Abstract
The aim of the study was to determine the effects of land use management on changes in the fecal contamination of water in the Łyna River, one of the main lowland watercourses in the southern watershed of the Baltic Sea (northern Poland). A total of 120 water samples were collected in different seasons of 2011 and 2012 at 15 sites where the river intersected forest (FA), agricultural (AA), and urbanized (UA) areas. Fecal indicator bacteria (FIB), the counts of Enterobacteriaceae and Escherichia coli, total bacterial counts (TBCs), and domain Bacteria (EUB338) were determined by culture-dependent and culture-independent methods. Temperature, pH, chemical oxygen demand, dissolved oxygen, total dissolved solids, ammonia nitrogen, nitrite nitrogen, nitrate nitrogen, orthophosphate, and total phosphorus were also determined. The lowest bacterial counts were noted in water samples collected in FA, and the highest in samples collected in UA. Statistically significant differences were determined between bacterial populations across the analyzed land use types and in different sampling seasons. Significant correlations were also observed between the populations of FIB and physicochemical parameters. The results indicate that land use type influenced FIB concentrations in river water. The combined use of conventional and molecular methods improves the accuracy of fecal contamination analyses in river ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers are dynamic ecosystems that undergo continuous changes between the source and the river mouth. Those modifications are induced by the main current and river runoffs, both natural and those associated with human activities. The above factors influence the trophic state, microbiological quality, and epidemiological safety of river ecosystems.
As sources of drinking and domestic and recreational water, rivers have to be continuously monitored. Water quality is determined primarily by the type of land use in a catchment area. Potential sources of pollution include surface runoffs from agricultural land, including pastures, fields, and meadows fertilized with liquid manure (Bu et al. 2014; Edge et al. 2009; Wilkes et al. 2011). Rivers are receptacles of domestic and industrial effluents as well as municipal and rural rainwater (Daly et al. 2013; Kacar 2011; Servais et al. 2007). Effluents discharged to rivers contain fecal pathogens such as viruses, bacteria, and protozoa. Those microorganisms can be transported across significant distances, and they pose a serious threat to public health (Gotkowska-Płachta et al. 2013; Harnisz 2013; Harnisz et al. 2011; Korzeniewska and Harnisz 2013; Korzeniewska et al. 2013). The monitoring of water resources and sanitary risk identification are priority tasks in many countries. The European Union has introduced the Water Framework Directive (WFD 2000/60/EC) and the Bathing Water Directive (2006/7/EC) to protect, monitor, and rationally manage the quality of surface waters. In line with these directives, water quality is evaluated based on various physicochemical and ecological indicators. Sanitary risks in water reservoirs are identified by determining the concentrations of fecal indicator bacteria (FIB). Fecal microbes are easy to detect, and their presence in water samples can be associated with other gut pathogens and the risk of infection (Haller et al. 2009; Kay et al. 2007; Oliver et al. 2009; Shah et al. 2011). Escherichia coli can be easily identified under environmental conditions. It is regarded as the most universal, reliable, and mandatory indicator of contamination. The abundance of E. coli and other FIB in surface waters is determined by various physicochemical and biological factors, including water temperature, pH, solar radiation, content of mineral and organic compounds, predation, and competition (Huachang et al. 2010; Juhna et al. 2007; Wilkes et al. 2011; Zhu et al. 2011).
The most popular methods for identifying coliform bacteria include relatively rapid methods that rely on specific marker enzymes (beta-D-galactosidase for coliform bacteria and beta-D-glucuronidase for E. coli) and chromogenic media (Bonadonna et al. 2007; Rompré et al 2002), as well as molecular methods such as fluorescence in situ hybridization (FISH) (Amann et al. 1996; Garcia-Arminsen and Servais 2004). In the FISH method, specific oligonucleotide probes are used to identify bacterial species under an epifluorescence microscope (Amann et al. 1996). This method supports rapid detection (6–8 h) and identification of viable but non-culturable (VBNC) bacteria which lose the ability to proliferate on standard growth media but remain viable in the environment (Lew et al. 2010; Rompré et al. 2002). The combined use of conventional and molecular techniques improves the accuracy of microbiological contamination analyses in aquatic habitats (Korzeniewska and Harnisz 2012; Servais et al. 2007).
The Łyna, one of the main lowland rivers flowing into the southern Baltic Sea, is exposed to anthropogenic pressure. The sources of water pollution have not been accurately identified in the catchment area of the Łyna. During four seasons between autumn 2011 to summer 2012, water samples were collected for physicochemical and FIB analyses from the headwater section in a forest and from downstream sites in agricultural and urban sections of the catchment area. A combination of conventional and molecular analytical techniques was used to accurately describe FIB contamination levels in the Łyna River. Discharges from urban sections and farm fields were expected to increase chemical indicators and FIB levels in river water. The correlations between physicochemical parameters and microbiological indicators of river water were determined.
Materials and methods
Study area
The study was carried out in the Łyna River, the main watercourse in northeastern Poland (Fig. 1) with a total length of 263.1 km. Łyna feeds into the Pregolya River in the Kaliningrad Region. On the Polish territory, Łyna has the length of 200 km and a catchment area of about 5700 km2. Łyna intersects a lake region, and it is a slowly flowing and strongly meandering watercourse that locally resembles a mountain stream. The width of the river channel ranges from less than 20 m to around 5 m. Łyna has an average depth of 1.5–2.5 m. The average annual flow volume ranges from 7 to 35 m3 s−1, and the highest flows are noted in spring (Glińska-Lewczuk and Burandt 2011). The catchment area is occupied by arable land, meadows, and pastures in 52.92 %, and by forests in 26.30 %. The southern and central sections of the catchment are dominated by forests that occupy 68 % of the basin area, whereas the northern part features mostly arable land. Łyna intersects urbanized areas (UA; numerous villages and five towns), including Olsztyn, the capital city of the Region of Warmia and Mazury with the population of 176,000. Treated municipal waste (approximately 41,000 m3/d from five wastewater treatment plants) is discharged into the river. The source of the Łyna River is situated at an altitude of 153 m above sea level on the territory of the Łyna Spring nature reserve. The Łyna source was formed by headward erosion, a phenomenon that is rarely observed in lowlands. In its upper course, the river intersects the Warmia Forest nature reserve with an area of 1656 ha, the habitat of many protected wildlife species. The reserve is part of the Central Łyna Valley Protected Landscape Area. In its middle course, the Łyna flows through the Sępopol Plain, which is part of the Warmia Bird Refuge of the Natura 2000 network.
Sampling sites
Water samples collected along the 200-km-long Polish section of the Łyna River were subjected to microbiological and chemical analyses. The samples were collected at 15 sampling sites at specific points along the river, situated between the river source and the Polish-Russian border (Fig. 1). Four sampling sites were established in forest areas (FA) and agricultural areas (AA) each. Seven sampling sites were created in urban areas to assess the influence of urban agglomerations on the catchment area. The sites were selected to reflect variations in the microbiological and physicochemical parameters of water, with special emphasis on the varied effects of FA, AA, and UA on the catchment. The characteristic features of 15 sampling sites are presented in Table 1.
Water samples were collected from the main river stream at a depth of 0.3–0.5 m, two times in each of the four sampling seasons between winter 2011 and autumn 2012. The samples were collected in winter (December, January), spring (March, May), summer (July, August), and autumn (October, November). Three water samples were collected from each site on every sampling date. The samples were combined in equal proportions, and the resulting pool sample was subjected to microbiological analysis. A total of 32, 32, and 56 combined water samples were acquired from FA, AA, and UA, respectively. Overall, 120 samples of river water were collected and analyzed separately. All samples were placed in sterile bottles at 4 °C, transported to the laboratory, and assayed within 12 h of collection.
Physicochemical parameters
The following physicochemical parameters of river water samples were determined: temperature, pH, chemical oxygen demand (COD), dissolved oxygen (DO), total dissolved solids (TDS), ammonia nitrogen (NH4-N), nitrite nitrogen (NO2-N), nitrate nitrogen (NO3-N), orthophosphate (PO4-P), and total phosphorus (PT).
All microbiological and physicochemical determinations were carried out in the same water samples. Temperature, pH, and DO (mg O2 mL−1) were determined with the YSI 556 MPS Handheld Multiparameter Instrument (Multiprobe System) with measurement precision of ±0.1 °C, ±0.01 pH, and ±0.01 mg O2 L−1. COD, TDS, NH4-N, NO2-N, NO3-N, PO4-P, and PT were determined in accordance with the methods described by Hermanowicz et al. (1999), and the results were calculated and expressed in mg L−1.
Microbiological analysis
Determination of Enterobacteriaceae and E. coli counts by the culture-dependent method
Enterobacteriaceae and E. coli counts were determined by membrane filtration. Bacteria were passed through sterile polycarbonate filters (47 mm in diameter, 0.45-μm pore size) (Millipore) into water samples of 10 to 100 mL, depending on the expected microbial concentrations. Membrane filters were transferred to Petri dishes filled with Chromocult coliform agar (CCA) and incubated for 24 h at 35 ± 2 °C. The CCA medium is used to isolate and enumerate Enterobacteriaceae and E. coli in analyses of contamination with human and animal feces (Finney et al. 2003). After incubation, Enterobacteriaceae and E. coli colonies were counted based on the color reaction of the CCA medium, and they were identified based on colony morphological parameters, Gram stain results, and the production of indole and cytochrome oxidase. Oxidase-negative bacteria forming pale green or violet colonies (resulting from salmon-galactoside cleavage by β-d-galactosidase) were identified as Enterobacteriaceae. Dark blue colonies (resulting from the cleavage of salmon-galactoside and X-glucuronide by β-d-galactosidase and β-d-glucuronidase) were identified as E. coli.
Analyses were performed according to the standard microbiological method for testing water quality in Poland and the Standard Methods for the Examination of Water and Wastewater (APHA 1998). All determinations were performed in three replicates in the same water sample. Plate counts were expressed as colony-forming units (CFU) per 100 mL of the sample.
Determination of total bacterial counts (DAPI staining) and in situ hybridization (FISH)
The samples for the quantification of bacterial communities by the FISH method and 4′,6′-diamidino-2-phenyloindole (DAPI) staining were fixed according to the protocol described by Korzeniewska and Harnisz (2012) and Loy et al. (2007). After preliminary analyses, selected hybridization conditions were modified to optimize the results. This approach increased the efficiency of FISH analysis to 75–90 % of total cell counts.
Total bacterial counts were determined in 20–40-mL samples of river water (subject to the sampling site and suspension concentrations) which were homogenized for 2 × 1.5 min at 7000 rpm (Silent Crusher, Heidolph) to separate bacterial cells. The samples were fixed with freshly prepared paraformaldehyde (PFA, pH 7.4) to a final concentration of 4 % (v/v) and were stored at the temperature of 4 °C for 12 h. Fixed samples were passed through white polycarbonate filters (0.2-μm pore size, 47-mm in diameter) (GTTP, Millipore) under low negative pressure and with the use of base filters (0.45-μm pore size, Sartorius) to distribute cells evenly on filters. Filters were rinsed with 10 mL of ultrapure water (MQ Millipore) to remove excess paraformaldehyde and were dried for several minutes at room temperature.
Total bacterial counts (TBCs) in samples were determined by staining filtered water samples with 4′,6′-diamidino-2-phenyloindole (DAPI, showing affinity for cellular DNA) with a final concentration of 0.1 ng mL−1 (Pernthaler et al. 2001).
The samples fixed on filters were subjected to the FISH procedure with the use of oligonucleotide probes complementary to the conservative region of 16S rRNA or 23S rRNA of the analyzed bacteria. Enterobacteriaceae were identified with the ENT183 probe (Friedrich et al. 2003), E. coli with the ECO1167 probe (Neef et al. 1995), and the probe specific for the domain Bacteria (EUB338) was applied to identify all bacterial species (Amann et al. 1990). All samples were simultaneously screened with the NON338 probe (Wallner et al. 1993), complementary to EUB338, to control autofluorescence and non-specifically stained cells, the mean value of which did not exceed 5 % for the analyzed samples. All oligonucleotide probes were labeled with Cy3 dye.
For the hybridization process, 2 μL (50 ng μL–1) of EUB338 and ENT183 or ECO1167 probe solution was combined with 18 μL of the hybridization buffer containing 1 mM Tris HCl pH 7.4, 5 M NaCl, 10 % SDS, and formamide. Formamide concentrations varied subject to the applied probe (20 % for ENT183, 35 % for EUB338, and 40 % for ECO1167). The prepared solution (20 μL) was applied to filter pieces and hybridized at 46–50 °C for 1.5–3 h, subject to the applied probe. The probe was cleaned with a warm rinse buffer (48 °C) whose composition was identical to that of the hybridization buffer with the exception of formamide, which was replaced with 0.5 M EDTA, pH 8. Filters were rinsed with ultrapure water; they were dried and stained with the DAPI solution (50 μL) for 3 min at room temperature in dark. Stained filters were thoroughly rinsed with 80 % ethanol for more than 10 s to remove non-specific bonds; after which, they were rinsed with sterile distilled water and dried in air. Filter pieces were attached to slides with Citifluor (Agar Scientific, Essex, UK) and Vectashield (Vector Laboratories, Burlingame, CA) immersion oils, applied 4:1, and covered with coverslips.
The specimens were viewed under a fluorescent microscope (Olympus BX61) equipped with a × 100 oil immersion lens, UV lamp, DAPI and CY3 filters, and a CCD camera (Olympus). Images were analyzed in the Cell F application (Olympus). More than 100 hybridized bacterial cells (FISH) and 1000 to 10,000 DAPI-stained bacterial cells were identified per every evaluated sample.
Mean values and standard deviation were calculated from 20 random fields of view in every filter section and expressed in terms of 100 mL of water. Additionally, the ENT183 and ECO1167 probe-specific cell counts were presented as the number and percentage of cells visualized by DAPI.
Statistical analysis
The significance of differences in bacterial counts and physicochemical parameters of water samples from the Łyna River between sampling sites and seasons were determined by one-way analysis of variance (ANOVA). Leven’s test was used to assess the homogeneity of variance. When Leven’s test produced statistically significant results, the verified hypothesis was rejected. The Kruskal-Wallis test, a non-parametric version of classical one-way ANOVA (Stanisz 2006), was then applied. The correlations between the counts of the analyzed bacterial groups and physicochemical parameters of water samples were determined by Spearman’s non-parametric rank correlation test (p < 0.05, p < 0.01). Statistical analyses were performed in the Statistica 10.0 (StatSoft, Poland) application.
Results
Physicochemical parameters of river water
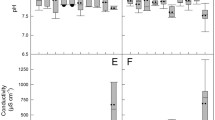
The mean values and standard deviation of the evaluated physicochemical parameters of water samples (temperature, pH, COD, DO, TDS, NH4-N, NO2-N, NO3-N, PO4-P, and PT) are presented in Table 2. These parameters varied during the seasons and land use management. Seasonal fluctuations (p < 0.05, p < 0.01, or p < 0.001) in the analyzed parameters over the entire period of the study (2011–2012) were confirmed by the Kruskal-Wallis test, except for NO2-N and NO3-N. No significant differences (ANOVA, p < 0.05) in values of temperature, pH, and NO3-N were observed between land use throughout the experimental period.
Surface water temperature ranged from 3.8 °C in winter to 25.6 °C in summer (not shown in the table), and the observed differences were statistically significant (p < 0.001). The lowest pH was noted in AA samples collected in winter, whereas the highest pH was observed in FA samples collected in summer. The observed differences in pH were statistically significant (p < 0.001) only between seasons. Significant differences in COD and DO values of water samples collected in the upper and lower course of the Łyna River were noted between land use types (p < 0.05) and seasons (p < 0.001). COD concentrations were highest (25.7 mg O2 L−1) in summer samples from UA, whereas the highest DO levels (11.3 mg O2 L−1) were observed in winter samples from FA (not shown in the table). Significant differences in TDS concentrations were observed between the analyzed samples, subject to land use type (p < 0.05) and season (p < 0.01). The lowest TDS values were noted in samples from FA or UA and the highest in samples obtained from AA in summer and spring, respectively. The concentrations of NH4-N and NO2-N (p < 0.05 or p < 0.001) varied significantly between sites characterized by different land use types and between seasons, respectively. The lowest NH4-N and NO2-N values were reported in samples obtained from FA in winter and autumn, respectively. The highest NH4-N and NO2-N concentrations were noted in water samples collected from UA in summer and spring, respectively. The concentrations of NO3-N tended to decrease along the river, but no statistically significant differences were observed between samples collected in sites with different land use types and in different seasons. The concentrations of PO4-P and PT varied between sites characterized by different land use types. Statistically significant differences (p < 0.01, p < 0.001) between PO4-P and PT levels were observed between seasons. The concentrations of PO4-P and PT differed significantly (p < 0.05, p < 0.001) between sampling sites. The lowest concentrations of both forms of phosphorus were determined in spring in FA samples, whereas water sampled from AA and UA in autumn was most abundant in PO4-P and PT.
Bacterial counts in water samples
The mean values and standard deviation of total Enterobacteriaceae and E. coli counts determined by the culture-dependent method, as well as TBC, EUB338, ENT183, and ECO1167 values determined in a culture-independent analysis of water samples, are presented in Table 3. Bacterial counts differed by 7 orders of magnitude, subject to the applied analytical method, the analyzed bacterial species, sampling site, land use type, and season. Significant differences (p < 0.05, p < 0.01, or p < 0.001) in the counts of the analyzed bacterial species were observed between land use type and seasons. Throughout the entire experimental period, the lowest TBC values were noted in water samples collected in FA. Somewhat higher bacterial concentrations were reported in samples from AA, whereas the highest TBC values were observed in water samples collected in UA.
In water samples collected in FA, Enterobacteriaceae and E. coli counts were determined by the culture-dependent method at 2 × 103 to 9 × 103 CFU 100 mL−1 and 0.1 × 103 to 0.2 × 103 CFU 100 mL−1, respectively. The abundance of Enterobacteriaceae and E. coli bacteria in water samples from AA reached 12 × 103 to 68 × 103 CFU 100 mL−1 and 0.4 × 103 to 2.5 × 103 CFU 100 mL−1, respectively. The size of Enterobacteriaceae and E. coli populations in water samples collected in UA was determined at 79 × 103 to 163 × 103 CFU 100 mL−1 and 1.7 × 103 to 5.3 × 103 CFU 100 mL−1, respectively.
The counts of Enterobacteriaceae (ENT183) and E. coli (ECO1167) determined by the fluorescence method were approximately 102–103 times higher than the size of those bacterial populations determined by the culture-dependent method. The lowest bacterial counts were noted in water samples collected in FA. The highest bacterial concentrations were determined in samples collected in UA. Despite the above, similar differences in bacterial counts were noted between samples collected in sites characterized by different types of land use. In samples obtained from FA, TBC, and the counts of bacteria hybridized with probes EUB338, ENT183, and ECO1167 did not exceed 5.3 × 108, 1.9 × 108, 0.03 × 108, and 0.009 × 108 cells 100 mL−1, respectively. The maximum abundance of the analyzed bacteria, TBC, and bacteria hybridized with probes EUB338, ENT183, and ECO1167 in water sampled from AA was determined at 7.3 × 108, 3.6 × 108, 0.08 × 108, and 0.019 × 108 cells 100 mL−1, respectively. Water samples collected in UA were characterized by the highest TBC and the highest counts of bacteria hybridized with probes EUB338, ENT183, and ECO1167 at 10.1 × 108, 4.5 × 108, 0.71 × 108, and 0.058 × 108 cells 100 mL−1, respectively.
Significant fluctuations (p < 0.05 or p < 0.001) in the abundance of the evaluated bacteria were noted between seasons. The counts of Enterobacteriaceae and E. coli determined by the culture-dependent method and FISH technique were lowest in winter (FA) when water temperature reached 3.8–9.0 °C, and they were determined at 0.11 × 104 and 0.1 × 103 CFU 100 mL−1, and 0.1 × 107 and 0.2 × 106 cells 100 mL−1, respectively. The highest Enterobacteriaceae and E. coli counts were noted in summer (UA) when water temperature ranged from 19.4 to 25.6 °C. They were determined at 2.2 × 105 and 6.5 × 103 CFU 100 mL−1, and 9.0 × 107 and 6.9 × 106 cells 100 mL−1, respectively (Fig. 2).
Counts of Enterobacteriaceae and Escherichia coli determined by a the FISH technique (bacteria hybridized with probes ENT183 and ECO1167) and b the culture-dependent method in water samples collected at different sites throughout the study. Independent variable (assembling): season. RMS random mean square, SD standard deviation, N number of samples, p significance level
The percentage of DAPI-stained bacteria visualized by probes ENT183 and ECO1167 did not exceed 0.65 and 0.17 % of TBC, respectively, in water samples from FA, 1.30 and 0.32 % of TBC, respectively, in samples from AA, and 7.0 and 0.68 % of TBC, respectively, in samples from UA (Fig. 3). The percentages of the evaluated bacteria varied subject to the type of land use, and they increased with a rise in the concentrations of PT and NH4-N.
Correlations between bacterial counts and physicochemical parameters of river water
The correlations between the counts of the analyzed bacterial groups identified in samples of river water during the entire period of the study and selected physicochemical parameters of river water are shown in Table 4. Spearman’s test revealed statistically significant (p < 0.05, p < 0.01) positive correlations between most bacterial groups. Significant (p < 0.05, p < 0.01) negative and positive correlations were noted between all bacterial groups versus water temperature, COD, DO, NH4-N, NO2-N, and PT concentrations. Correlations were also observed between PO4-P levels versus Enterobacteriaceae counts determined by the culture-dependent method, TBC, and the counts of EUB338-visualized bacteria, and between water pH versus the counts of EUB338-visualized bacteria, Enterobacteriaceae, and E. coli determined by the culture-independent method.
Discussion
The monitoring of water resources and sanitary risk identification are priority tasks in many countries around the world. The abundance, type, and origin of microbial pollutants may be very difficult to determine in aquatic habitats (Haller et al. 2009; Huachang et al. 2010; Łuczkiewicz et al. 2013; Oliver et al. 2009). The counts of Enterobacteriaceae and E. coli indicator bacteria are determined to assess epidemiological risks in water ecosystems (Korzeniewska and Harnisz 2012). An increase in the abundance of indicator bacteria could point to the presence of other potentially pathogenic microorganisms (Kacar 2011; Olapade and Weage 2010).
In this study, the concentrations of indicator bacteria in water samples from the Łyna River differed between sampling sites and experimental seasons. The observed variations were attributed to different types of land use in the surveyed parts of the catchment area as well as environmental factors. Statistical analyses revealed significant (p < 0.05, p < 0.01, p < 0.001) differences in the abundance of indicator bacteria in samples collected in different seasons from sites characterized by different types of land use. Statistically significant (p < 0.05, p < 0.01) positive and negative correlations were also observed between the analyzed bacterial groups, and between bacterial counts and the analyzed physicochemical parameters of river water.
Enterobacteriaceae and E. coli concentrations determined by the culture-dependent method
Statistical analyses revealed significant differences between the counts of indicator bacteria (Enterobacteriaceae and E. coli) in water samples obtained in different sites and seasons. Significant correlations were noted between the populations of Enterobacteriaceae and E. coli and between the concentrations of indicator bacteria versus TBC, EUB338, ENT183, and ECO1167 counts determined by the fluorescence technique. In this study, the concentrations of Enterobacteriaceae and E. coli did not exceed 220 × 105 and 10 × 105 CFU 100 mL−1, respectively, and were similar to or different from the values noted in other river ecosystems, subject to the degree and type of river contamination, type of land use in the catchment area, and the applied analytical method. The populations of indicator bacteria were approximately 10-fold lower in the Czarna Hańcza River in the Wigry National Park (Gotkowska-Płachta et al. 2005). In the Vistula River, Enterobacteriaceae and E. coli counts were estimated in the range of 102 to 104 CFU 100 mL−1 (Donderski and Wilk 2002a, b). In the Kalamazoo River in the USA, the size of indicator bacteria populations was determined in the range of 104 to 105 CFU 100 mL−1 by Olapade and Weage (2010). The abundance of indicator bacteria in the Seine River (France) polluted with effluents was estimated at 106 to 108 CFU 100 mL−1 by Savichtcheva and Okabe (2006). In a study of more than 10 Canadian rivers, Wilkes et al. (2009) determined the counts of indicator bacteria at 107 to 108 CFU 100 mL−1. In the Łyna River, significant seasonal variations were observed in the size of Enterobacteriaceae and E. coli populations. Seasonal differences in the counts of indicator bacteria were also noted by Kacar (2011) in five rivers in western Turkey and by Okeke et al. (2011) in the Tallapoosa River in the USA.
In our study, the minimum and maximum counts of Enterobacteriaceae and E. coli bacteria in the analyzed sampling sites and seasons could be attributed to the stimulating or inhibiting effects of environmental factors as well as differences in the type of land use in the catchment area, which contributed to the inflow of organic and mineral substances from external sources. This conclusion is corroborated by the results of our statistical analysis showing significant positive or negative correlations between the populations of Enterobacteriaceae and E. coli versus water temperature, COD, DO, NH4-N, NO2-N, and PT. Increased concentrations and loads of PT, NH4-N and FIB in stream water are considered to be universal indicators in urban streams, even at low levels of catchment urbanization (Hatt et al. 2004; Tryland et al. 2002). The counts of Enterobacteriaceae and E. coli were 10- to 50-fold higher in water samples collected in AA and UA than in samples obtained from FA, which indicates that the type of land use influences the microbiological quality of water in the Łyna River. Due to higher levels of bacterial contamination, the quality of water samples from AA and UA were classified as “poor,” whereas the quality of water from FA was classified as “excellent” in line with the criteria set out by the Bathing Water Directive (2006/7/EC). Higher concentrations of E. coli in urban catchments were also reported by Bu et al. (2014) and Daly et al. (2013). Similar correlations between the type of land use in the catchment area and the physicochemical and/or microbiological parameters of river water were observed by Economou et al. (2013), Nnane et al. (2011), and Wilkes et al. (2009, 2011).
Bacterial concentrations determined by DAPI staining and the FISH method
Total bacterial counts in the Łyna River were determined at 106–108 cells 100 mL−1, and they were similar to or insignificantly different from the most recent values reported in other rivers around the world. Somewhat lower microbial counts (0.13 × 107 to 330 × 107 cells 100 mL−1) were observed in the Rouge River (USA) by Tiquia (2010), in whose study microbial populations differed significantly between water samples collected in the analyzed seasons. Total bacterial counts of 0.27–8.1 × 109 cells 100 mL−1 were determined by Bouvy et al. (2010) in the Senegal River and its 13 tributaries, and similar microbial concentrations were observed by Araújo and Godinho (2008) in the Pitimbu River in Brazil. In our study, the differences in TBC values between seasons and sites confirm that aquatic microorganisms are highly sensitive to environmental changes. The physicochemical parameters of water and the size of heterotrophic bacterial populations in water bodies are determined by the type of land use, degree of urbanization, and the intensity of human activities in the catchment area (Glińska-Lewczuk 2006; Gołaś et al. 2008a, b, 2009). In the Łyna River, TBC values were severalfold higher in samples collected from AA and UA than from FA, which clearly indicates that the type of land use in the catchment area influences the microbiological quality of water reservoirs.
The abundance of Enterbacteriaceae and E. coli determined by the FISH method in the Łyna River was approximately 102–103 higher than that determined with the use of a culture-dependent method (Table 1). The above results indicate that the analyzed river is a substantial reservoir of non-culturable fecal bacteria. Fecal bacteria may cease to proliferate under stress. Intestinal microorganisms that reach river waters with evacuated effluents are particularly exposed to changes in habitat conditions, such as temperature, oxygen concentrations, pH, and nutrient availability, and such bacterial cells could become non-culturable. The above poses a significant problem when pathogenic and opportunistic bacteria are concerned, because such microorganisms are generally characterized by higher virulence than cells not exposed to environmental stressors (Rowan 2004). In many cases, E. coli bacteria carry drug resistance genes that can be transferred to other microorganisms in water, soil, and air (Korzeniewska and Harnisz 2013; Korzeniewska et al. 2013). The concentrations of indicator bacteria in aquatic habitats should be accurately determined to eliminate epidemiological and public health risks in rivers. Fluorescent in situ hybridization supported the determination of the actual contamination of the Łyna River with Enterobacteriaceae and E. coli. The highest Enterobacteriaceae and E. coli concentrations were noted in water samples from UA, and they were approximately 10-fold lower in FA samples. Bacterial counts were positively correlated (p < 0.01) with COD, NH4-N, NO2-N, and PT levels which are indicative of water pollution associated with domestic, municipal, and land use activities. The correlations between Enterobacteriaceae and E. coli levels versus PT and NH4-N (Fig. 3) values suggest that effluents discharged by wastewater treatment plants in urban areas and runoffs from farm fields fertilized with phosphate and ammonia were the combined sources of pollution. Our results indicate that urban areas (UA) situated along the Łyna River significantly contributed to the deterioration in downstream water quality.
Conclusions
The concentrations of fecal indicator bacteria in the Łyna River varied significantly subject to the type of land use in the catchment area and environmental factors. The populations of all analyzed bacterial groups were higher in water samples collected in UA where treated municipal waste was discharged directly to the Łyna River. Microbial concentrations were also higher in water samples collected in AA. At sampling sites characterized by high levels of bacterial contamination, the quality status of water samples was reclassified from excellent to poor in line with the criteria set out by the Bathing Water Directive (2006/7/EC).
The concentrations of fecal indicator bacteria (Enterobacteriaceae and E. coli) in the Łyna River were most highly correlated with water temperature and COD, DO, NH4-N, NO2-N, and PT concentrations, which could adversely influence self-purification processes in the studied ecosystem. The above contributes to eutrophication and poses greater risk to public health. In the future, the results of microbiological analyses could constitute a valuable reference point for evaluating the rate and directions of changes in the bacteriological profile of the Łyna River.
The combination of conventional and molecular analytical techniques supported accurate determinations of contamination with Enterobacteriaceae and E. coli indicator bacteria in the Łyna River. The FISH technique successfully identified bacteria that had not been determined by the culture-dependent method. The results of the FISH analysis contributed to a reliable assessment of fecal contamination in the studied river and an evaluation of the suitability of river water for drinking, recreational, and domestic use. Our results indicate that the combined use of conventional and molecular methods improves the accuracy of microbiological contamination analyses in river ecosystems, eliminates the risk of underestimation of pollution levels, and contributes to epidemiological and public health safety.
References
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56(6):1919–1925
Amann R, Snaidr J, Wagner M, Ludwig W, Schleifer KH (1996) In situ visualization of high genetic diversity in a natural microbial community. J Bacteriol 178(12):3496–3500
APHA (American Public Health Association) (1998) Standard method for the examination of water and wastewater, 19th edn. American Public Health Association, Washington DC
Araújo MFF, Godinho MJL (2008) Seasonal and spatial distribution of bacterioplankton in a fluvial-lagunar system of a tropical region: density, biomass, cellular volume and morphologic variation. Braz Arch Biol Technol 51(1):203–212, ISSN 1516–8913
Bonadonna L, Cataldo C, Semproni M (2007) Comparison of methods and confirmation tests for the recovery Escherichia coli in water. Desalination 213(1–3):18–23
Bouvy M, Arfi R, Bernard C, Carré C, Got P, Pagano M, Troussellier M (2010) Estuarine microbial community characteristics as indicators of human-induced changes (Senegal River, West Africa). Estuar Coast Shelf Sci 87(6):573–582
Bu H, Meng W, Zhang Y, Wan J (2014) Relationships between land use patterns and water quality in the Taizi River basin. Chin Ecol Indic 41:187–197
Daly E, Kolotelo P, Schang C, Osborne CA, Coleman R, Deletic A, McCarthy DT (2013) Escherichia coli concentrations and loads in an urbanised catchment: the Yarra River, Australia. J Hydrol 497:51–61
Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality. Off J Eur Commun L64: 37–51
Donderski W, Wilk I (2002a) Bacteriological studies of water and bottom sediments of the Vistula River between Wyszogród and Toruń. Pol J Environ Stud 11(1):33–40
Donderski W, Wilk I (2002b) The sanitary state of water in the River Vistula between Wyszogród and Toruń. Pol J Environ Stud 11(5):509–515
Economou V, Gousia P, Kansouzidou A, Sakkas H, Karanis P, Papadopoulou C (2013) Prevalence, antimicrobial resistance and relation to indicator and pathogenic microorganisms of Salmonella enterica isolated from surface waters within an agricultural landscape. Int J Hyg Environ Health 216(4):435–444
Edge TA, Droppo I, El-Shaarawi A, Gannon V, Hewitt M, Kent R, Khan I, Koning W, Lapen D, Marcogliese D, Marvin C, Miller J, Neumann N, Phillips R, Robertson W, Schreier H, Shtepani I, Topp E, van Bochove E (2009) An evaluation of Escherichia coli as a potential agri-environmental waterborne pathogen standard. Environment Canada, Gatineau, Quebec, Synthesis report 14. National Agri- Environmental Standards Initiative, p 48
Finney M, Smullen J, Foster HA, Brokx S, Storey DM (2003) Evaluation of chromocult coliform agar for the detection and enumeration of Enterobacteriaceae from faecal samples from healthy subjects. J Microbiol Methods 54(3):353–358
Friedrich U, Van Langenhove H, Altendorf K, Lipski A (2003) Microbial community and physicochemical analysis of an industrial waste gas biofilter and design of 16S rRNA-targeting oligonucleotide probes. Environ Microbiol 5(3):183–201
Garcia-Arminsen T, Servais P (2004) Enumeration of viable E. coli in rivers and wastewaters by fluorescent in situ hybridization. J Microbiol Method 58:269–279
Glińska-Lewczuk K (2006) Effect of land use and lake presence on chemical diversity of the Lyna river system. Pol J Environ Stud 15(2):259–269
Glińska-Lewczuk K, Burandt P (2011) Effect of river straightening on the hydrochemical properties of floodplain lakes: observations from the Łyna and Drwęca Rivers, N Poland. Ecol Eng 37:786–795
Gołaś I, Zmysłowska I, Harnisz M, Korzekwa K, Skowrońska A, Teodorowicz M, Górniak D, Gros M, Brzozowa S (2008a) Nitrogen cycle bacteria in the waters of the Drwęca River. Pol J Environ Stud 17(2):215–225
Gołaś I, Zmysłowska I, Harnisz M, Korzekwa K, Skowrońska A, Teodorowicz M, Górniak D, Dudziec E (2008b) Anthropogenic impact on quantitative differentiation of nitrogen cycling bacteria in waters of the River Drwęca. Pol J Nat Sci 23(3):667–680
Gołaś I, Korzekwa K, Harnisz M, Zmysłowska I, Teodorowicz M, Terech-Majewska E, Rodziewicz W, Bieńkowska M (2009) Influence of fishery management and environmental factors on occurrence of heterotrophic, hemolytic and mesophilic bacteria and Aeromonas hydrophila in waters of Drwęca River, Poland. Arch Environ Protect 35(2):27–40
Gotkowska-Płachta A, Niewolak S, Korzeniewska E (2005) Pollution degree and sanitary state indicator bacteria as the indicators of the purity of Lake Hańcza waters. Arch Environ Protect 31(2):53–68
Gotkowska-Płachta A, Filipkowska Z, Korzeniewska E, Janczukowicz W, Dixon B, Gołaś I, Szwalgin D (2013) Airborne microorganisms emitted from wastewater treatment plant treating domestic wastewater and meat processing industry wastes. CLEAN Soil Air Water 41(5):429–436
Haller L, Pote’ J, Loizeau J-L, Wildi W (2009) Distribution and survival of faecal indicator bacteria in the sediments of the Bay of Vidy, Lake Geneva, Switzerland. Ecol Indic 9:540–547
Harnisz M (2013) Total resistance of native bacteria as an indicator of changes in the water environment. Environ Pollut 174:85–92
Harnisz M, Gołaś I, Pietruk M (2011) Tetracycline-resistant bacteria as indicators of antimicrobial resistance in protected waters—the example of the Drwęca River nature reserve (Poland). Ecol Indic 11:663–668
Hatt BE, Fletcher TD, Walsh CJ, Taylor SL (2004) The influence of urban density and drainage infrastructure on the concentrations and loads of pollutants in small streams. Environ Manag 34:112–124
Hermanowicz W, Dożańska W, Dojlido J, Koziorowski B (1999) Physicochemical methods of water and wastewater examination. (in Polish). Arkady, Warsaw
Huachang H, Jianwen Q, Liang Y (2010) Environmental factors influencing the distribution of total and fecal coliform bacteria in six water storage reservoirs in the Pearl River Delta Region. Chin J Environ Sci 22(5):663–668
Juhna T, Birzniece D, Rubulis J (2007) Effect of phosphorus on survival of Escherichia coli in drinking water biofilms. Appl Environ Microbiol 73(11):3755–3758
Kacar A (2011) Analysis of spatial and temporal variation in the levels of microbial fecal indicators in the major rivers flowing into the Aegean Sea, Turkey. Ecol Indic 11:1360–1365
Kay D, Edwards AC, Ferrier RC, Francis C, Kay C, Rushby L, Watkins J, McDonald AT, Wyer M, Crowther J, Wilkinson J (2007) Catchment microbial dynamics: the emergence of a research agenda. Prog Phys Geogr 31(1):59–76
Korzeniewska E, Harnisz M (2012) Culture-dependent and culture-independent methods in evaluation of emission of Enterobacteriaceae from sewage to the air and surface water. Water Air Soil Pollut 223:4039–4046
Korzeniewska E, Harnisz M (2013) Extended-spectrum beta-lactamase (ESBL)-positive Enterobacteriaceae in municipal sewage and their emission to the environment. J Environ Manag 128:904–911
Korzeniewska E, Korzeniewska A, Harnisz M (2013) Antibiotic resistant Escherichia coli in hospital and municipal sewage and their emission to the environment. Ecotoxicol Environ Saf 91:96–102
Lew S, Lew M, Mieszczyński T, Szarek J (2010) Selected fluorescent techniques for identification of the physiological state of individual water and soil bacterial cells—review. Folia Microbiol 55(2):107–118
Loy A, Maixner F, Wagner M, Horn M (2007) ProbeBase—an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Res 3(1):00–804
Łuczkiewicz A, Felis E, Ziembińska A, Gnida A, Kotlarska E, Olańczuk-Neyman K, Surmacz-Górska J (2013) Resistance of Escherichia coli and Enterococcus spp. to selected antimicrobial agents present in municipal wastewater. J Water Health 11(4):600–612
Neef A, Amann R, Schleifer KH (1995) Detection of microbial cells in aerosols using nucleic acid probes. Syst Appl Microbiol 18(1):113–122
Nnane DE, Ebdon JE, Taylor HD (2011) Integrated analysis of water quality parameters for cost-effective faecal pollution management in river catchments. Water Res 45(6):2235–2246
Okeke BC, Thomson MS, Moss EM (2011) Occurrence, molecular characterization and antibiogram of water quality indicator bacteria in river water serving a water treatment plant. Sci Total Environ 409(23):4979–4985
Olapade OA, Weage EA (2010) Comparison of fecal indicator bacterial populations in surface waters of the Kalamazoo River, USA. Microbes Environ 25(1):41–44
Oliver DM, Fish RD, Hodgson CJ, Heathwaite AL, Chadwick DR, Winter M (2009) A cross-disciplinary toolkit to assess the risk of faecal indicator loss from grassland farm systems to surface waters. Agric Ecosyst Environ 129(4):401–412
Pernthaler J, Glöckner FO, Schönhuber W, Amann R (2001) Fluorescence in situ hybridization with rRNA-targeted oligonucleotide probes. In: J Paul (ed), London: Academic Press Ltd. Methods in Microbiology 30: 207–226
Rompré A, Servais P, Baudart J, De Roubin MR, Laurent P (2002) Methods of detection and enumeration of coliforms in drinking water a review. J Microbiol Methods 49(1):31–54
Rowan NJ (2004) Viable but non-culturable forms of food and waterborne bacteria: Quo vadis? Trends Food Sci Technol 15:462–467
Savichtcheva O, Okabe S (2006) Alternative indicators of fecal pollution: relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res 40(13):2463–2476
Servais P, Garcia-Armisen T, George I, Billen G (2007) Fecal bacteria in the rivers of the Seine drainage network (France): sources, fate and modeling. Sci Total Environ 375(1–3):152–167
Shah AH, Abdelzaher AM, Phillips M, Hernandez R, Solo-Gabriele HM, Kish J, Scorzetti G, Fell JW, Diaz MR, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidle ML, Wanless D, Ager A, Lui J, Stewart JR, Plano LRW, Fleming LE (2011) Indicator microbes correlate with pathogenic bacteria, yeasts and helminthes in sand at a subtropical recreational beach site. J Appl Microbiol 110(6):1571–1583
Stanisz A (2006) A straightforward course in statistics based on the STATISTICA PL program as applied in medicine. Volume Basic statistics . (In Polish). StatSoft Poland. Cracow, 2006. ISBN 83-88724-18-5
Tiquia SM (2010) Metabolic diversity of the heterotrophic microorganisms and potential link to pollution of the Rouge River. Environ Pollut 158(5):1435–1443
Tryland I, Surman S, Berg JD (2002) Monitoring faecal contamination of the Thames estuary using semiautomated early warning system. Water Sci Technol 46:25–31
Wallner G, Amann R, Beisker W (1993) Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14(2):136–143
WFD, (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. J Eur Union
Wilkes G, Edge T, Gannon V, Jokinen C, Lyautey E, Medeiros D, Neumann N, Ruecker N, Topp E, Lapen DR (2009) Seasonal relationships among indicator bacteria, pathogenic bacteria, Cryptosporidium oocysts, Giardia cysts, and hydrological indices for surface waters within an agricultural landscape. Water Res 43(8):2209–2223
Wilkes G, Edge TA, Gannon VPJ, Jokinen C, Lyautey E, Neumann NF, Ruecker N, Scott A, Sunohara M, Topp E, Lapen DR (2011) Associations among pathogenic bacteria, parasites, and environmental and land use factors in multiple mixed-use watersheds. Water Res 45(18):5807–5825
Zhu X, Wang JD, Solo-Gabriele HM, Fleming LE (2011) A water quality modeling study of non-point sources at recreational marine beaches. Water Res 45(9):2985–2995
Acknowledgments
This study was supported by grants No. 528-0807-0806 and No. 1010.0804 from the Ministry of Science and Higher Education (Poland).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Gotkowska-Płachta, A., Gołaś, I., Korzeniewska, E. et al. Evaluation of the distribution of fecal indicator bacteria in a river system depending on different types of land use in the southern watershed of the Baltic Sea. Environ Sci Pollut Res 23, 4073–4085 (2016). https://doi.org/10.1007/s11356-015-4442-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4442-6