Given et al. (2006) estimated total losses for Los Angeles County beaches to range from $14.2 to $35.1 million depending on the dose–response model used. To obtain water quality standards, the US Environmental Protection Agency has implemented various regulatory actions to reduce microbial pollution of marine and estuarine waters listed as impaired under Section 303(d) of the Federal Clean Water Act. Chief among these is the total maximum daily load (TMDL) strategy where the assimilative capacity of a body of water for a target pollutant is determined numerically, and then loads for that pollutant are allocated among point, nonpoint, and natural sources in order to achieve the water quality objective (Cal EPA 2010).
Santa Monica Bay in Southern California is impacted by runoff from the urbanized Ballona Creek Watershed, the largest draining into the bay. This watershed has an area of 340 km2, is 83 % developed, and encompasses several cities including a major portion of Los Angeles (Bay et al. 1999). Its waterways have been transformed into a series of underground pipes conveying runoff to Ballona Creek, an open concrete channel in the lower portion of the watershed. An annual flow of approximately 65 million m3 of runoff with mean daily winter/spring flows of 2.3 m3/s and mean daily summer/fall flows of 0.6 m3/s impacts receiving waters and habitats of the Ballona Wetlands and Estuary, Del Rey and Ballona Lagoons, Santa Monica Bay, and beaches adjacent to the mouth of Ballona Creek (Dojiri et al. 2003; PWA 2006). To date, TMDLs for metals (Cd, Cu, Pb, Ag, and Zn), toxic organic compounds (chlordane, DDTs, PCBs, and total PAHs), trash, and FIB have been established by the state to control these pollutants in the Ballona Creek and Estuary (see http://www.waterboards.ca.gov/losangeles/water_issues/programs/tmdl/ for details).
One component of the Ballona Estuary bacterial TMDL is to determine if the Del Rey Lagoon contributes significant concentrations of FIB to the estuary, thus contributing to water quality impairment not only of the estuary but to adjacent recreational beaches. According to the TMDL plan for the Ballona Estuary, the Del Rey Lagoon is considered a nonpoint source for bacteria entering waters of the estuary. However, no studies have been conducted to understand how FIB levels fluctuate within the lagoon, especially during tidal exchanges, and how this relates to the lagoon serving as a sink or source of FIB for the estuary. Studies of FIB dynamics within tidal channels of the nearby Ballona Wetlands showed that concentrations of these bacteria can fluctuate up to three orders of magnitude over a 24-h period, and that the wetlands can act as a FIB sink under daytime flood–tide conditions or a source during stronger ebb flows when sediments are suspended (Dorsey 2006; Dorsey et al. 2010). Wetland processes in general have been demonstrated to reduce FIB concentrations through exposure to UV light (Jillson et al. 2001; Karathanasis et al. 2003; Whitman et al. 2008), settling onto submerged plant surfaces (Karathanasis et al. 2003), and predation by protozoans (Surbeck et al. 2010). Conversely, ebb flows from a salt marsh wetland can result in elevated FIB densities in receiving surf zone waters of adjacent beaches (Grant et al. 2001; Steets and Holden 2003).
Given the popularity of the lagoon shoreline for recreation, it is important to have knowledge of its microbial water quality. This study therefore addresses two questions:
-
1.
Is the lagoon a sink or source of FIB to the adjacent estuary?
-
2.
Are potentially pathogenic bacteria present within the lagoon waters, and does their diversity differ tidally?
To address the first question, densities of FIB (total coliforms, Escherichia coli, and enterococci) were measured in water entering the lagoon during flood tides and leaving on the ebb flows. Here we define the lagoon as sink when FIB concentrations are reduced through various processes. Conversely, the lagoon would act as a source if FIB concentrations increase and are released to the adjacent receiving waters of the estuary. If the lagoon was a source of FIB, then the ratio of flood to ebb FIB densities would be <1.0, indicating that more FIB outwell from the lagoon on ebb tide flows. Conversely, ratios >1.0 indicate that FIB densities in the lagoon's surface water were reduced through various processes such as inactivation by UV light, sedimentation, or predation by microorganisms. This sampling scheme was conducted over a variety of tides ranging from spring to neap tides to examine how ebb and flood densities; hence, the ratio may differ with varying tidal conditions. This approach was used to help elucidate FIB dynamics in the nearby Ballona Wetland tidal channels (Dorsey 2006).
The second question was approached by attaining a partial measure of the bacterial diversity of flood and ebb flows from water samples collected for the FIB analyses. Comparing tidal diversity may provide information on the source of bacteria, particularly pathogens, and lead to measures to control their presence thus reducing risk to people exposed to the lagoon water. Although only a fraction of culturable bacteria could be isolated and identified using the same media as used for the FIB analyses, this approach was treated as a preliminary screening method, providing a measure of the relative diversity of resident bacteria and the presence of possible pathogens.
Methods
Del Rey Lagoon
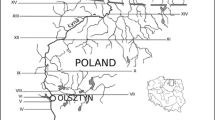
The Del Rey Lagoon is in the City of Los Angeles' Playa Del Rey suburb located just south of Marina Del Rey (Fig. 1). The lagoon is the remnant of a once larger coastal lagoon system located at the mouth of Ballona Creek. It was split into a southern (Playa Del Rey Lagoon and Regional Park) and northern portion (Ballona Lagoon) after Ballona Creek was straightened and its banks armored as part of a flood-control project by the US Army Corps of Engineers in the early 1930s (PWA 2006).
Presently, the lagoon is approximately 0.02 km2 (5.2 ac) in area and surrounded by a residential community with parkland at its southern end (Fig. 1). It is a full tidal system influenced by mixed semidiurnal tides. Water enters and departs the lagoon from the Ballona Estuary via a single tide gate positioned at the north end of the lagoon. Runoff enters the lagoon from the surrounding drainage area of 0.10 km2 (24.5 ac)(CLA 2007). Although the parkland at the southern end of the lagoon is popular for recreation, no swimming is allowed within the lagoon due to impaired water quality based on historically elevated levels FIB.
Field sampling
FIB densities were measured for 16 days from February 2008 through March 2009 (Table 1). Only dry-weather days were sampled to elucidate tidal patterns among the FIB groups. If rain occurred, then sampling was postponed to 4 days after the rain event, presumably enabling all rain-related runoff to flush from the lagoon.
During each sampling day, sets of replicate samples (n = 3) were collected during the flood and ebb flows. Sampling days were selected to include a variety of tidal conditions ranging from neap to spring tides with tidal ranges ranging from 0.14 to 2.73 m, respectively (Table 1). Sampling was done approximately 1 h prior to each high and low tide peaks to ensure that water was moving and not at slack level. Only dry-weather days were sampled to avoid confounding effects from runoff.
Samples were collected at the north end of the lagoon by the tide gate (Fig. 1). Replicates were collected within 2 m of the tide gate over a period of about 1 min. Samples were collected in sterile 120 mL polypropylene containers, placed on ice, transported back to the laboratory, and tested within 1 h of collection.
Water quality measurements of temperature (in degree Celsius), salinity (in parts per thousand), pH, and dissolved oxygen (in milligrams per liter) were made in situ using an YSI 600 sonde (YSI Yellow Springs, OH). In the laboratory, turbidity in nephelometric turbidity units (NTU) was measured for each replicate sample using a HACH model 2100 N turbidimeter (HACH, Loveland, CO).
Beginning March 2008, estimates were made of bird species and densities to determine their use of the lagoon. Estimates were made during each flood and ebb flow sample collection time on each sampling date.
Bacterial analyses
FIB concentrations were determined using chromogenic substrate tests (APHA et al. 1998, Standard Methods Section 9223 B). Idexx media Colilert®-18 was used for total coliforms and E. coli and Enterolert® media for enterococci (IDEXX, Westbrook, Me). Samples were quantified using Idexx Quanti-Tray® 2000 97-well trays.
A variety of other bacterial species will grow in the media used in defined substrate methods, many of which occur mainly in environmental samples (Stevens et al. 2003). In comparing traditional lactose fermentation with methods based upon β-d-galactosidase, Fricker and Eldred (2009) isolated bacteria from Colilert-18® liquid extracted from Quanti-Tray® wells testing positive for coliforms and then identified them using the Vitek microbial identification system. This approach was used to obtain a relative measure bacterial richness in the lagoon for each tidal flow during the latter part of the study for six sampling events beginning with the 16 September 2008 collection. From each set of three replicate Quanti-Trays®, 10 wells testing positive for coliforms and 10 for enterococci were randomly selected. Positive wells were sampled to ensure that we would isolate bacteria and to identify species of Enterococci, many of which are important from a public health perspective. A volume of 10 μL was extracted, mixed into 10 mL of sterile DI water to yield a 0.001 % suspension, immediately streaked onto tryptic soy agar (TSA), and then incubated either at 35 °C for suspensions from the E. coli wells or 41 °C for those from the enterococci wells. After 24–48 h of incubation, one colony of each morphological type was selected from each TSA plate, suspended in 3 mL of sterile DI water, streaked onto nutrient blood agar (5 % sheep blood in TSA) to obtain pure isolates, and then incubated as done for the initial TSA plates. One isolate from each blood agar plate then was identified using the Vitek® 2 Compact (V2C) biochemical identification system (bioMérieux Vitek Systems Inc., Hazelwood, MO) according to manufacturer's specifications. Isolates acquired from the Colilert®-18 and Enterolert® medias were identified using Vitek gram-negative and gram-positive cards, respectively.
Data analyses-FIB
For each tidal event, the mean concentration of each FIB group was tested between flood and ebb conditions using the Student's t test on log10-transformed data. Ratios of flood to ebb FIB mean concentration (R F/E) were calculated for each sampling event to determine if the lagoon was acting as a sink (R F/E > 1) or source (R F/E < 1) of FIB.
Pearson correlations were performed between FIB densities and water quality parameters to elucidate relationships among variables. Regression analyses were done to determine the extent to which variation in bacterial density was explainable by salinity, temperature, pH, dissolved oxygen, mean NTU, and tidal height. The data set used for these analyses began with 32 records, each record representing either the flood or ebb tide collection for a date. Each record included mean densities for each FIB group, mean turbidity, tidal height, and YSI measurements (temperature, salinity, pH, and dissolved oxygen). Three incomplete records were deleted from the analyses due to missing data for the 20 March 2008, 14 May 2008, and 4 June 2008 collections, so the final data set equaled 29 records for both the Pearson correlations and regression analyses.
Data analyses-bacterial community
Bacterial isolates identified using the V2C provided a relative measure of the bacterial species richness sampled during flood or ebb tide flows, while acknowledging that these isolates represent only a fraction of the bacteria community present.
Alpha diversity rarefaction, and rank abundance
The alpha diversity (α-diversity) of an ecological community is an evaluation of both the number of species (i.e., species richness) and the number of individuals in each species (species abundance) that are sampled at a specified site (Whittaker 1972). To assess the α-diversity of bacterial communities, we used the Shannon diversity index (H), which is calculated as follows:
Where S is the total number of species sampled and p i represents the proportional species abundance. The term p i is calculated as follows:
Where n i is the number of individuals in the ith species and N is the total number of individuals encountered across all S species. Shannon diversity index values can range from 0 → ln(S), and large values of H are indicative of sites with both high species richness and high equitability among relative species abundances (species dominance is low).
To compare the α-diversity of different bacterial communities, it is necessary to employ a rarefaction subsampling technique that will allow for the evaluation of diversity index values that are computed with equal population sizes (Sanders 1968). For each of the sites, the rarefaction procedure reduces N at random intervals and computes a respective value of H, until N = 0. The resulting plot between the computed values of N and resulting values of H is known as a rarefaction curve (Gotelli and Colwell 2001), which serves to compare diversity indices based on equal sampling sizes. The rarefaction curves for this study were computed with EstimateS v8.2 using data triplets of sampling date, species identification, and species abundance for each of the flood and ebb flow tidal states.
Rank–abundance curves were used to evaluate the species richness and species abundances equitability (species evenness) in both flood and ebb bacterial assemblages. A rank–abundance curve is a plot of the proportional species abundance (p i ) and the respective rank in abundance, such that the highest value of p i receives a rank of 1, the second highest a rank of 2, and so on.
Richness estimates beta diversity, and cluster analysis
Estimating species richness is an important tool in surveying species-rich communities and is commonly used as a technique where it is impractical to sample exhaustively (Gotelli and Colwell 2001). The program EstimateS v8.2 was used to predict (i.e., estimate) the species richness of both flood and ebb collections based on the species occurrence patterns in the sampling data gathered during each sampling event. This program with description of the indices used herein is available at the EstimateS web site (http://viceroy.eeb.uconn.edu/EstimateSPages/AboutEstimateS.htm). The beta diversity (β-diversity) of an ecological community is an evaluation of the number of unique species among specified sites and measures the rate of change in species composition (Whittaker 1972). In order to assess the β-diversity of the bacterial assemblages in both the flood and ebb flows, similarity indices comparing shared species were calculated using EstimateS v8.2 and included the Bray–Curtis, Morisita-Horn, Chao-Sorenson, Chao-Jaccard, Sorensen Classic, and Jaccard Classic. A cluster analysis was then used to evaluate the temporal variability of the species composition in the bacterial assemblages for the different flood and ebb tidal conditions encountered. The proportional species abundance (p i ) was calculated for each sampling date during each flood and ebb state, which was used to generate a similarity matrix that formed the source for a Euclidean distance cluster diagram using Statistica v9.1 (StatSoft 2010).
Results
Water quality measurements
Summary statistics for the water quality parameters during the flood and ebb tide flows are presented in Table 2. Water temperatures ranged from 12.9 to 25.3 °C reflecting seasonal changes. The mean temperatures differed significantly (p = 0.04) between flood and ebb tide conditions with the flood tide water being cooler by 2.3 °C on average with up to a 7.3 °C difference as recorded during the 20 March 2008 sampling event. Salinities were typical of estuarine and marine waters, ranging from 24.3 to 33.6 ppt. Water often was more saline by up to 2.5 ppt (13 February 2008 sampling event) as it entered the lagoon from the Ballona Estuary, but overall mean salinities did not differ significantly (p = 0.60) between flood and ebb flows. However, a pattern was evident that greater salinities occurred during the more extreme high and low tidal levels (Fig. 2). Dissolved oxygen (DO) displayed the greatest variation with values ranging from 2.6 to 25.4 mg/L. The mean DO values for flood vs. ebb flows did not differ significantly (p = 0.92). When DO values regressed against tidal levels, dissolved oxygen tended to increase with higher tide level, however, this trend was not significant (R 2 = 0.02, p = 0.53). Values of pH were consistent (p = 0.93), averaging 7.96 and 7.95 for flood and ebb tide flows, respectively. The mean values of turbidity (NTU) differed significantly between flood and ebb flows (p < 0.01) with flood conditions averaging 1.96 NTU compared to 10.76 during ebb flows. Correlations among the various water quality parameters generally were weak with the exception of those for turbidity vs. tidal height (r = −0.56, p < 0.01) and salinity vs. pH (r = −0.55, p = 0.02). In the case of turbidity, waters became more turbid as the tide receded, especially during spring tide conditions, presumably due to sediments suspended during swifter ebb flows.
FIB concentrations
Densities of total coliforms, E. coli, and enterococci differed in that the lagoon tended to act nearly equally as a sink and source for total coliforms, while mainly a source for E. coli and enterococci (Table 3). Mean densities of total coliforms ranged from 102 to 104 most probable number (MPN)/100 mL with overall flood and ebb flow densities being similar. Over the 16 sampling events, R F/E for total coliforms averaged 2.9 with values >1.0 occurring 56 % of the time, suggesting that the lagoon tended to act both as a sink or source for total coliforms nearly equally. Densities of both E. coli and enterococci tended to be greater during the ebb flows (Table 3), ranging from 101 to 102 MPN/100 mL. Although the mean R F/E for E. coli was 3.6, due mainly to a single event in October 2008, 69 % of the events had ratios <1.0 suggesting that the lagoon was acting as a source of E. coli on the ebb tidal flows. The average R F/E for enterococci was 0.9, and like E. coli, the majority of the sampling events (69 %) had ratios <1.0, indicating that the lagoon was acting mainly as a source of enterococci.
Multiple regressions between the FIB groups and water quality parameters resulted in significant results for enterococci and total coliforms. Increasing enterococci concentrations was best explained by increasing levels of turbidity and salinity (R 2 = 0.69, p < 0.01). Total coliform variation was best explained by diminished levels of salinity and dissolved oxygen (R 2 = 0.54, p < 0.01), possibly associated with cooler water entering the lagoon during flood flows. Regression models for total coliforms and E. coli were similar in that both FIB groups were negatively associated with diminishing levels of salinity and pH, although the regression model for E. coli was not significant (R 2 = 0.05, p = 0.31).
Birds
The most common birds observed on or around the lagoon were ring-billed gulls Larus delawarensis, American coots Fulica americana, and mallards Anas platyrhynchos (Table 4), particularly during spring and fall. During low tides, various species of shorebirds were observed feeding on the exposed mudflat, the most common being whimbrels Numenius phaeopus, Dowitchers Limnodromus sp., and various species of plovers. The most conspicuous large birds were a resident group of seven graylag geese Anser anser.
For each FIB group, Spearman's rank correlations were done between the number of birds per acre (Table 4) and mean concentrations of FIB during ebb flows (Table 3). However, there was no significant relationship between bird densities and the FIB groups (total coliforms: r = −0.34, p > 0.05; E. coli: r = 0.14, p > 0.05; enterococci: r = 0.07, p = > 0.05).
Bacterial diversity
Of the 277 isolates cultured from the IDEXX Quanti-Tray wells, approximately 54 species representing 24 genera were identified using the V2C system (Table 5). Nine isolates, 3.2 % of the total, were unidentifiable using the V2C, while 19 taxa (6.9 %) were reported as species complexes requiring additional tests to acquire final species designations. Genera with the greatest number of isolates included species of Enterococcus with 87 and Vibrio with 54. Species of Vibrio tended to be more prevalent during the months of September and October when water temperatures were warmer relative to the cooler winter months (Pearson r = 0.61, p = 0.03). About half (55 %) of the species represented by the isolates have been implicated as opportunistic or direct human pathogens (Table 6), but detailed molecular tests would be required to determine if these strains were actually pathogenic.
The rarefaction curves illustrate that the Shannon diversity index of the isolates collected during ebb flows was higher than for the flood flows (Fig. 3a). The rank–abundance curves also demonstrate that ebb flows had higher species richness and higher species evenness than the flood flows (Fig. 3b). However, estimates of species richness based on sampling patterns show that flood flows are projected to have a greater number of species (Fig. 4). Samples from both ebb and flood flows had 21 bacterial species in common. Similarity analyses show that indices of shared species vary between 38 and 92 % (Fig. 5). Additionally, the cluster analysis indicates that there was high variation in terms of both species abundance and composition of bacterial species with respect to both sampling date as well as ebb- and flood flow events (Fig. 6).
The variation among bacterial assemblages (in terms of both species composition and species abundance) with respect to both tidal flow (FLD or EBB) and sampling date (expressed as year–month–day or YYMMDD). For each date, the neap (N) or spring (S) tidal conditions are given along with the tidal range between the high and low tides sampled that day
Discussion
Differences were apparent in the bacterial composition of water flooding in from the estuary compared to water draining from the lagoon during ebb flows. These differences reflected the varying FIB concentrations and diversity of other bacterial species identified in this study.
FIB variation
Concentrations of all FIB groups often differed by up to an order of magnitude over a 6-h period from flood through ebb tide. Concentrations of total coliforms tended to diminish after flooding into the lagoon while E. coli and enterococci concentrations could increase during strong ebb flows during spring tides. Dorsey (2006) and Dorsey et al. (2010) measured similar tidal variation in FIB concentrations upstream in the Ballona Wetlands.
The observed tidal variation could have implications for FIB compliance testing at recreational beaches near the mouths of tidally influenced drainage areas. In California, most agencies monitoring beach water quality collect daily or weekly shoreline water samples (one grab sample per station) to determine if FIB concentrations meet state standards. Samples typically are collected during morning hours and then processed by mid-afternoon with results available within 18–24 h. FIB concentrations could be in compliance with standards during the morning collection but exceed standards in the afternoon as tidal conditions change. This situation was demonstrated in the lagoon. When looking at individual replicate data, E. coli were within compliance of California recreational water standards (CDPH 2010) for single samples (400 MPN/100 mL) during the morning flood flows but failed these standards on eight occasions during the afternoon ebb flows. Likewise, enterococci failed the standard of 103 MPN/100 mL 12 times during the afternoon ebb flows even though standards were met for this FIB group earlier in the day during the flood flow. As discussed below, elevated densities in the afternoon ebb flows probably were associated with suspended sediments occurring during periods of low spring tides.
These results are inconsequential for the lagoon since swimming is banned but could be important for Dockweiler and Venice Beaches, popular recreational beaches in Santa Monica Bay lying adjacent to the mouth of the Ballona Estuary (Fig. 1). Ballona Creek is a significant source of FIB (Dorsey and Lindaman 2004; Stein and Tiefenthaler 2005; Tiefenthaler et al. 2009) as is the estuary (Dorsey 2006), particularly during ebb flows (Dorsey et al. 2010). Morning compliance sampling during a flood tide could indicate that standards are met, but 6 h later during the ebb flow, standards could be exceeded, thus placing swimmers at greater risk of exposure to possible pathogens. Boehm et al. (2002) measured FIB variability along the open ocean beach at Huntington Beach where the surf zone was impacted with flow from the Santa Ana River and Talbert Marsh. FIB varied over time scales ranging from minutes to hours depending on tidal and daylight conditions. Given this variability, especially around the mouths of major drainages, Boehm et al. (2002) recommend that compliance monitoring focus on obtaining a geometric mean from a series of sample collected over a longer period, such as a week. This approach would enable beach managers to judge water quality based on 30-day standards while still being able to respond to spikes in FIB concentrations measured from instantaneous samples.
The lagoon as a sink for FIB
The Ballona Estuary tended to be a major source of FIB for the lagoon, especially for total coliforms whose mean RF/E value over the study period equaled 1.83 (Table 3). In turn, the estuary is contaminated mainly by runoff from Ballona Creek. Within the creek itself, Stein and Tiefenthaler (2004) and Tiefenthaler et al. (2009) measured dry weather geometric mean concentrations of E. coli and enterococci ranging from 102 to 103 MPN/100 mL and 104 for total coliforms. In the lower reaches of the creek, Dorsey and Lindaman (2004) measured similar densities for E. coli and enterococci during dry weather with total coliforms averaging 104–105 MPN/100 mL. During FIB loading studies within the Ballona Wetlands, concentrations in the estuary generally were an order of magnitude greater than those found in the wetlands, following similar trends with mean concentrations ranging from 102 to 104 MPN/100 mL for total coliforms and 101–103 MPN/100 mL for both E. coli and enterococci (Dorsey et al. 2010).
The lagoon tended to be a sink for total coliforms based on values of R F/E >1.0 for 56 % of the sampling events and a mean ratio of 2.9. Exposure to sunlight could explain this trend. During this study, sampling usually was done during daylight hours with flood tides occurring during morning hours, ebb flows later in afternoons or early evenings (Table 1). During the day, solar UV light likely was a factor in reducing densities of FIB within the surface waters of the lagoon. Sunlight has been demonstrated to reduce FIB densities within wetland and other water systems (Jillson et al. 2001; Boehm et al. 2002; Stinton et al. 2002; Karathanasis et al, 2003; Noble et al. 2004; Mayo 2004; Vymazal 2005; Whitman et al. 2008).
During this study, there were three occasions when total coliform densities did not diminish between flood and subsequent ebb flows. These events occurred when all samples were collected at night (3 June 2008 and 14–15 July 2008), or the flood flow samples were collected before sunrise (20 March 2009). For these events, R F/E values for total coliforms were <1.0 with significantly greater mean densities during the ebb tides (Table 3). More than likely, there was insufficient exposure of total coliforms to UV light over this period to effectively reduce their concentrations.
Noble et al. (2004) determined light inactivation coefficients (k D) for FIB in a series of experiments where sea- and freshwater matrices were inoculated with sewage and runoff, then exposed to full and reduced (simulating winter insolation) sunlight for a period of about 1 week. Results indicated that inactivation rates were similar in both fresh and seawater matrices, and that enterococci degraded more rapidly in sunlight than E. coli. Because the study of Noble et al. was conducted at a facility about 4.5 km from the Del Rey Lagoon, we used the k D coefficients generated from this study to see if FIB in the lagoon were inactivated at similar rates during the daylight hours (Table 7). Densities of total coliforms fell on 9 out of 14 occasions. These reductions averaged 54.7 %, slightly more than the average of 30.4 % predicted by the k D coefficients. In contrast, E. coli and enterococci had reduced densities on only 4 of the 14 dates examined, indicating that the lagoon may act as a source for these bacteria as discussed below. On the four occasions that densities did fall over the day, the observed percent reductions were similar to the predicted values. We conclude from this analysis that light does reduce densities of FIB, particularly total coliforms in this case, but that other factors, discussed below, tend to reintroduce FIB back into the lagoon's water.
The lagoon as a source for FIB
Over the 16 sampling events, R F/E values for E. coli and enterococci were <1 on 11 occasions or 68 % of the time (Table 3), suggesting that the lagoon was a source for these FIB groups. It was not apparent why E. coli displayed this pattern since it did not significantly correlate with other FIB groups, water quality parameters, or tidal levels. It is possible that a source for this FIB would be the many species of birds using the lagoon (Table 4) as discussed below. In contrast, densities of enterococci were associated with greater salinities, turbidity, and lower tidal levels. Greater salinities were measured during both higher and lower tidal levels associated with spring tides (Fig. 2). Flood flows would bring more saline water in from the estuary with corresponding loads of enterococci. During spring ebb flows, salinities also were relatively greater, possibly from evaporation in the tidal channels as water levels and volume fell throughout the day. During these spring tide ebb flows, densities of enterococci were elevated as tidal heights diminished, presumably due to suspension of enterococci with bottom sediments. During spring low tides when water levels are less than 1.0 m mean lower low water, water within the lagoon drains from a narrow central channel approximately 3–5 m wide. At the tide gate, swifter ebb flows within the channel tend to suspend sediments, increasing turbidity and associated FIB. Based on a regression between enterococci and low tide heights (R 2 = 0.36, p < 0.01, y = −0.61X + 1.76), a tidal height of ≤0.35 m would result in enterococci densities ≥103 MPN/100 mL, exceeding bathing water standards for the State of California (CDPH 2010). A similar situation was documented in the nearby Ballona Wetlands where Dorsey et al. (2010) measured greater current speeds, turbidity, and FIB spikes associated with spring tide ebb flows.
Sediments can have viable populations of enterococci (Ferguson et al. 2005; Lee et al. 2006; Yamahara et al. 2009) and E. coli (Lee et al. 2006; Ishii et al. 2007), resulting in increased concentrations within the water column if sediment FIB reservoirs are suspended through storm or tidal actions (Sanders et al. 2005). Evanson and Ambrose (2006) found that within a wetland system impacted by urban runoff, the sediment-associated FIB populations may be distinct from those in the overlying water column based on the ratio of total coliforms/E. coli (TC/EC). In their study, sediment populations generally had ratios >10 compared to overlying water where TC/EC ratios were <10, suggesting possible human fecal contamination (Haile et al. 1999). Within the Del Rey Lagoon, additional studies like that of Evanson and Ambrose (2006) would be required to conclusively determine if increased FIB concentrations during ebb flows are caused by tidally suspended sediments.
Birds using the lagoon as a resource are another potentially significant source of FIB impacting lagoon water. Bird feces have been shown to be a prime source of fecal material, driving up FIB densities within the water and shoreline sediment (Alderisio and Deluca 1999; Ricca and Cooney 1998). During this study, we observed flocks of American coots and mallards reaching densities of 100 individuals or more, while various species of gull collectively approached similar densities (Table 4). A variety of shorebird species were common, especially during low tides when they were observed foraging along the lagoon's exposed mudflat. American coots, mallards, Western gulls, and killdeers are year-round residents of the area while the other species of waterfowl, gulls, and shorebirds observed are mainly winter residents (Cooper 2006). Based on correlation analyses, there was no significant relationship between the densities of FIB groups and the densities of birds observed at the lagoon.
Regarding enterococci specifically, Ferguson et al. (2011) and von Bitner et al. (2011) found Enterococcus faecalis and Enterococcus faecium to predominate in sediments where flocks of shorebirds fed and roosted, suggesting that the source of this species were the birds. In Del Rey Lagoon, these two species represented 24 % of the 87 Enterococcus isolates (Table 5). Further studies at the lagoon should document the seasonal use of the lagoon by birds, more closely linking their densities per unit area with changing FIB concentrations.
A final source of FIB entering the lagoon could be runoff from the local community and adjacent park. The City of Los Angeles has noted four small storm drains that empty directly into the lagoon (CLA 2007). Although flows from these drains are unknown, we have observed them to convey only a small volume of runoff, so their contribution of FIB to the lagoon may be irrelevant. Nonetheless, their flows should be measured to estimate FIB loading. Irrigation of the parkland adjacent to the lagoon also could result in contaminated runoff entering the water. For example, when American coots are present, they often congregate in large groups on the parkland located at the southern end of the lagoon. During irrigation, water not soaking into the ground could carry their feces into the lagoon.
Despite the FIB spikes during spring tide ebb flows, water flowing from the lagoon still might be diluting water flowing from the more contaminated estuary into Santa Monica Bay. Dorsey et al. (2010) reported this situation for ebb flows departing the Ballona Wetlands' west and east tide gates located at 206 and 388 m, respectively, upstream from the lagoon's tide gate. Further measurements would need to be conducted comparing ebb flows from the lagoon with those of the estuary to determine if dilution occurs and under what tidal circumstances. If additional investigations indicate that lagoon ebb flows dilute those of the estuary, then we would not consider the lagoon to be a significant source of FIB to the adjacent Ballona Estuary.
Bacterial isolates and public health considerations
Bacterial isolates identified from the Idexx Quanti-Trays® using the V2C system provided a measure of the bacterial community composition even though the following factors could affect the diversity measurements:
-
1.
Only a fraction of potentially culturable species was sampled from the Quanti-Tray wells.
-
2.
A selectivity bias would be present due to components in the Colilert®-18 and Enterolert® media. Both these broth-type media are subject to species selection bias as opposed to solid media, allowing for faster growing or hardier species and strains to predominate. However, this limitation did not appear to be a major problem in this study given the high diversity of species measured (Table 5).
-
3.
By sampling only positive Quanti-Tray® wells, E. coli and species of enterococci would be enriched, thus biasing the diversity results.
Despite these drawbacks, a variety of species were isolated, providing a measure of diversity for the various tidal flows. Also, identifying various Enterococcus species was of public health interest since many have been implicated as human pathogens (Teixeira et al. 2007).
This method indicated that during ebb flow conditions, the assemblage of bacterial isolates were more α-diverse than when water flooded into the lagoon from the estuary (Fig. 3a), and ebb flows had a greater evenness (relative species abundance) in the community composition. Rarefaction curves for the ebb and flood flows (Fig. 3a) never reached their respective asymptotes, indicating that there are more species yet to be encountered at the two tidal conditions using this identification system. Based on sampling patterns, all species richness estimates predict that flood flow will overtake ebb flow in terms of the greatest number of bacterial species (Fig. 4). However, it is unclear how a greater richness estimate would impact species evenness, a measure of the equitability of individuals among flood flow species. This complexity is important because changes in species evenness during flood flow conditions will also serve to impact (by either increasing or decreasing) the bacterial species index of diversity. Species unique to flood and ebb flows reflect a medium-to-low β-diversity of shared species between the two bacterial assemblages. However, the cluster analysis (Fig. 6) points to a dynamic assemblage that changes species composition with respect to both day-to-day fluctuations and tidal action, as represented by mix of tidal flows (ebb vs. flood) and ranges (neap vs. spring) within clusters. The variability and dynamic nature of the bacterial assemblages measured in the flood and ebb flows is not surprising given the potentially great number of bacterial species present in the lagoon and estuary systems. Bacterial assemblages would represent a mix of marine indigenous species and those externally introduced from contaminated urban runoff.
Of the total number of species identified, approximately 56 % represent species that have strains reported to be associated with human illnesses (Table 6). These included natural marine pathogens, mainly Vibrio spp., and many opportunistic pathogens implicated in nosocomial infections. The presence of these species could be of public health concern under the following defining conditions for the disease triangle (Scholthof 2007): (1) the pathogenic strain of the species must be present and in sufficient abundance to cause infections, (2) the person must be exposed to the pathogen and susceptible to infection, and (3) environmental conditions must conducive for maintaining the pathogen.
With regard to the first condition above, Stewart et al. (2008) point out that not all strains of an infectious bacterial species are equally pathogenic. For example, results presented herein showed that species of Vibrio, particularly Vibrio cholerae, were more common in the lagoon during warmer water months (Table 5). Further molecular testing is required to determine whether the Vibrio strains found in this study are toxigenic. Of the 103 recognized strains of V. cholerae, only V. cholerae O1 and V. cholerae O130 cause the severe gastrointestinal illness associated with many epidemics (Abbott et al. 2007). A third strain, V. cholerae Non-O1, causes not only a milder form of watery diarrhea but also has been implicated in cases of severe septicemia (Abbott et al. 2007). Therefore, microbial risk analyses in bodies of water where people can be exposed to natural and introduced pathogens need to include genomic analyses or other techniques such as agglutination for pathogenic species of Vibrio, to identify those strains of microorganisms expressing virulence genes required for human illness (Stewart et al. 2008).
The second condition above, human exposure and degree of susceptibility to pathogens, is limited in the lagoon since it is posted for no swimming, and shellfish consumption is banned during the warm water months of May through October when this source of seafood can be contaminated by blooms of harmful dinoflagellates and other toxic alga. However, children playing in the sand along the lagoon's shoreline could be at greater risk for illness and infections. A child would have less of an immune response compared to an adult, conceivably acquiring wound infections from digging in the sand or gastrointestinal illness if ingesting contaminated sand. Bacteria capable of producing such illnesses were present in the lagoon (Tables 5 and 6), but the question remains—were environmental conditions favorable for sustaining pathogenic strains in sufficient concentrations to cause increased health risks? This question would also apply to the bathing beaches surrounding the mouth of the Ballona Estuary, especially during periods of ebb flows when swimmers would be exposed to potentially greater levels of pathogens. With regard to shellfish consumption, we recommend that this source of seafood be banned year around given the potentially pathogenic species of bacteria recorded in this study.
Knowledge of pathogen loading into coastal waters via nonpoint source runoff is limited (Stewart et al. 2008), and the association with human illness may not be predicted using traditional FIB indicators (Colford et al. 2007). Stewart et al. (2008) discuss how coastal habitats and their resident populations of marine organisms (e.g., shellfish) can harbor pathogens introduced from terrestrial sources like contaminated runoff or sewage effluents. These habitats and organisms act as sentinels to be monitored, providing information on emerging pathogens, potential health impacts to human users, and general health of the ecosystem. The Del Rey Lagoon and adjacent Ballona Estuary should be considered as such a habitat sentinel. Periodic monitoring of the non-FIB microbial assemblage utilizing molecular methods such as PCR or microarrays (Girones et al. 2010) should be performed on key ecosystem components of this system such as flood and ebb flow water, sediments, and edible seafood (clams and mussels). Also included in such monitoring would be the contribution of microorganisms by birds into this habitat. Such sentinel monitoring would provide valuable information to resource managers and regulators on the overall health of the system and aid in assessing progress towards improved water quality as regulatory programs like TMDLs are implemented and conducted.
Conclusions
-
1.
Concentrations of all FIB groups often differed by up to an order of magnitude over a tidal period.
-
2.
The lagoon tended to act as a sink for densities of total coliforms. In contrast, E. coli and enterococci concentrations were greater during ebb flow conditions for the majority of the sampling events. Enterococci were associated with the stronger spring tide ebb flows, suggesting that the lagoon was a source for this FIB group during these tidal conditions. Also, another potentially significant source of these two FIB groups could be from the many species of birds using the lagoon as a resource.
-
3.
Ebb flow conditions showed the highest α-diversity of bacterial isolates. We also detected a medium-to-low β-diversity of shared species between ebb and flood flows. Rarefaction curves indicated that bacterial diversity for both ebb and flood flow conditions are still increasing. Estimates of species richness (based on patterns in the sampling data) projected that flood flow will eventually overtake ebb flow in terms of species richness. The cluster analysis of bacterial isolates also reflected a complex and dynamic bacterial assemblage of both marine indigenous and externally introduced species that varied in terms of species abundance and composition between sampling dates and ebb vs. flood flow conditions.
-
4.
Approximately 56 % of the identified isolates represented potential opportunistic human pathogens; many of which have been implicated in nosocomial infections. Pathogenic strains within a few of these species groups, such as Vibrio alginolyticus could be harmful to people recreating along the lagoon's shoreline (mainly children) due to infections of open wounds, or consuming shellfish during winter months when harvesting is permitted.
References
Abbott, S. L. (2007). Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and other Enterobacteriaceae. In P. R. Murray (Ed.), Manual of clinical microbiology (pp. 698–715). Washington: American Society for Microbiology.
Abbott, S. L., Janda, M., Johnson, J. A., & Farmer, J. J. (2007). Vibrio and related organisms. In P. R. Murray (Ed.), Manual of clinical microbiology (pp. 723–733). Washington: American Society for Microbiology.
Ackerman, D., Stein, E. D., & Schiff, K. C. (2005). Dry-season water quality in the San Gabriel River Watershed. Bulletin of the Southern California Academy of Sciences, 104, 125–145.
Alderisio, K. A., & DeLuca, N. (1999). Seasonal enumeration of fecal coliform bacteria from the feces of ring-billed gulls (Larus delawarensis) and Canada geese (Branta canadensis). Applied and Environmental Microbiology, 65, 5628–5630.
American Public Health Association, American Water Works Association, and Water Environment Federation. (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington: American Public Health Association.
Baffone, W., Pianetti, A., Bruscolini, F., Barbieri, E., & Citterio, B. (2000). Occurrence and expression of virulence-related properties of Vibrio species isolated from widely consumed seafood products. International Journal of Food Microbiology, 54, 9–18.
Bannerman, T. L., & Peacock, S. J. (2007). Staphylococcus, Micrococcus, and other catalase-positive cocci. In P. R. Murray (Ed.), Manual of clinical microbiology (pp. 390–411). Washington: American Society for Microbiology.
Bay, S., Jones, B. H., & Schiff, K. (1999). Study of the impact of stormwater discharge on the beneficial uses of Santa Monica Bay. Los Angeles, CA: Prepared for the Los Angeles County Department of Public Works.
Boehm, A. B., Grant, S. B., Kim, J. H., Mowbray, S. L., McGee, C. D., Clark, C. D., Foley, D. M., & Wellman, D. E. (2002). Decadal and shorter period variability of surf zone water quality at Huntington Beach, California. Environmental Science and Technology, 36, 3885–3892.
Cabelli, V. J., Dufour, A. P., McCabe, L. J., & Levin, M. A. (1982). Swimming-associated gastroenteritis and water quality. American Journal of Epidemiology, 115, 606–616.
Cal EPA (2010). California Environmental Protection Agency, State Water Resources Control Board. Total maximum daily load program: background and information. http://www.swrcb.ca.gov/water_issues/programs/tmdl/background.shtml.
Calderon, R. L., Mood, E. W., & Dufour, A. P. (1991). Health effects of swimmers and nonpoint sources of contaminated water. International Journal of Environmental Health Research, 1, 21–31.
Carrero, P., Garrote, J. A., Pacheco, S., Garcia, A. I., Gil, R., & Carbajosa, S. G. (1995). Report of six cases of human infection by Serratia plymuthica. Journal of Clinical Microbiology, 33, 275–276.
Champsaur, H., Andremont, A., Mathieu, D., Rottman, E., & Auzepy, P. (1982). Cholera-like illness due to Aeromonas sobria. Journal of Infectious Diseases, 145, 248–254.
CDPH (2010). California Department of Public Health. California Department of Health Services [now CDPH] Regulations for ocean beaches and ocean water-contact sports areas pursuant to AB 411. Available at: http://www.cdph.ca.gov/HealthInfo/environhealth/water/Pages/Beaches.aspx.
CLA (2007). City of Los Angeles, Del Rey Lagoon Drainage Area Map. Department of Public Works, Watershed Protection Division. Map file location: S:/Admin/Layers/Projects/Marina/DelReyLagoonDrainiage. Revised 02/14/07.
Colford, J. M., Wade, T. J., Schiff, K. C., Wright, C. C., Griffith, J. F., Sandhu, S. K., Burns, S., Sobsey, M., Lovelace, G., & Weisberg, S. B. (2007). Water quality indicators and the risk of illness at beaches with nonpoint sources of fecal contamination. Epidemiology, 18, 27–35.
Cooper, D. S. (2006). Annotated checklist of extirpated, reestablished, and newly-colonized avian taxa of the Ballona Valley, Los Angeles County. California Bulletin Southern California Academy of Sciences, 105, 91–112.
Craun, G. F., Calderon, R. L., & Craun, M. F. (2005). Outbreaks associated with recreational water in the United States. International Journal of Environmental Health Research, 15, 243–262.
Daily, O. P., Joseph, S. W., Coolbaugh, J. C., Walker, R. I., Merrell, B. R., Rollins, D. M., Seidler, R. J., Colwell, R. R., & Lissner, C. R. (1981). Association of Aeromonas sobria with human infection. Journal of Clinical Microbiology, 13, 769–777.
Davies, C. M., Long, J. A., Donald, M., & Ashbolt, N. J. (1995). Survival of fecal microorganisms in marine and freshwater sediments. Applied and Environmental Microbiology, 61, 1888–1896.
de Champs, C., Bonnet, R., Sirot, D., Chanal, C., & Sirot, J. (2000). Clinical relevance of Proteus mirabilis in hospital patients: a two year survey. Journal of Antimicrobial Chemotherapy, 45, 537–539.
Deguchi, K., Yokota, N., Koguchi, M., Nakane, Y., Suzuki, Y., Fukayama, S., Ishihara, R., & Oda, S. (1991). Antibacterial activities of ofloxacin against recent isolates from patients with community acquired infections. The Japanese Journal of Antibiotics, 44, 941–57.
Dojiri, M., Yamaguchi, M. M., Weisberg, S. B., & Lee, H. J. (2003). Changing anthropogenic influence on the Santa Monica Bay watershed. Marine Environmental Research, 56, 1–14.
Dorsey, J. H. (2006). Densities of fecal indicator bacteria in tidal waters of the Ballona Wetlands, Los Angeles, County. Bulletin of the Southern California Academy of Sciences, 105, 59–75.
Dorsey, J. H., Carter, P. M., Bergquist, S., & Sagarin, R. (2010). Reduction of fecal indicator bacteria (FIB) in the Ballona Wetlands saltwater marsh (Los Angeles County, California, USA) with implications for restoration actions. Water Research, 44, 4630–4642.
Dorsey, J. H., & Lindaman, E. (2004). Water quality. In D. McNeill & J. Landry (Eds.), Ballona Creek trail and bikeway: Environmental monitoring and assessment (pp. 62–81). Los Angeles: Baldwin Hills Conservancy. Report submitted to the California State Legislature.
Dufour, A. (1984). Health effects criteria for fresh recreational waters. EPA-600/1-84-004.
Engler, H. D., Troy, K., & Bottone, E. J. (1990). Bacteremia and subcutaneous abscess caused by Proteus penneri in a neutropenic host. Journal of Clinical Microbiology, 28, 1645–1646.
English, J.C. (2010). Pitted Keratolysis. eMedicine http://emedicine.medscape.com/article/1053078-overview
Evanson, M., & Ambrose, R. (2006). Sources and growth dynamics of fecal indicator bacteria in a coastal wetland system and potential impacts to adjacent waters. Water Research, 40, 475–486.
Facklam, R., & Elliott, J. A. (1995). Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clinical Microbiology Reviews, 8, 479–495.
Fefera, J. J., Ratzana, K. R., Sharpb, S. E., & Saizc, E. (1998). Lactococcus garvieae endocarditis: report of a case and review of the literature. Diagnostic Microbiology and Infectious Disease, 32, 127–130.
Ferguson, D. M., Zhowandai, M. H., Getrich, M. A., Moore, D. F., Lissner, A., Haimann, R., Linger, D. W. (2003). Bacteriological monitoring studies to identity sources of fecal pollution at Baby Beach, Dana Point Harbor, California. OCEANS 2003. Proceedings (pp2797–P2800).
Ferguson, D. M., Moore, D. F., Getrich, M. A., & Zhowandai, M. H. (2005). Enumeration and speciation of enterococci found in marine and intertidal sediments and coastal water in Southern California. Journal of Applied Microbiology, 99, 598–608.
Ferguson, D., Griffith J., Cao, Y. (2011). Assessing natural sources and regrowth of Enterococcus in urban runoff impacting coastal beaches in San Diego. Headwaters to Ocean (H2O) Conference, May 24-26, 2011, San Diego, CA. http://www.coastalconference.org/h20_2011/2011_presentations.php.
Facklam, R. (2002). What happened to the streptococci: overview of taxonomic and nomenclature changes. Clinical Microbiology Reviews, 15, 613–630.
Fricker, C. R., & Eldred, B. J. (2009). Identification of coliforms genera recovered from water using different technologies. Letters in Applied Microbiology, 49, 685–688.
Girones, R., Ferrús, M. A., Alonso, J. L., Rodriquez-Manzano, J., Calgua, B., Corrêa, A. A., Hundesa, A., Carratala, A., & Bofill-Mas, S. (2010). Molecular detection of pathogens in water—the pros and cons of molecular techniques. Water Research, 44, 4325–4339.
Given, S., Pendleton, L. H., & Boehm, A. B. (2006). Regional Public Health cost estimates of contaminated coastal waters: a case study of gastroenteritis at Southern California Beaches. Environmental Science & Technology, 40, 4851–4858.
Gotelli, N. J., & Colwell, R. K. (2001). Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters, 4, 379–391.
Gordon, S., Swenson, J. M., Hill, B. C., Pigott, N. E., Facklam, R. R., Cooksey, R. C., Thornsberry, C., Jarvis, V. R., & Tenover, F. C. (1992). Antimicrobial susceptibility patterns of common and unusual species of enterococci causing infections in the United States. Enterococcal Study Group. Journal of Clinical Microbiology, 30, 2373–2378.
Grant, S. B., Sanders, B. F., Boehm, A. B., Redman, J. A., Kim, J. H., Mrse, R. D., Chu, A. K., Gouldin, M., McGee, C. D., Gardiner, N. A., Jones, B. H., Svejkovsky, J., Leipzig, G. V., & Brown, A. (2001). Generation of enterococci bacteria in a coastal saltwater marsh and its impact on surf zone water quality. Environmental Science and Technology, 35, 2407–2416.
Haile, R. W., Witte, J. S., Gold, M., Cressey, R., McGee, C. D., Millikan, R. C., Glasser, A., Harawa, N., Ervin, C., Harmon, P., Harper, J., Dermand, J., Alamillo, J., Barrett, K., Nides, M., & Wang, G. Y. (1999). The health effect of ocean water contaminated by storm drain runoff. Epidemiology, 10, 355–363.
Hardle, J. M., & Whlley, R. A. (1997). Classification and overview of the genera Streptococcus and Enterococcus. J. Appl. Microbiol. Symposium Supplement, 83, 1S–11S.
Halda-Alija, L., Hendricks, S. P., & Johnston, T. C. (2001). Spatial and temporal variation of Enterobacter genotypes in sediments and the underlying hyporheic zone of an agricultural stream. Microbial Ecology, 42, 286–294.
Hardy, W. G., & Klontz, K. C. (1996). The epidemiology of Vibrio infections in Florida, 1981–1993. Scandinavian Journal of Infectious Diseases, 173, 1176–1183.
Harwood, V. J., Gandhi, J. P., & Wright, A. C. (2004). Methods for isolation and confirmation of Vibrio vulnificus from oysters and environmental sources: a review. Journal of Microbiological Methods, 59, 301–316.
Hlavsa, M. C., Roberts, V. A., Anderson, A. R., Hill, V. R., Kahler, A. M., Orr, M. et al. (2011). Surveillance for waterborne disease outbreaks and other health events associated with recreational water---United States, 2007–2008. Surveillance Summaries, September 23, 2011/60(ss12); 1–32
Huycke, M. M., Sahm, D. F., & Gilmore, M. S. (1998). Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerging Infectious Diseases, 4, 239–249.
Ishii, S., Hansen, D. L., Hicks, R. E., & Sadowsky, M. J. (2007). Beach sand and sediments are temporal sinks and sources of Escherichia coli in Lake Superior. Environmental Science & Technology, 41, 2203–2209.
Jillson, S. J., Dahab, M. F., Woldt, W. E., & Surampalli, R. Y. (2001). Pathogen and pathogen indicator removal characteristics in treatment wetlands systems. Practice Periodical of Hazardous, Toxic, and Radioactive Waste Management, 5, 153–160.
Jiang, S., Noble, R., & Chu, W. (2001). Human adenoviruses and coliphages in urban runoff-impacted coastal waters of Southern California. Applied and Environmental Microbiology, 67, 179–184.
Karathanasis, A. D., Potter, C. L., & Coyne, M. S. (2003). Vegetation effects on fecal bacteria, BOD, and suspended solid removal in constructed wetlands treating domestic wastewater. Ecological Engineering, 20, 157–169.
Krajden, S., Fuksa, M., Petrea, C., Crisp, L. J., & Penner, J. L. (1987). Expanded clinical spectrum of infections caused by Proteus penneri. Journal of Clinical Microbiology, 25, 578–579.
Kloos, W. E., & Bannerman, T. L. (1994). Update on clinical significance of coagulase-negative staphylococci. Clinical Microbiology Reviews, 7, 117–140.
Lee, C. M., Lin, T. Y., Lin, C.-C., Kohbodi, G. A., Bhatt, A., Lee, R., & Jay, J. A. (2006). Persistence of fecal indicator bacteria in Santa Monica Bay beach sediments. Water Research, 40, 2593–2602.
Lockhart, S. R., Abramson, M. A., Beekmann, S. E., Gallagher, G., Riedel, S., Diekema, D. J., Quinn, J. P., & Doern, G. V. (2007). Antimicrobial resistance among Gram negative bacilli as causes of infections in intensive care unit patients in the United States between 1993 and 2004. Journal of Clinical Microbiology, 45, 3352–3359.
Maier, R. M., Pepper, I. L., & Gerba, C. P. (2000). Environmental microbiology. New York: Academic.
Mayo, A. W. (2004). Kinetics of bacterial mortality in granular bed wetlands. Physics and Chemistry of the Earth, Parts A/B/C, 29, 1259–1264.
Moellering, R. C., Jr. (1992). Emergence of Enterococcus as a significant pathogen. Clinical Infectious Diseases, 14, 1173–1176.
Murray, P., Rosenthal, K., Kobayashi, G., & Pfaller, M. (1998). Medical microbiology (3rd ed., p. 719). St. Louis: Mosby.
Noble, R. T., Dorsey, J. H., Leecaster, M., Reid, D., Schiff, K. C., & Weisberg, S. (2000). A regional survey of the microbiological water quality along Southern California bight shoreline. Environmental Monitoring and Assessment, 81, 435–447.
Noble, R. T., Weisberg, S. B., Leecaster, M., McGee, C. D., Dorsey, J. H., Vainik, P., & Orozco-Borbon, V. (2003). Storm effects on regional beach water quality along the Southern California shoreline. Journal of Water Health, 1, 23–31.
Noble, R. T., Lee, I. M., & Schiff, K. C. (2004). Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater. Journal of Applied Microbiology, 96, 464–472.
Perola, O., Nousiainenc, T., Suomalainend, S., Aukeea, S., Kärkkäinena, U.-M., Kauppinenb, J., Ojanena, T., & Katilaa, M.-L. (2002). Recurrent Sphingomonas paucimobilis-bacteraemia associated with a multi-bacterial water-borne epidemic among neutropenic patients. Journal of Hospital Infection, 50, 196–201.
PWA (2006). Phillip Williams & Associates, Ltd. Ballona Wetlands existing conditions report. Report submitted to California Coastal Conservancy. 184 p.
Reeves, R. L., Grant, S. B., Mrse, R. D., Oancea, C. M., Sanders, B. F., & Boehm, A. B. (2004). Scaling and management of fecal indicator bacteria in runoff from a coastal urban watershed in Southern California. Environmental Science and Technology, 38, 2637–2648.
Ricca, D. M., & Cooney, J. J. (1998). Coliphages and indicator bacteria in birds around Boston Harbor. Journal of Industrial Microbiology and Biotechnology, 21, 28–30.
Ruoff, K. L. (2002). Miscellaneous catalase-cegative, gram-positive cocci: emerging opportunists. Journal of Clinical Microbiology, 40, 1129–1133.
Sanders, B. F., Arega, F., & Sutula, M. (2005). Modeling the dry-weather tidal cycling of fecal indicator bacteria in surface waters of an intertidal wetland. Water Research, 39, 3394–3408.
Sanders, H. (1968). Marine benthic diversity: a comparative study. American Naturalist, 102, 243–283.
Sanders, W. E., Jr., & Sanders, C. C. (1997). Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clinical Microbiology Reviews, 10, 220–41.
Schmidt, U., Chme, H., & Cobbs, C. (1979). Vibrio alginolyticus infections in humans. Journal of Clinical Microbiology, 10, 666–668.
Scholthof, K.-B. G. (2007). The disease triangle: pathogens, the environment and society. Nature Reviews Microbiology, 5, 152–156.
Shashikala, S., Kavitha, R., Prakask, K., Chithra, J., Shailaja, T., Karim, P. S. (2008). Kocuria varians infective endocarditis. The Internet J. Microbiol. 5(2). http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijmb/vol5n2/kocuria.xml.
Singh, G., & Naik, C. L. (2005). Pitted keratolysis. Indian Journal of Dermatology, Venereology and Leprology, 71, 213–215.
Spellerberg, B., & Brandt, C. (2007). Streptococcus. In P. R. Murray (Ed.), Manual of clinical microbiology (pp. 412–429). Washington: American Society for Microbiology.
StatSoft, Inc. (2010). Statistica (Data Analysis Software System), Version 9.1. http://www.statsoft.com.
Steets, B. M., & Holden, P. A. (2003). A mechanistic model of runoff-associated fecal coliform fate and transport through a coastal lagoon. Water Research, 35, 589–608.
Stein, E.D., Tiefenthaler, L.L. (2004). Characterization and source identification of dry-weather metals and bacteria in Ballona Creek. In S. Weisberg and D. Elmore (Ed.). Southern California Coastal Water Research Project, Biennial Report 2003-2004.
Stein, E. D., & Tiefenthaler, L. L. (2005). Dry-weather metals and bacteria loading in an arid, urban watershed: Ballona Creek, California. Earth and Environmental Science, 164, 367–382.
Stepanovic, S., Jeek, P., Vukovic, D., Dakic, I., & Petrá, P. (2003). Isolation of members of the Staphylococcus sciuri group from urine and their relationship to urinary tract infections. Journal of Clinical Microbiology, 41, 5262–5264.
Stevens, M., Ashbolt, N., & Cunliffe, D. (2003). Recommendations to change the use of coliforms as microbial indicators of drinking water quality (p. 42p). Australia: National Health and Medical Research Council.
Stewart, J. R., Gast, R. J., Fujioka, R. S., Solo-Gabriele, H. M., Meschke, J. S., Amaral-Zettler, L. A., del Castillo, E., Polz, M. F., Collier, T. K., Strom, M. S., Sinigalliano, C. D., Moeller, P. D., & Holland, A. F. (2008). The coastal environment and human health: microbial indicators, pathogens, sentinels and reservoirs. Environmental Health, 7(suppl), 1–14.
Stinton, L. W., Hall, C. H., Lynch, P. A., & Davies-Colley, R. J. (2002). Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Applied and Environmental Microbiology, 68, 1122–1131.
Surbeck, C. Q., Jiang, S. C., Ahn, J. H., & Grant, S. B. (2006). Flow fingerprinting fecal pollution and suspended solids in stormwater runoff from an urban coastal watershed. Environmental Science and Technology, 40, 4435–4441.
Surbeck, C. Q., Jiang, S. C., & Grant, S. B. (2010). Ecological control of fecal indicator bacteria in an urban stream. Environmental Science & Technology, 44, 631–637.
Teixeira, L. M., Carvalho, M. G. S., & Facklam, R. R. (2007). Enterococcus. In P. R. Murray (Ed.), Manual of clinical microbiology (pp. 430–442). Washington: American Society for Microbiology.
Tiefenthaler, L. L., Stein, E. D., & Lyon, G. S. (2009). Fecal indicator bacteria (FIB) levels during dry weather from Southern California reference streams. Environmental Monitoring and Assessment, 155, 477–492.
Vandenesch, F., Eykyn, S. J., & Etienne, J. (1995). Infections caused by newly-described species of coagulase-negative staphylococci. Review of Medicine Microbiology, 6, 94–100.
von Bitner, T., Lewengrub, J., von Bitner, T., Ferguson, D. (2011). Seasonal and natural sources of fecal indicator bacteria to the coastal ocean from San Juan Creek watershed. Headwaters to Ocean (H2O) Conference, May 24-26, 2011, San Diego, CA. http://www.coastalconference.org/h20_2011/2011_presentations.php
Vymazal, J. (2005). Removal of enteric bacteria in constructed treatment wetlands with emergent macrophytes: a review. J. Environ. Sci. & Hlth., Part A: Toxic/Hazard. Substances & Environ. Engineer, 40, 1355–1367.
Wade, T. J., Pai, N., Eisenberg, J. N. S., & Colford, J. M. (2003). Do US Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environmental Health Perspectives, 111, 1102–1109.
Whitman, R. L., Przybyla-Kelly, K., Shively, D. A., Nevers, M. B., & Byappanahalli, M. N. (2008). Sunlight, season, snowmelt, storm, and source affect E. coli populations in an artificially ponded stream. Science of the Total Environment, 390, 448–455.
Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon, 21, 213–251.
Yamahara, K. M., Walters, S. P., & Boehm, A. B. (2009). Growth of enterococci in unaltered, unseeded beach sands subjected to tidal wetting. Applied and Environmental Microbiology, 75, 1517–1524.
Zhang, Q., Kwoh, C., Attorri, S., & Clarridge, J. E. (2000). Aerococcus urinae in urinary tract infections. Journal of Clinical Microbiology, 38, 1703–1705.
Acknowledgments
We wish to acknowledge the hard work and dedication of Marcus Raymond and Bridget Hellige, our students who spent many hours in the field and lab making this study possible. Donna Ferguson, Dr. Yiping Cao (Southern California Coastal Water Research Project), and Dr. David Turbow (TUI University, College of Health Sciences) reviewed the manuscript, providing valuable comments and suggestions. We wish to thank Dr. Gerald McGowan (City of Los Angeles, Bureau of Sanitation, Environmental Monitoring Division) for providing initial suggestions for the study along with Idexx and Vitek testing supplies. A Loyola Marymount University Continuing Faculty Grant (to JHD), Rains Research Assistant Grant, and a grant from the Southern California Academy of Sciences (Research Training Program) helped support this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorsey, J.H., Carmona-Galindo, V.D., Leary, C. et al. An assessment of fecal indicator and other bacteria from an urbanized coastal lagoon in the City of Los Angeles, California, USA. Environ Monit Assess 185, 2647–2669 (2013). https://doi.org/10.1007/s10661-012-2737-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2737-3