Abstract
Soil contamination with Cd and Pb is a worldwide problem which not only degrades the environment but also poses a serious threat for human and animal health. Phytoremediation of these contaminated soils using halophytic plants like quinoa presents an opportunity to clean the soils and use them for crop production. The current experiment was performed to evaluate the Cd and Pb tolerance potential of quinoa and subsequently its implications for human health. Three weeks old quinoa seedlings were exposed to Cd (30, 60 and 90 mg kg−1) and Pb (50, 100 and 150 mg kg−1) levels along with a control. The results revealed that plant height decreased at highest levels of soil Cd and Pb. Shoot, root and seed dry weight decreased with increasing levels of soil Cd and Pb. Tissue Cd and Pb concentrations increased with increasing levels of Cd and Pb in soil, the highest Cd was found in roots while the lowest in seeds. The highest Pb concentration was found in shoots at low Pb level, while in roots at high level of Pb. Increasing levels of Cd and Pb stimulated the activities of measured antioxidant enzymes and decreased membrane stability index. The health risk assessments of Cd and Pb revealed that hazard quotient was < 1 for both the metals. However, the results of total hazard quotient showed that value was < 1 for Pb and 1.19 for Cd showing potential carcinogenicity. This study demonstrates that quinoa has good phytoremediation potential for Cd and Pb however, the risk of Cd toxicity is challenging for human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unrestricted and untreated discharge of industrial effluent in peri urban areas of developing countries is an emerging issue of toxicity of heavy metals and a threat to agricultural land (Wahid et al. 2009). Excessive use of fertilizers, untreated waste water irrigation and industrial wastes are major causes of heavy metal contaminated soils. Heavy metals cause toxicity in the food chain and disturbs the ecological balance (Abdollahi et al. 2011). Unlike organic pollutants, heavy metals can sustain in the environment for a long time, as they are non-degradable. Due to a half-life of these heavy metals of more than 20 years, they may alter food chains and pose serious threats to life systems (Ruiz et al. 2009).
Lead (Pb) is a non-essential, potentially toxic and a known protoplasmic poison. It is mainly used as an additive of pigments, pesticides, fertilizers and gasoline (Wuana and Okieimen 2011). Its concentration in soil is usually < 50 mg kg−1 (Nazar et al. 2012) and at higher levels it is lethal for growth and development of plants (Sharma and Dubey 2005). It interrupts water balance and mineral nutrition, stops enzymatic activities, hinders photosynthesis, delays cell division and eventually causes cell death in plants (Sharma and Dubey 2005; Singh et al. 2016).
Cadmium (Cd) accumulation in soil occurs from both natural and human activities (Pan et al. 2016). Weathering is a natural source of Cd accumulation in soil (Liu et al. 2013). However, human activities include waste water irrigation, industrial activities, agrochemicals, manufacturing and mining which contribute to accumulation of Cd in soil (Nawab et al. 2016). Its concentration ranges between 3 and 30 mg kg−1 in plant tissues (Liang et al. 2011), and it reduces germination and plant growth, causes chlorosis, root browning, necrosis, and leads to plant death (Abbas et al. 2017; Chang et al. 2013). It also adversely affects respiration (Smiri et al. 2009), photosynthesis (Feng et al. 2010), water uptake (Polle et al. 2013) and transport of nutrients in plants (Polle et al. 2013).
Cadmium and Pb toxicity in plants causes oxidative stress via excessive production of reactive oxygen species (ROS) (Ahmadi et al. 2018). The ROS consists of H2O2, OH• and O2− and their production occurs due to membrane linked electron transport activities (Sharma et al. 2012). The ROS are cytotoxic and can harm proteins, nucleic acid, membrane lipids, enzymes, and chloroplast pigments (Gill and Tuteja 2010).
Several ecological and environmental processes like metals solubilization, their availability and transport are influenced by metal–organic matter complexes (Shahid et al. 2012). Likewise, the presence of different metals at higher concentrations in eatable plant parts is a great deal of concern across the world (Han et al. 2018; Jia et al. 2018; Shahid et al. 2018). Henceforth, the intake of metal-contaminated cereal crops is a worldwide hazard for the people that are consuming them (Xiong et al. 2016). The threshold concentration of Cd and Pb for many grain crops is 0.10 and 0.20 mg kg−1, respectively. European Commission Regulation (2006) reported that depending upon the crop type; metals are uptaken and subsequently bioaccumulated in plant tissues like grains, roots and shoots in different concentrations and thus enter the food chain. It not only poses serious health risk for humans and animals in the food chain but also dangerous for environmental sustainability.
Phytoremediation is a process in which plants degrade the contaminants from nature (Visioli and Marmiroli 2013). It includes transfer, immobilization, removal and degradation of contaminants from soil and water (Ahmadpour et al. 2012). Phytoremediation of metal contaminated soils depends on time to reduce metal toxicity (Paz-Ferreiro et al. 2014). Compared with other traditional techniques, this technique is latest and has potential to provide necessary green technology for contaminated environment. According to Halder and Ghosh (2014), phytoremediation can be classified as phytostabilisation, phytofiltration, phytotransformation, phytoextraction and phytovolatilization. The success ratio of the phytoextraction is mainly reliant on numerous factors including metal bioavailability, plant species and soil properties (Walliwalagedara et al. 2010).
Quinoa (Chenopodium quinoa Willd.) belongs to the Amaranthaceae family, which has been recently introduced in Pakistan. Quinoa can grow in contaminated soils because it is a facultative halophyte (Matiacevich et al. 2006). The nutritional content of quinoa as well as its ability to grow in harsh environments are high. It is reported that halophytes have the potential to extract heavy metals (Vara Prasad and de Oliveira Freitas 2003). Quinoa may be a good choice to ensure food security, as it can tolerate abiotic stresses like salinity, drought and low temperature (Jacobsen et al. 2005, 2007; Jacobsen 2014; Mujica et al. 2001; Nanduri et al. 2019; Razzaghi et al. 2015).
It was hypothesized that quinoa being a halophyte and able to survive under diverse environmental conditions would survive under heavy metals toxicity and would be safe for human consumption. The experiment was performed to assess the tolerance and physiological changes in quinoa against Cd and Pb toxicity, to evaluate the phytoremediation potential, and to estimate health risk from Cd and Pb toxicity.
Material and methods
Soil sampling and preparation
Soil was collected from experimental site of COMSATS University Islamabad, Vehari Campus (latitude, 30°-1.9998′N, longitude 72°-21′E, and altitude 184.4 m) with average a minimum and maximum temperature of 15–26.4 °C and a relative humidity of 57.5–62.7%. Five samples were collected, air dried, ground, passed through a 2 mm sieve and different physicochemical properties were analyzed (Table 1). The soil was spiked with Cd (30, 60 and 90 mg kg−1 designated as Cd-30, Cd-60 and Cd-90 respectively) by using the CdCl2 and Pb (50, 100 and 150 mg kg−1 designated as Pb-50, Pb-100 and Pb-150 respectively) by using PbCl2. The earthen pots were lined with polythene sheets and filled with spiked soil (6 kg/pot) for each treatment along with a control treatment where no Cd or Pb was applied.
Plant nursery establishment and transplantation
The experiment was performed in the glasshouse of the Department of Environmental Sciences. The glasshouse has no control over temperature and humidity; however, it restricts the entrance of insects. Healthy and sterilized quinoa seeds (cv. Titicaca) were grown in polythene lined iron trays having quartz sand. Seedlings were watered daily by distilled water for three weeks until they were ready for transplantation. Two healthy seedlings were transplanted in each pot. Nitrogen and phosphorous (67 and 33 mg kg−1 respectively) was applied as urea and diammonium phosphate and mixed respectively in each pot (Iqbal and Afzal 2014). Plants were irrigated with tap water and all the physiochemical properties of the water were analyzed (Table 1). At the onset of flowering stage one plant was harvested from each pot for determination of different physiological parameters.
Determination of antioxidant enzyme activity
Leaf extract
For leaf extract, 0.5 g of leaf samples was homogenized in potassium phosphate buffer (pH = 7) in pre-cooled mortar pestle. Samples were then centrifuged at 4 °C for 20 min at 10,000 × g and supernatant was collected for the determination of antioxidant enzymes activity.
Superoxide dismutase
Superoxide dismutase (SOD) activity was determined by estimating photochemical reduction of nitro blue tetrazolium (NBT) according to (Gupta et al. 1993). The reaction mixture consisted of 0.05 μL of enzyme extract, 1.5 ml of 100 mM phosphate buffer, 0.1 ml of 200 mM EDTA, 0.1 ml of 200 mM methionine and 0.01 ml of 2.25 mM NBT, 1 ml of distilled water and 0.1 ml of 60 µl riboflavin. In order to start the reaction, the reaction mixture was kept under 30 W fluorescent lamp for 15 min, the distance between lamp and reaction mixture was 30 cm. The absorbance was measured at 560 nm using UV–VIS spectrometer (PerkinElmer Lambda 25). The formation of blue formasan is restricted as superoxide anions present in the sample are scavenged by the SOD activity.
Catalase
Catalase (CAT) activity was measured according to (Aebi 1984). Assay mixture was prepared by using 40 μL enzyme extract, 1 ml of 50 mM phosphate buffer and 5 µl of H2O2. The decomposition of H2O2 was measured by recording the absorbance at 240 nm for 1 min.
Ascorbate peroxidase activity
Ascorbate peroxidase (APX) activity was measured as described by (Amako et al. 1994). Assay mixture was prepared by adding 40 μl enzyme extract, 1 ml of 50 mM phosphate buffer, 100 µl of 0.5 mM ascorbate and 100 μl of H2O2 to start the reaction. The reduction in absorbance was recorded at 290 nm and this decrease in absorbance was used to calculate the APX activity.
Peroxidase
The activity of Peroxidase (POD) was measured according to (Panda et al. 2003). Assay mixture was prepared by using 40 μl of enzyme extract, 15 μl of H2O2, 20 μl of guaiacol. The decrease in concentration of guaiacol was analyzed at 436 nm. The enzyme activity was expressed in μmol guaiacol per minute per milligram of protein.
Membrane stability index
Membrane stability index (MSI) was estimated by the method described by (Sairam et al. 2002). Two leaf samples having weight of 0.1 g were taken and then they were kept in 10 ml distilled water and put into water bath. To calculate the membrane stability index the electrical conductivity of samples were noted down separately for samples kept at 40 °C for 30 min and for samples kept at 100 °C for 10 min. The formula to calculate the membrane stability is:
Plant biomass and yield
At seed maturity, plants were harvested and separated into roots, shoots and seeds. Seeds were cleaned, sun dried and their weight was measured on an analytical balance. Plant shoots and roots were measured separately after hot air oven drying at 65 °C for 48 h till constant weight was obtained.
Bioconcentration factor and tolerance Index
The Bioconcentration factor (BCF) was determined as the ratio of plant metal concentrations (Pb and Cd) in plant biomass to that in the soil.
Cadmium and Lead tolerance index (TI) was calculated on the basis of dry weight stressed and control plants.
Health risk assessment
Average Daily Intake
Average Daily Intake (ADI) was estimated from the average values of metal concentration for quinoa by using Eq. (5) (Khan et al. 2014; Rehman et al. 2016).
In Eq. (5), Cf = Concentration of metals in edible parts, IR = Ingestion rate that is standard 100.4 g day−1, EF = exposure frequency (180 days), ED = Everage exposure duration (70 years globally), BW = average body weight (70 kg), and AT = Average life expectancy.
Hazard quotient
The hazard quotient (HQ) for heavy metals is the ratio of ADI of heavy metals in quinoa to the reference dose (RfD). The HQ values were estimated by using Eq. (6) (Rehman et al. 2016).
In Eq. (6), ADI = average daily intake, RfD = reference dose of a metal which is 0.001 for Cd and 0.004 for Pb.
Cancer risk assessment
It is important to find the life time cancer risk (LTCR) associated with the consumption of quinoa, Therefore, the values of LTCR from quinoa ingestion throughout the life were calculated by using Eq. (7) (Rehman et al. 2016).
In Eq. (7), ADI = average daily dose calculated from Eq. (5), and CSF = cancer slope factor which is 6.1 mg kg−1 day −1 for Cd and 0.0085 mg kg−1 day −1 for Pb.
Target hazard quotient
The target hazard quotient (THQ) for health risk assessment through contaminated food was calculated with Eq. (7) (Zeng et al. 2015). The value of THQ < 1 is considered safe.
In Eq. (7), EF = average exposure frequency, ED = average exposure duration, IR = average ingestion rate, Cf = conversion factor, RfD = reference dose, BW = average body weight, AT = average life expectancy.
Statistical analysis
The complete randomized design (CRD) was followed to perform experiments with five replicates. The statistical significance of various parameters for Cd and Pb treatments was estimated with Analysis of variance (ANOVA). The means of different parameters were compared by using Tukey’s test with STATISTIX 8.1 software (http://www.statistix.com).
Results
Plant growth, tolerance index and bioconcentration factor
The results revealed that different levels of Cd and Pb significantly decreased shoot and root lengths, dry weights and seed yield as compared to the control (Table 2). The lowest values of these parameters were found at highest levels of these metals i.e. Cd-90 and Pb-150. The percent reductions at Cd-90 and Pb-150 were in shoot length 34 and 38%, shoot dry weight 70 and 63%, root dry weight 66 and 63%, and seed yield 57 and 47%, respectively.
The tolerance index (TI) was calculated based upon dry weight of plants which showed a significant decreasing trend with increasing Cd and Pb concentration. Table 2 showed that the TI was lowest (34.50% and 51.58%) at highest concentration of Cd (Cd-90) and Pb (Pb-150) respectively. Similarly, the BCF values also decreased with increasing levels of Cd and Pb treatments and the values were < 1, except Pb-50 treatment (1.09).
Calcium, potassium and sodium concentration
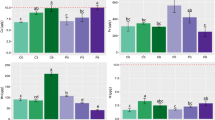
The results revealed that Cd caused a significant decline in Ca concentration in roots and shoots but increased Ca level in seeds compared to the control (Fig. 1a). However, root and shoot Ca concentration significantly increased by increasing Pb levels whereas, seed Ca concentration remained unchanged (Fig. 1d).
Accumulation of Ca, K and Na in root, shoot and seeds in response to increasing levels of Cd: 30, 60 and 90 mg kg−1 designated as Cd-30, Cd-60, Cd-90 (a–c) and Pb: 50, 100 and 150 mg kg−1 designated as Pb-5, Pb-10, Pb-15 (d–f) stress. All the values are means of five replicates ± SE and Tukey’s range test has been used to identify the significant differences between different means. The bars sharing the same letters (a, b, c, etc.) show the non-significant differences at p < 0.05
The results revealed that roots K concentration showed a significant decrease in shoots at Cd-30 (Fig. 1b). The increasing Pb in soil significantly increased roots K concentration, whereas, K concentration significantly decreased at Pb-100 and Pb-150 and increased at Pb-50 (Fig. 1e).
The results showed that Na accumulation in roots and shoots significantly increased with increasing Cd in soil while, it significantly decreased at Cd-90 (Fig. 1c). Increasing level of Pb significantly increased accumulation of Na in root and decreased in shoot at Pb-50 and Pb-100 (Fig. 1f).
Lipid peroxidation and membrane stability index
A significant decrease in MSI was observed with increasing levels of Cd and Pb in soil. The lowest MSI was observed at the highest level of Cd and Pb i.e. Cd-90 and Pb-150 (Fig. 2a, b).
Effect of increasing levels of Cd: 30, 60 and 90 mg kg−1 designated as Cd-30, Cd-60, Cd-90 and Pb: 50, 100 and 150 mg kg−1 designated as Pb-5, Pb-10, Pb-15 stress on membrane stability index (a, b) and MDA contents (c, d) in quinoa. The bars are mean values of five replicates ± SE and Tukey’s range test has been used to identify the significant differences between different means. The bars sharing the same letters (a, b, c, etc.) show the non-significant differences at p < 0.05
The concentration of MDA as an estimate of lipid peroxidation showed a significant increase with increasing Cd and Pb stress with highest contents at Cd-90 and Pb-150 (Fig. 2c, d).
Antioxidant enzyme activity
The activity of SOD significantly increased with increasing both Cd and Pb levels in the soil and was highest at Cd-90 and Pb-150 (Fig. 3a, b).
Effect of increasing levels of Cd: 30, 60 and 90 mg kg−1 designated as Cd-30, Cd-60, Cd-90 and Pb: 50, 100 and 150 mg kg−1 designated as Pb-5, Pb-10, Pb-15 stress on activity of SOD (a, b), CAT (c, d), APX (e, f) and POX (g, h) activity in quinoa. The bars are means of five replicates ± SE and Tukey’s range test has been used to identify the significant differences between different means. The bars sharing the same letters (a, b, c, etc.) show the non-significant differences at p < 0.05
A significant increase in CAT activity with increasing Cd stress was observed in quinoa leaves, and significant increase at Pb-100 and Pb-150 (Fig. 3c, d).
The activity of APX significantly increased at higher levels of both Cd (Cd-30, Cd-60 and Cd-90) and Pb (Pb-50, Pb-100 and Pb-150) as compared to control however, it was non-significant among higher levels of Cd and Pb levels (Fig. 3e, f).
There was a non-significant difference regarding POX activity between control, Cd-30 and Cd-60 levels however, it significantly decreased at Cd-90 compared to control and other levels of Cd. Contrarily, increasing Pb levels caused a significant increase in POX activity with highest activity at Pb-150 (Fig. 3g, h).
Tissues cadmium and lead concentration and translocation
Increasing Cd levels in soil significantly increased Cd accumulation in roots and shoots as compared to control with highest accumulation at Cd-60. Whereas, seeds Cd concentration showed a non-significant effect with increasing soil Cd levels (Fig. 4a). Root to shoot translocation of Cd decreased at Cd-30 compared to control while, gradually increased at Cd-30 to Cd-90. Whereas, translocation of Cd from shoot to seed unaltered by the increasing Cd levels in soil except at Cd-60 where it slightly decreased with respect to other levels [Supplementary Fig. 1(i)].
Accumulation of cadmium (a) and lead (b) in different plant tissues in response to increasing levels of Cd: 30, 60 and 90 mg kg−1 designated as Cd-30, Cd-60, Cd-90 stress and Pb: 50, 100 and 150 mg kg−1 designated as Pb-5, Pb-10, Pb-15 stress in quinoa. The bars are means of five replicates ± SE and Tukey’s range test has been used to identify the significant differences between different means. The bars sharing the same letters (a, b, c, etc.) show the non-significant differences at p < 0.05
Roots and shoots accumulation of Pb showed a significant increase with increasing Pb levels in soil as compared to control with highest accumulation at Pb-150. While, a non-significant effect was observed in seeds as compared to control (Fig. 4b). Moreover, Pb translocation from root to shoot decreased with increasing concentration of Pb in soil While, shoot to seed translocation showed a minor decrease at Pb-5 compared to other levels of Pb [Supplementary Fig. 1(ii)].
Health risk assessment
Cadmium health risk assessment
The highest Cd concentration in grains was observed at Cd-90 (0.833 mg kg−1). The estimated average daily intake of Cd from the quinoa grains by humans was 0.411. From the average values of Cd concentration HQ, ILTCR and THQ was calculated for humans. The average of Cd concentration for HQ was 0.050 that was < 1 and poses no danger for humans on consumption. The permissible limits of HQ for humans are < 1. For the ILTCR calculation average values of Cd concentration are 0.003 and showed cancer risk. It will cause cancer, if humans’ intake the quinoa grains grown in Cd contaminated soil. The permissible limits for cancer risk are < 10–4. For THQ the average concentration for Cd was 0.590 that was < 1 and poses no danger for humans (Table 3).
Health risk assessment associated with lead
The highest concentration of Pb in grains was observed at Pb-150 while the accumulation of Pb in grains decreased at Pb-100 as compared with the control. Assessment of the estimated daily intake of Pb from the grains of quinoa was lowest (2.82E-07) in control and highest (3.38E-04 at Pb-150 consumed by humans. The HQ, ILTCR and THQ for the humans were calculated from the average values of Pb concentrations. The average of Pb concentration for HQ was 4.40E-02 that was < 1 and posed no danger for humans. The permissible limits for the HQ for Pb are < 1 for humans. For the ILTCR calculation, average values of Pb concentration are 0.00001 and posed no danger for cancer risk at all Pb concentrations. The permissible limits for cancer risk are < 10–4. For THQ, the average concentration at all levels of Pb was 0.518 that was < 1 and posed no danger for humans (Table 3).
Discussion
Heavy metals have been widely reported to induce phytotoxic effects in plants. Plants upon exposure to heavy metals accumulate them in different tissues up to toxic levels. Heavy metals induce several toxic effects in plants such as ROS generation, oxidation of lipid macromolecules, and pigment reduction, and they may lead to cell death (Natasha et al. 2019; Rafiq et al. 2018; Shahid et al. 2017). The Pb and Cd are widely reported to cause the overproduction of ROS in plants. The ROS oxidize lipid membranes and decrease membrane stability (Zhang et al., 2018). The Pb and Cd concentrations in roots were 41.47 and 12.06 when Cd-60 mg/L and Pb-15 mg/L was applied in the soil. The accumulation of metals mainly depends on metal type and plant genotype. Muhammad et al. (2018) reported that Pb accumulation in plants tissue was different in different quinoa genotypes. It was observed that A1 variety accumulated 2.9–9.82 mg/kg while A9 accumulated 1.84–5.16 mg/kg when 50 and 100 mg/kg Pb was applied, respectively (Muhammad et al. 2018). Scoccianti et al. (2016) showed that Cr accumulation was 3.73 mg/g after 3 days and 11.603 mg/g after 7 days when 5 mM Cr was applied. Zoufan et al. (2018) reported Zn accumulation as 1.66 and 0.43 mg g−1 DW, respectively in Chenopodium murale roots and shoots when grown at 600 µM Zn concentration. Sidhu et al. (2018) conducted a study to assess the Chenopodium murale response to Pb for 8 weeks. The results showed that the shoot Pb concentration was 685–2301 mg kg−1 DW, while root Pb concentrations was 1678–2513 mg kg−1 DW, respectively under 300–500 mg kg−1 Pb concentrations. Bhargava et al. (2008) reported that quinoa has ability to accumulate large amount of heavy metals in its leaves. It was indicated that quinoa can accumulate metals in roots and shoots, whereas seeds are not affected to the same degree (Zoufan et al. 2018). It has been reported that the plants showing BCF values greater than 1 are considered good phytoremediation materials (Wu et al. 2018; Farooq et al. 2020). In the current experiment, the results showed that the quinoa plant can be used for phytoremediation of Pb at Pb-50 having sufficiently high TI (74.78) and BCF (1.09).
The presence of heavy metals in the growth medium interferes the uptake of other nutrients like sodium (Na), potassium (K), and calcium (Ca) (Xiong et al. 2016). Since, there is no report of specific Cd transporter it is assumed to be transported by specific and non-specific transporters of essential elements (Llugany et al. 2012), such as Fe2+, Ca2+, Zn2+, Cu2+ and Mg2+ (Mendoza-Cózatl et al. 2011; Shahid et al. 2016). In this study, it was observed that seed Ca concentration significantly increased at Cd-60 and Cd-90 as compared with control whereas it significantly decreased in root and shoot. Calcium provides intermolecular linkages being bound to cell and exterior surface of plasma membrane (Jiang et al. 2004. Thus, it becomes utmost important for plasma membrane integrity (Wang et al. 2002). An increase in tissues Ca concentration under Cd stress has been reported to be one of the possible mechanisms to reduce its toxicity (Jiang et al. 2004). While, tissues Na and K concentration were non-significantly affected by Cd toxicity suggesting less competition of Cd ions with monovalent (Na+ and K+) as compared to divalent ions (Ca2+).
The Pb may enter the roots through several pathways, and a particular pathway is through ionic channels. Inhibition of Pb absorption by Ca is well-known (Kim et al. 2006) and is associated with competition between these two cations for Ca channels (Mendoza-Cózatl et al. 2011). Several authors have demonstrated that Ca2+ permeable channels are the main pathways by which Pb enters roots (Pourrut et al. 2008; Wang et al. 2007). However, in this study we did not observe any significant difference in Ca uptake by plants under increasing Pb levels. While there was a slight decrease in Na and K contents in the plants when Pb uptake was increased. Hence, it is evident that Pb utilizes selective Na and K ionic channels to enter the plants (Pourrut et al. 2011).
In the present study, MDA content increased and MSI decreased with increasing concentration of Pb and Cd. The enhanced production of MDA indicates the degradation of membrane and the loss of membrane stability. Zoufan et al. (2018) reported that MDA content was increased with increasing concentration of Zn. Highest content was observed at 600 µM dose. Oxidative stress during the ROS production increases MDA and decreases MSI which ultimately leads to cell death (Anjum et al. 2014; Sidhu et al. 2017).
Plants utilize many defensive mechanisms when metals enter in the plant cell. Plants respond to metals induced oxidative stress by antioxidative defense system having enzymatic (SOD, CAT, POX and APX etc.) and non-enzymatic species as is the case in the current study. It has also been reported by other researchers in different plant species (Malar et al. 2016; Tauqeer et al. 2016; Zhang et al. 2007). Zoufan et al. (2018) reported that SOD and CAT activity increased with increasing concentration of Zn. Sidhu et al. (2018) conducted experiment to examine the quinoa physiological response to Pb. Their results showed that SOD, CAT and POX activity increased in quinoa plant with increasing concentration of Pb. Hinojosa et al. (2018) reported that antioxidant enzyme activity increased when quinoa was exposed to abiotic stress.
The translocation factor was higher for Cd than Pb, because Cd was readily collected and translocated. Moreover, due to higher solubility of salts of Cd than Pb, its concentration is higher in the soil solution and thus higher uptake (Verbruggen et al. 2009). The higher concentration of heavy metals in grains is a serious concern as it poses severe health hazards for humans. The threshold of Cd and Pb concentration in grains is 0.013–0.22 mg kg−1 (Nazar et al. 2012) and 0.2–0.5 mg kg−1 (Kabata-Pendias 2010), respectively. In the present study, Pb and Cd levels in quinoa grains were lower than the threshold levels with all the treatments. This shows a potential to grow quinoa on metal contaminated soil where other crops such as wheat will uptake metals above the accepted levels.
The THQ and HQ of Pb and Cd for humans was < 1 suggesting food safety. Similarly, Wang et al. (2012) reported that the THQ and HQ by the ingestion of food grown on Pb and Cd contaminated soil was safe i.e. < 1. The Pb concentration for cancer risk posed no danger for humans. While Cd concentration for cancer risk is higher than acceptable level. It is safe to intake quinoa grains obtained from the Pb contaminated soil. But in Cd contaminated soil, quinoa plants cannot be used for food purpose as grains Cd concentration was higher than acceptable level.
Conclusion
Results revealed that the quinoa plant shows different response to Cd and Pb toxicity such as physiological changes, membrane stability index and antioxidant enzymes activities. However, the Cd accumulation in seeds is higher than permissible limits suggesting that seeds grown on Cd contaminated soils not suitable for edible purpose. The index for health hazard shows that Cd values were greater than Pb for human health risk. Based on this study, it is concluded that quinoa plant is good for phytoremediation of Pb and Cd. While, seeds Pb accumulation is under permissible limits and thus does not pose significant risk to human health. From this study, we suggest that quinoa plants can be used for phytoremediation of Cd and Pb. Quinoa plants used for phytoremediation of Pb can be used as a food, because Pb is not accumulated in grains. However, Cd is accumulated in grains, therefore quinoa plants used for phytoremediation of Cd cannot be used as food.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Abbas, T., et al. (2017). Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicology and Environmental Safety, 140, 37–47.
Abdollahi, H., Fekri, M., & Mahmodabadi, M. (2011). Effect of heavy metals pollution on pistachio trees. International Journal of Agriculture and Biology, 13, 599–602.
Aebi, H. (1984). Catalase in vitro. Methods enzymology, 105, 121–126.
Ahmadi, H., et al. (2018). CAX1 suppresses Cd-induced generation of reactive oxygen species in Arabidopsis halleri. Plant, Cell and Environment, 41(10), 2435–2448.
Ahmadpour, P., et al. (2012). Phytoremediation of heavy metals: A green technology. African Journal of Biotechnology, 11, 14036–14043.
Amako, K., et al. (1994). Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant and Cell Physiology, 35, 497–504.
Anjum, N. A., et al. (2014). Assessment of cadmium accumulation, toxicity, and tolerance in Brassicaceae and Fabaceae plants—implications for phytoremediation. Environmental Science and Pollution Research, 21, 10286–10293.
Bhargava, A., et al. (2008). Chenopodium: A prospective plant for phytoextraction. Acta Physiologiae Plantarum, 30, 111–120.
Chang, Y.-S., et al. (2013). Nitrogen fertilization promotes the phytoremediation of cadmium in Pentas lanceolata. International Biodeterioration and Biodegradation., 85, 709–714.
Farooq, A., et al. (2020). Cadmium partitioning, physiological and oxidative stress responses in marigold (Calendula calypso) grown on contaminated soil: Implications for phytoremediation. Bulletin of Environmental Contamination and Toxicology, 105(2), 270–276.
Feng, J., et al. (2010). Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Scientia Horticulturae, 123, 521–530.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
Gupta, A. S., et al. (1993). Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiology, 103, 1067–1073.
Halder, S., & Ghosh, S. (2014). Wetland macrophytes in purification of water. International Journal of Environmental Sciences, 5, 432–437.
Han, W., et al. (2018). Ecological and health risks assessment and spatial distribution of residual heavy metals in the soil of an e-waste circular economy park in Tianjin, China. Chemosphere, 197, 325–335.
Hinojosa, L., et al. (2018). Quinoa abiotic stress responses: A review. Plants, 7, 106.
Iqbal, S. M. B. S., & Afzal, I. (2014). Evaluating the response of nitrogen application on growth development and yield of quinoa genotypes. International Journal of Agriculture and Biology, 16(5), 886–892.
Jacobsen, S.-E., et al. (2005). Plant responses of quinoa (Chenopodium quinoa Willd.) to frost at various phenological stages. European Journal of Agronomy, 22, 131–139.
Jacobsen, S.-E., et al. (2007). Frost resistance mechanisms in quinoa (Chenopodium quinoa Willd.). European Journal of Agronomy, 26, 471–475.
Jacobsen, S. E. (2014). New climate-proof cropping systems in dry areas of the Mediterranean region. Journal of Agronomy and Crop Science, 200, 399–401.
Jia, Z., et al. (2018). Assessment of soil heavy metals for eco-environment and human health in a rapidly urbanization area of the upper Yangtze Basin. Scientific Reports, 8, 3256.
Jiang, X., Luo, Y., Liu, Q., et al. (2004). Effects of cadmium on nutrient uptake and translocation by Indian mustard. Environmental Geochemistry and Health, 26, 319–324.
Kabata-Pendias, A. (2010). Trace elements in soils and plants. London: CRC Press.
Khan, S., et al. (2014). Application of biochar to soil reduces cancer risk via rice consumption: A case study in Miaoqian village, Longyan, China. Environment International, 68, 154–161.
Kim, D.-Y., Bovet, L., Kushnir, S., Noh, E. W., Martinoia, E., & Lee, Y. J. P. P. (2006). AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiology, 140, 922–932.
Liang, C., et al. (2011). Cadmium accumulation and translocation in two Jerusalem artichoke (Helianthus tuberosus L.) cultivars. Pedosphere, 21, 573–580.
Liu, Y., et al. (2013). High cadmium concentration in soil in the three gorges region: Geogenic source and potential bioavailability. Applied Geochemistry, 37, 149–156.
Llugany, M., Miralles, R., Corrales, I., Barceló, J., & Poschenrieder, C. J. (2012). Cynara cardunculus a potentially useful plant for remediation of soils polluted with cadmium or arsenic. Journal of Geochemical Exploration, 123, 122–127.
Malar, S., et al. (2016). Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Botanical Studies. https://doi.org/10.1186/s40529-014-0054-6.
Matiacevich, S. B., et al. (2006). Water-dependent thermal transitions in quinoa embryos. Thermochimica Acta, 448, 117–122.
Mendoza-Cózatl, D. G., Jobe, T. O., Hauser, F., & Schroeder, J. I. (2011). Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Current Opinion in Plant Biology, 14, 554–562.
Muhammad, H., et al. (2018). Quinoa response to lead: Growth and lead partitioning. International Journal of Agriculture and Biology, 20, 338–344.
Mujica, A., et al. (2001). Resultados de la Prueba Americana y Europea de la Quinua (p. 51). UNA-Puno, CIP: FAO.
Nanduri, K. R., et al. (2019). Quinoa: A New Crop for Harsh Environments. In Bilquees Gul, Benno Böer, M. AjmalKhan, Miguel Clüsener-Godt, & Abdul Hameed (Eds.), Sabkha Ecosystems (pp. 301–333). Cham: Springer.
Natasha, et al. (2019). Foliar uptake of arsenic nanoparticles by spinach: An assessment of physiological and human health risk implications. Environmental Science and Pollution Research, 26, 20121–20131.
Nawab, J., et al. (2016). Organic amendments impact the availability of heavy metal (loid) s in mine-impacted soil and their phytoremediation by Penisitum americanum and Sorghum bicolor. Environmental Science and Pollution Research, 23, 2381–2390.
Nazar, R., et al. (2012). Cadmium toxicity in plants and role of mineral nutrients in its alleviation. American Journal of Plant Sciences, 3, 1476.
Pan, L.-B., et al. (2016). Heavy metals in soils from a typical county in Shanxi Province, China: Levels, sources and spatial distribution. Chemosphere, 148, 248–254.
Panda, S., et al. (2003). Does aluminium phytotoxicity induce oxidative stress in greengram (Vigna radiata). Bulgarian Journal of Plant Physiology, 29, 77–86.
Paz-Ferreiro, J., et al. (2014). Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth, 5, 65–75.
Polle, A., et al. (2013). Impact of cadmium on young plants of Populus euphratica and P.× canescens, two poplar species that differ in stress tolerance. New Forests, 44, 13–22.
Pourrut, B., Perchet, G., Silvestre, J., Cecchi, M., Guiresse, M., & Pinelli, E. (2008). Potential role of NADPH-oxidase in early steps of lead-induced oxidative burst in Vicia faba roots. Journal of Plant Physiology, 165, 571–579.
Pourrut, B., Shahid, M., Dumat, C., Winterton, P., & Pinelli, E. (2011). Lead uptake, toxicity, and detoxification in plants. In David M. Whitacre (Ed.), Reviews of Environmental Contamination and Toxicology (Vol. 213, pp. 113–136). NewYork: Springer.
Rafiq, M., et al. (2018). A comparative study to evaluate efficiency of EDTA and calcium in alleviating arsenic toxicity to germinating and young Vicia faba L. seedlings. Journal of Soils and Sediments, 18, 2271–2281.
Razzaghi, F., et al. (2015). Ionic and photosynthetic homeostasis in quinoa challenged by salinity and drought–mechanisms of tolerance. Functional Plant Biology, 42, 136–148.
Rehman, Z. U., et al. (2016). Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan. Science of the Total Environment, 550, 321–329.
Ruiz, J. M., et al. (2009). Distribution and efficiency of the phytoextraction of cadmium by different organic chelates. Terra Latinoamericana, 27(4), 295–301.
Sairam, R. K., et al. (2002). Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Science, 163, 1037–1046.
Scoccianti, V., et al. (2016). Oxidative stress and antioxidant responses to increasing concentrations of trivalent chromium in the Andean crop species Chenopodium quinoa Willd. Ecotoxicology and Environmental Safety, 133, 25–35.
Shahid, M., Dumat, C., Khalid, S., Niazi, N. K., & Antunes, P. M. (2016). Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. In Pim De Voogt (Ed.), Reviews of Environmental Contamination and Toxicology (Vol. 241, pp. 73–137). Cham: Springer.
Shahid, M., et al. (2017). Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. Journal of Hazardous Materials, 325, 36–58.
Shahid, M., et al. (2018). A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environmental Pollution, 234, 915–934.
Shahid, M., et al. (2012). Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. Journal of Hazardous Materials, 219, 1–12.
Sharma, P., & Dubey, R. S. (2005). Lead toxicity in plants. Brazilian Journal of Plant Physiology, 17, 35–52.
Sharma, P., et al. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012, 1–26.
Sidhu, G. P. S., et al. (2018). Bioaccumulation and physiological responses to lead (Pb) in Chenopodium murale L. Ecotoxicology and Environmental Safety, 151, 83–90.
Sidhu, G. P. S., et al. (2017). Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicology and Environmental Safety, 135, 209–215.
Singh, S., et al. (2016). Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Frontiers in Plant Science, 6, 1143.
Smiri, M., et al. (2009). Respiratory metabolism in the embryonic axis of germinating pea seed exposed to cadmium. Journal of Plant Physiology, 166, 259–269.
Tauqeer, H. M., et al. (2016). Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicology and Environmental Safety, 126, 138–146.
Vara Prasad, M. N., & de Oliveira Freitas, H. M. (2003). Metal hyperaccumulation in plants: Biodiversity prospecting for phytoremediation technology. Electronic Journal of Biotechnology, 6, 285–321.
Verbruggen, N., et al. (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist, 181, 759–776.
Visioli, G., & Marmiroli, N. (2013). The proteomics of heavy metal hyperaccumulation by plants. Journal of Proteomics, 79, 133–145.
Wahid, A., et al. (2009). Cadmium phytotoxicity: Responses, mechanisms and mitigation strategies: A review. In Eric Lichtfouse (Ed.), Organic Farming, Pest Control and Remediation of Soil Pollutants (pp. 371–403). Dordrecht: Springer.
Walliwalagedara, C., et al. (2010). Differential expression of proteins induced by lead in the Dwarf Sunflower Helianthus annuus. Phytochemistry, 71, 1460–1465.
Wang, H.-H., Shan, X.-Q., Wen, B., Owens, G., Fang, J., Zhang, S.-z, & Botany, E. (2007). Effect of indole-3-acetic acid on lead accumulation in maize (Zea mays L.) seedlings and the relevant antioxidant response. Environmental and Experimental Biology, 61, 246–253.
Wang, X., Becker, F. F., & Gascoyne, P. R. (2002). Membrane dielectric changes indicate induced apoptosis in HL-60 cells more sensitively than surface phosphatidylserine expression or DNA fragmentation. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1564, 412–420.
Wang, Y., Qiao, M., Liu, Y., & Zhu, Y. (2012). Health risk assessment of heavy metals in soils and vegetables from wastewater irrigated area, Beijing-Tianjin city cluster, China. Journal of Environmental Sciences, 24, 690–698.
Wu, M., et al. (2018). Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicology and Environmental Safety, 162, 35–41.
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecology. https://doi.org/10.5402/2011/402647.
Xiong, T., et al. (2016). Measurement of metal bioaccessibility in vegetables to improve human exposure assessments: Field study of soil–plant–atmosphere transfers in urban areas, South China. Environmental Geochemistry and Health, 38, 1283–1301.
Zeng, X., et al. (2015). Health risk assessment of heavy metals via dietary intake of wheat grown in Tianjin sewage irrigation area. Ecotoxicology, 24, 2115–2124.
Zhang, F.-Q., et al. (2007). Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere, 67, 44–50.
Zhang, M., et al. (2018). Effect of heat shock protein 90 against ROS-induced phospholipid oxidation. Food Chemistry, 240, 642–647.
Zoufan, P., et al. (2018). Oxidative damage and antioxidant response in Chenopodium murale L. exposed to elevated levels of Zn. Brazilian Archives of Biology and Technology. https://doi.org/10.1590/1678-4324-2018160758.
Funding
The research was supported by COMSATS University Islamabad (CUI) research grant program.
Author information
Authors and Affiliations
Contributions
MMI, experimental work; MA, conceptualization, supervision and data analysis; GA, data analysis; ABUF and MAN, investigation and measurements; MI and BM, methodology, MN, Health risk assessment analysis; S-EJ, provided seeds and article reviewing.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amjad, M., Iqbal, M.M., Abbas, G. et al. Assessment of cadmium and lead tolerance potential of quinoa (Chenopodium quinoa Willd) and its implications for phytoremediation and human health. Environ Geochem Health 44, 1487–1500 (2022). https://doi.org/10.1007/s10653-021-00826-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00826-0