Abstract
Atmospheric contamination by heavy metal(loid)–enriched particulate matter (metal-PM) is highly topical these days because of its high persistence, toxic nature, and health risks. Globally, foliar uptake of metal(loid)s occurs for vegetables/crops grown in the vicinity of industrial or urban areas with a metal-PM-contaminated atmosphere. The current study evaluated the foliar uptake of arsenic (As), accumulation of As in different plant organs, its toxicity (in terms of ROS generation, chlorophyll degradation, and lipid peroxidation), and its defensive mechanism (antioxidant enzymes) in spinach (Spinacia oleracea) after foliar application of As in the form of nanoparticles (As-NPs). The As-NPs were prepared using a chemical method. Results indicate that spinach can absorb As via foliar pathways (0.50 to 0.73 mg/kg in leaves) and can translocate it towards root tissues (0.35 to 0.68 mg/kg). However, health risk assessment parameters showed that the As level in the edible parts of spinach was below the critical limit (hazard quotient < 1). Despite low tissue level, As-NP exposure caused phytotoxicity in terms of a decrease in plant dry biomass (up to 84%) and pigment contents (up to 38%). Furthermore, several-fold higher activities of antioxidant enzymes were observed under metal stress than control. However, no significant variation was observed in the level of hydrogen peroxide (H2O2), which can be its possible transformation to other forms of reactive oxygen species (ROS). It is proposed that As can be absorbed by spinach via foliar pathway and then disturbs the plant metabolism. Therefore, air quality needs to be considered and monitored continuously for the human health risk assessment and quality of vegetables cultivated on polluted soils (roadside and industrial vicinity).

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric contamination by metal-enriched particulate matter (metal-PM) is of pronounced concern to the health, environment, public, and government organizations worldwide owing to the highly toxic nature of these metal-PM (Di Vaio et al. 2018; Lerner et al. 2018; Liu et al. 2017). Metal-PM is emitted into the atmosphere by both anthropogenic activities and natural processes (Kermani et al. 2018; Liu et al. 2017). The major sources of atmospheric metal-PM are smelters, power plants, mining operations, and automobiles. The situation of atmospheric contamination by metal-PM is highly worsening in the regions with high industrial activities (Corrêa et al. 2017; Douay et al. 2009; Fritsch et al. 2010), and various metropolitan in the world, especially in Africa, Asia, and Latin America (Mukherjee and Agrawal 2017). Metal-PM released from various sources into the atmosphere can deposit on different terrestrial ecosystems (soil, water, plant, and animals/humans) (Uzu et al. 2009; Wang et al. 2018).
The presence of high levels of these metal-PM in the atmosphere is confronting the quality of environment and animal health (including humans) worldwide (Liu et al. 2010; Xiong et al. 2018). Metal-PM can negatively affect human health via two major pathways: direct inhalation (Goix et al. 2014; Uzu et al. 2011) or ingestion of contaminated vegetables after foliar deposition and uptake (Xiong et al. 2016a; Xiong et al. 2017). However, the data regarding the latter aspect is negligible compared to the direct inhalation of metal-PM. The potential of these metal-PM to transport over long-range along with high persistency has made the situation more complex and worse. These metal-PM can cause adverse effects on the ecosystems and human health many miles away from the source of their origin (Shahid et al. 2017a; Xiong et al. 2016b).
Metal-PM can be adsorbed on the plant’s aerial organs and get transported inside the plant (Schreck et al. 2014; Uzu et al. 2010). This shows that plants can uptake and accumulate metal(loid)s directly via foliar tissues, in addition to absorption via root uptake (Schreck et al. 2012). Studies indicate that plants can absorb metal(loid)s via stomata, lenticels, cuticle, cracks, and aqueous pores (Schreck et al. 2013). After foliar uptake, plants can transfer metal(loid)s towards root tissues (Xiong et al. 2017). However, there is relatively fewer data available regarding foliar uptake of metal(loid)s compared to root uptake.
Among all heavy metal(loid)s, As is a highly toxic substance naturally present in water (Bakhat et al. 2017; Shahid et al. 2018; Tabassum et al. 2018). Moreover, high concentrations of As have been reported to exist in the atmosphere (Shahid et al. 2017c; Xu et al. 2016). It is reported that As concentration in the air at the global scale is > 0.02–4 ng/m3, 3–200 ng/m3, and 1000 ng/m3, respectively, for rural, urban, and industrial areas (Facts 2008). In Pakistan, As level in the air is about 230–2230 ng/m3 (Shigeta 2000). Arsenic is non-essential to plants and is capable to induce toxic effects even at low/moderate applied levels (Abbas et al. 2018; Rafiq et al. 2017b).
Plants under As stress show numerous toxicological symptoms such as low plant growth, biomass and chlorophyll content, genetic mutation, genotoxicity, fragmentation of DNA, and enhanced production of reactive oxygen species (ROS) (Abbas et al. 2018; Khalid et al. 2017b; Suriyagoda et al. 2018). Arsenic accumulation by plants also causes possible food chain contamination and health risks (Carey et al. 2010; Shahid et al. 2017c). Plant responds to As toxicity via activation of various enzymatic and non-enzymatic antioxidants (Abbas et al. 2018; Khalid et al. 2017b). However, plant responses to As stress under foliar application are not yet well-elucidated.
Numerous studies have been conducted on the plant’s soil-root uptake and accumulation of heavy metal(loid)s, but information regarding foliar metal uptake is comparatively limited, especially when applied in the form of nanoparticles (NPs) compared to foliar solution application. Therefore, it is highly necessary to study the mechanisms of foliar uptake and accumulation of heavy-metal(loid) NPs and their noxious effects in plants as well as the defense mechanisms of plants in relation with human health risks associated with contaminated crop/vegetable consumption. As far as we know, this is the first study of its kind which assessed the foliar uptake and transport of As from shoot to roots by spinach and the associated toxic effects to plants as well as the health risks.
Materials and methods
Chemicals and reagents for arsenic nanoparticles
The starting materials for the synthesis of arsenic nanoparticles were sodium arsenite (NaAsO2) and sodium borohydride (NaBH4) (Analytical grade, BDH).
Synthesis of arsenic nanoparticles
The stock solution (0.1 M) of NaAsO2 was prepared in distilled water. The freshly prepared aqueous solution of 0.2 M NaBH4 (stored in the refrigerator at 4 °C) was used for the reduction of As(III) to As(0). The reduction process was performed in the solution mixture of NaAsO2 and NaBH4 at pH 7–9 under ambient condition (Pal et al. 2012). During the reduction process, the pH of the solution increases slightly which was maintained using 0.1 M solution of sulfuric acid. The mixture of NaAsO2 and NaBH4 was stirred at room temperature for 30 min. The light brown As(0) nanoparticles precipitated down which were filtered and dried in an oven. The scanning electron microscope (SEM) (JSM5910, JEOL, Japan) was used to study the surface morphology and size of synthesized arsenic nanoparticle (As-NP) (Fig. 1).
Plant material and growth conditions
In this study, spinach dry seeds were grown in the acid-washed sand for 1 week. After germination, when the spinach roots were approximately 3–4 cm long, the seedlings were transferred to the polyvinyl tubes (2.5 L capacity) containing a nutrient solution of various micro- and macronutrients (5 mM KNO3, 2 mM KH2PO4, 5 mM Ca(NO3)2, 1.5 mM MgSO4, 9.11 μM MnSO4, 0.235 μM CuSO4, 0.1 μM Na2MoO4, 1.53 μM ZnSO4, 24.05 μM H3BO3, and 268.6 μM Fe-EDTA). This nutrient solution has been used previously in various studies dealing with morphological and biochemical processes of plants (Rafiq et al. 2017a; Shahid et al. 2017c). After vegetative growth of 7 days in hydroponics, spinach plants were exposed to different treatments of As-NP via foliar application.
Exposure of plants to As-NP
The synthesized As-NPs were applied on the leaves of spinach. The applied treatments of As-NP were the following: control without As-NP, As-10 with 10 mg/plant of As-NP, and As-50 with 50 mg/plant of As-NP.
The adaxial surface of the upper three leaves was contaminated with As-NP. All the treatments were applied on leaves with the help of applicator brushes as previously used by Xiong et al. (2014). A polyethylene sheath was placed on the surface of each tub to protect nutrient solution contamination by As-NP during foliar application and to avoid the uptake of As through roots. After each treatment application, the polyethylene sheath was cleaned for preventing As contamination of the nutrient solution. As-NP treatments were applied once a week for 4 consecutive weeks.
After the specific time of treatment exposure, spinach plants were harvested and separated into root and shoot. Shoot samples were rapidly washed with distilled water and the As bound to the surface of leaves was removed using 0.01 M HCl. Plants were then stored for further analysis. Each treatment was applied to six different spinach plants. Three plants were used for dry weight and metal content analysis and the remaining three plants were used for physicochemical analysis.
Dry weight of root and shoot
After harvest, root and shoot samples were stored in paper bags and were air-dried for 1 week and then in the vacuum oven at 75 °C for 1 h and the dry weight was measured using a digital weighing balance.
Arsenic analyses in spinach root and shoot
The dried root and shoot samples were ground to fine powder (40 mesh) using a mechanical grinder. After that, about 500 mg of spinach samples was digested using a diacid mixture of perchloric acid and nitric acid (1:2). The digestion of plant material was carried out at 200–225 °C using the hot plate for about 3–4 h until the solution became colorless as described elsewhere (Shahid et al. 2017c). The digest was then cooled at room temperature and diluted up to 25 mL. After filtration, the concentration of As was determined using hydride generation atomic absorption spectroscopy (AAS, Thermo AA®, Solar-Series). The stock solution of AsCl3 (AsIII, 1000 mg/L) was used for preparing sub-stock and As standards in the range of 10 to 80 μg/L as used previously (Niazi et al. 2018a; Niazi et al. 2018b; Shakoor et al. 2018b) and one or two more refs. The limit of detection for the VGA 77 HGAAS was set at 6 μg/L. The residual standard deviation value for all the analyzed samples was < 2% (Shahid et al. 2017b; Shakoor et al. 2018a; Tabassum et al. 2018).
Pigment content analysis
After harvest, spinach samples were instantly frozen using liquid nitrogen to preserve the biophysiological activities. The frozen spinach leaf samples (1 g) were ground to fine powder under liquid nitrogen. Pigments (chl-a, chl-b, and carotenoid) were extracted using 80% hydro-acetone (v/v) as described by Lichtenthaler (1987). The extract was centrifuged at 3000g for 10 min. The absorbance of the mixture was noted at 663.2, 646.8, and 470 nm with the spectrophotometer (AA, Solar-Series). The contents of pigment were calculated using the equations stated by Lichtenthaler (1987).
Reactive oxygen species (H2O2) analysis
For H2O2 content analysis, about 1 g of frozen spinach leaves/roots was ground in liquid nitrogen. Plant extract was then centrifuged (3000g, 10 min) to precipitate the suspended particles. The absorbance mixture comprised of 1 mL of the plant extract, 1 mL of 10 mM potassium phosphate buffer and 1 mL of 2 M potassium iodide. The absorbance of mixture assay was recorded at 390 nm with the spectrophotometer and the H2O2 concentration was calculated using a standard curve stated by Islam et al. (2008).
Lipid peroxidation analysis
The possible lipid peroxidation in spinach roots/shoots was assessed by measuring the thiobarbituric acid–reactive substances (TBARS) (Hodges et al. 1999). About 1 g of frozen spinach samples were ground in liquid nitrogen followed by incubation at 95 °C with trichloroacetic acid (TCA) and butyl hydroxytoluene (BHT) in the presence and absence of thiobarbituric acid (TBA). After centrifugation (3000 rpm, 10 min), the absorbance was measured at 532 nm using a spectrophotometer. The amount of TBARS was determined according to the equations reported by Hodges et al. (1999).
Measurement of antioxidant enzyme activities
For determining antioxidant enzyme activities, 250 mg of frozen spinach leaf sample was weighed and crushed using a pre-cooled pestle and mortar under liquid nitrogen in 0.1 M phosphate buffer (pH—7.0). The samples were then centrifuged two times at 4500 rpm for 30 mins at 4 °C.
Superoxide dismutase
The activity of superoxide dismutase (SOD) was calculated by evaluating its capacity to stop the photo-chemical reduction of nitro blue tetrazolium as described by Dhindsa et al. (1981). The 3-mL reaction assay was composed of 0.1 mM EDTA, 13 mM methionine, 50 mM phosphate buffer (pH—7.8), 75 μM NBT, 60 μM riboflavin, and 200 μL enzyme extract. The activity of SOD was expressed as the amount of this enzyme essential to cause 50% NBT reduction.
Catalase
Catalase (CAT) activity was evaluated following the protocol described by Aebi (1984). The 3-mL mixture assay of spinach samples comprised of 200 μL enzyme extract in 50 mM phosphate buffer (pH—7.0) and 15 mM H2O2. The absorbance was measured at 240 nm for 45 s at 25 °C. The activity of CAT is presented as micromolar of H2O2 degraded per minute per milligram of protein.
Peroxidase
Guaiacol peroxidase (POD) was evaluated following the protocol of Hemeda and Klein (1990). The reaction mixture for POD analysis was comprised of 12 mM guaiacol, 50 mM phosphate buffer (pH—6), 15 mM H2O2, and 200 μL enzyme extract. The absorbance of the mixture was recorded at 470 nm for 90 s at 25 °C. The activity of POD is presented as micromolar of guaiacol oxidized per minute per milligram of protein.
Health risk assessment
Average daily intake of arsenic via spinach consumption
The average daily intake (ADI) of As under foliar application was estimated according to Rehman et al. (2016).
The detail of all the parameters and their values used for ADI calculations is described in Supplementary Table 1.
Hazard quotient
Hazard quotient (HQ) was calculated as described by Khalid et al. (2017a)
The detail of all the parameters and their values used for HQ calculations is described in Supplementary Table 1.
Risk assessment of cancer
The lifetime cancer risk (ILTCR) via consumption of As-contaminated spinach was measured using the equation described by Khalid et al. (2017a).
The detail of all the parameters and their values used for ILTCR calculations is described in Supplementary Table 1.
Choice/justification of experimental design, treatments, and parameters
In this study, spinach (a leafy vegetable) was selected due to its (i) wide cultivation at global scale, (ii) high consumption rate, (iii) nutritional value, (iv) use in previous scientific studies of metal toxicity, and (iii) small growth period. Arsenic nanoparticles were selected due to their highly toxic nature. Moreover, As-enriched particulate matter exists widely in the atmosphere. The plant biochemical parameters (H2O2, lipid peroxidation, pigment contents) were selected for their high sensitivity to metal stress and simplicity of their evaluation assays. These plant biochemical parameters have been used widely in previous studies (Shamshad et al. 2018). The antioxidant enzymes were preferred to evaluate the possible activation of spinach defense mechanism under foliar stress of As-NPs. Finally, the risk assessment parameters were performed to estimate any possible health hazards of consuming vegetables cultivated in areas of high atmospheric metal particulate matter contamination. Especially, the cancer risk was estimated due to the carcinogenic nature of As.
Statistical analysis
Statistix (Ver 8.1) was used to perform analysis of variance (ANOVA). XLSTAT (ver. 19.4) was used for Pearson correlation and principal component analysis (PCA).
Results
SEM analysis of As-NP
The SEM micrographs of synthesized As-NP using chemical method are shown in Fig. 1a–d in the scanning range of 5 to 0.2 μm. The SEM micrographs show that the surface of the particles present in the matrix of As-NP is smooth, unwrinkled, and spherical in shape. The size of the particles is uniform and even in the whole matrix. There are some aggregates in the whole matrix which are due to weak forces present among the As-NP. It was estimated that the particle size of synthesized As-NP is lying in the range of 40–60 nm which are comparable with the reported ones, i.e., 60 nm (Pal et al. 2012).
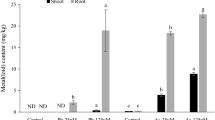
Foliar uptake of arsenic by spinach
Results revealed that uptake of As by spinach leaves increased (0.50 to 0.73 mg/kg) with increasing concentration of the applied As-NP on leaves from 10 to 50 mg/plant (Fig. 2). Likewise, increasing As concentration on leaves promoted the linear accumulation of As in roots (0.35 to 0.68 mg/kg) indicating the phloem mobility of As towards roots.
Effect of arsenic on pigment content
Foliar application of As-NP revealed a significant effect on photosynthetic pigments (Fig. 3). The lower level of As-NP (As-10) showed a slight non-significant reduction in pigment content compared to control, while the higher level (As-50) reduced the pigment content significantly.
Effect of arsenic on dry weight
Supplementary Figure 1 shows that the foliar application of As-NP caused a significant decrease in plant biomass at all applied levels compared to control. Application of As-10 and As-50 respectively caused 2.9 and 6.4 times decrease in dry weight of roots, and 3.7 and 4.0 times decrease in dry weight of leaves.
Effect of arsenic on H2O2 production and lipid peroxidation
In both leaves and roots of spinach treated with As-NP, no significant variation in H2O2 production was observed (Fig. 4a). In case of TBARS contents, As-NP (10 mg/plant) significantly induced lipid peroxidation in leaves but the TBARS contents decreased with increasing the treatment level (As-50) (Fig. 4b). In spinach roots, the TBARS contents decreased significantly for As-10 treatment, while there was no effect for As-50 treatment.
Effect of arsenic on antioxidant enzyme activities
Antioxidative enzyme activities were not measured for spinach roots due to insufficient root biomass required for enzyme analysis. In spinach leaves, enzyme activities were differently affected by As-NP application on leaves and the effect also differed with respect to the treatment level (Table 1). It has been observed that the activities of CAT and POD significantly increased, respectively, by 184% and 288% at the low level (10 mg), and by 31% and 605% at the high level (50 mg) of As, compared to control, developing a linear relation with applied As concentration. In contrast to other enzymes, SOD activity remained inhibited throughout the experiment and did not show any significant difference compared to control. The total protein content of spinach leaves treated with As increased significantly with the applied metal concentration in a linear way.
Health risk assessment
The values of health risk assessment parameters (ILTCR, HQ, and ADI) have been reported in Table 2. The values of these parameters depend on As contents in spinach edible parts (shoot). The ADI values were 0.019, 0.061, and 0.089 μg/kg/day, respectively, for control, As-10, and As-50. The low ADI values were due to low accumulation of As in spinach leaves (< 1 mg/kg). The HQ values were below the critical limit of HQ (< 1) for all the treatments: 0.065, 0.203, and 0.298, respectively, for control, As-10, and As-50. The ILTCR values were 0.000029, 0.000091, and 0.000134, respectively, for control, As-10, and As-50.
Discussions
Arsenic accumulation in roots and shoots of spinach
In the present study, As uptake by spinach leaves and its transfer towards roots increased with increasing the applied levels of As on leaves. Previously, As uptake by plants after foliar application in solution form has been reported. Bondada et al. (2004) demonstrated that the high level of As was absorbed by Chinese brake after foliar application. Schreck et al. (2012) also reported foliar absorption of As in three vegetables (lettuce, ryegrass, and parsley) after exposure to atmospheric fallouts resulting from the emissions of a battery-recycling factory. The results indicated high absorption of As by lettuce leaves having greater surface area as compared to other two vegetables. Moreover, Sachs and Michael (1971) reported the absorption and transport of As in Black Valentine Beans when the plants were sprayed with monosodium methanearsonate (MSMA). After 7 days of treatment, both root and shoot of the plants showed the almost the same concentration of As.
Previous studies also indicated the transport of foliar-applied metals into the roots. It is reported that after foliar metal(loid) application, leaves absorb the maximum part of metal(loid) and a little is transported towards the roots < 1% (Colle et al. 2009). Dollard (1986) reported 0.1%, 0.1–0.3%, and < 1% transfer of foliar-applied metal to the root tissues, respectively, in radish, carrot, and broad beans. In our study, although in low concentration (0.35 to 0.68 mg/kg), almost 40–50% of As applied to vegetable leaves was also translocated towards the roots (Fig. 2). The high shoot-root transfer of As in this study can be due to As application in the form of NP. The accumulation of foliar-applied As in root tissues has been reported by Zandstra and De Kryger (2007) when carrot plants were sprayed with MSMA. Results clearly indicated the translocation of foliar-applied As towards the root/edible part. So far, very less data is reported on the shoot-to-root transfer of metal(loid)s, especially when applied in the form of NP.
Effect of As-NP accumulation on pigment contents
Arsenic has been reported to induce severe noxious changes in pigment contents of plants upon exposure. Recently, Rafiq et al. (2017b) reported a significant decrease in pigment contents of vegetables due to As treatments. In our experimental conditions, high exposure of As-NP decreased the Chl-a, Chl-b, Chl-a+b, and carotenoid contents by 24, 38, 30, and 35% respectively (Fig. 3).
Our results are in agreement with the previous findings of Kováčik et al. (2012), who described the physiological changes in Tillandsia albida after foliar uptake of Cd and Ni. The significant decrease in sugar, water, and pigment contents was measured. Recently, Manaf (2016) reported the reduction in chlorophyll content due to the foliar spray of selenium (Se) on Vigna unguiculata, while Golob et al. (2017) did not indicate any change in pigment content of Triticum aestivum after foliar spray of Se. To the best of our knowledge, no data is available about As toxicity to pigment contents after foliar application in the form of NPs.
Effect of As-NP on dry weight
Metal toxicity and the decrease in dry weight due to the root uptake system have widely been reported in the literature (Rafiq et al. 2017b). But the toxicity after foliar absorption of As has not been well-established yet. In the present study, As accumulation in plants caused a significant decrease in plant dry biomass (Supplementary Figure 1). Previously, Sachs and Michael (1971) reported up to 89% reduction in dry biomass of different plant species when exposed to sodium arsenite, sodium arsenate, MSMA, and cacodylic acid via foliar application. Recently, Hong et al. (2016) reported significant toxic effects of Cu and Ce after foliar application on dry biomass of Cucumis Sativus. Hence, plants show biomass reduction when absorbing metal(loid)s through foliar organs.
Effect of As-NP on H2O2 induction and lipid peroxidation
Overproduction of ROS due to heavy-metal toxicity is a well-known fact nowadays (Abbas et al. 2018; Suriyagoda et al. 2018). In the present study, no significant increase in H2O2 production and lipid peroxidation was observed upon As treatments (Fig. 4a, b). The one possible reason could be the less accumulation of As in the tissues (< 1 mg/kg) capable to induce ROS production. The other possible reason could be the conversion of one form of ROS into other forms that have not determined in this study. Previously, several studies have reported that different antioxidative enzymes are capable to convert one form of ROS into another form in order to tolerate/avoid metal toxicity (Abbas et al. 2018). Therefore, H2O2 form of ROS might have been transferred to another form of ROS under current experimental conditions. Moreover, we could not compare our data due to the unavailability of previous studies about foliar application of As-NP.
Effect of metal accumulation on antioxidant enzyme activities
Once heavy metal(loid)s have entered the plant cell, plants may use numerous tolerance/defense mechanisms by which they can avoid toxic effects of metal(loid)s. This tolerance of plants against a specific heavy metal(loid) is governed by interrelated mechanisms operating at physiological and molecular levels (Mohan et al. 2016). Tiwari and Sarangi (2017) reported the enhanced activities of antioxidant enzymes in Pteris vittata and Vetiveria zizanioides under As stress. In the present study, the stimulation of CAT and POD in leaves confirmed the response of highly toxic As-NP accumulated in plants (Table 1). This shows that foliar absorbance of As-NPs even at very low level (< 1 mg/kg) is capable to induce toxicity and induce activation of antioxidative enzymes. The H2O2 is a very strong oxidant, which can induce various toxic effects in plants especially when it is converted to hydroxyl anion. Therefore, H2O2 requires quick and efficient removal to avoid its toxic effects on the plants. Generally, this scavenging of H2O2 is accomplished by the activities of POD and CAT in different cellular compartments (Abbas et al. 2018). The highest increase in CAT and POD activity was 165 and 349% observed at the lower dose of As solutions. There is a possibility that activation of CAT and POD has converted H2O2 into other forms. The decrease in activity recorded for SOD in the presence of As can be due to the reaction between As, CAT, and POD. However, no research/study has focused on the defense mechanism of plants against foliar As stress.
Health risk assessment
In the present study, the health risk assessment parameters (ADI, HQ, and ILTCR) indicated no sever potential risk to humans due to low As contents in edible parts of spinach. In contrast, some previous studies showed high metal accumulation in foliar tissues after foliar application of metals. The contrasting results of the current study can be due to the nature of metal(loid), duration of treatment exposure, and the type of applied metal (nanoparticles in this study compared to solution application in most previous studies). However, it was observed that increasing concentration of foliar-applied As-NP on spinach leaves increased its level in spinach roots and leaves. This highlighted a possible risk in areas with high atmospheric As contents such as along roadside or near mining and industrial areas. There is no previous data available regarding foliar uptake of spinach and its possible health hazards,
Principal component analysis and Pearson correlation
In order to evaluate the correlation between different variables, Pearson correlation matrix (correlation between two variables) and principal component analysis (combined correlation between all variables) were calculated (Fig. 5, Supplementary Table 2, 3). Results showed a strong positive correlation of As contents in spinach root and shoot with CAT, POD, and total protein contents (Supplementary Table 2). This was also confirmed by the combined grouping of these parameters in PCA (right side of Fig. 5). This showed that the increase in As concentration in root and leaves increased CAT and POD activities and total protein contents. However, the plant growth parameters (pigment contents and dry weights) were negatively and strongly correlated with As contents in root and shoot.
In PCA, the growth parameters have been placed opposite to As contents in spinach tissues (left side of Fig. 5a). This confirms that foliar As application can negatively affect plant growth and initiates plant defense mechanism by activating antioxidative enzymes (CAT and POD). However, weak correlation of As contents in root and shoot with H2O2 and lipid peroxidation confirms our earlier proposition of H2O2 conversion to some other forms of ROS (Supplementary Table 2).
Moreover, when the three treatments (control, As-10 ,and As-50) were compared (Fig. 5b) for their variance with respect to spinach response variables, it was observed that all the three treatments were grouped separately. This confirmed the differential effects of these treatments on growth and physiological and tolerance mechanisms of spinach. However, the effect was not linear with respect to the applied dose of As, as confirmed by their non-linear placement with respect to horizontal and vertical axes.
Conclusions
The present study evaluated that the deposition of arsenic in the form of nanoparticles on aerial plant parts is an important source of plant contamination and toxicity. The results proposed that the foliar As-NP deposition and uptake can be a source of their accumulation in different plant parts (shoot and root). The application of As-NP on spinach leaves can significantly reduce plant biomass and pigment contents and can increase the activities of antioxidative enzymes (SOD, CAT, POD). Based on the results, it is proposed that spinach growing near the industrial areas or along roadsides is susceptible to accumulate As inside its edible tissues. Moreover, there is also a risk of food chain contamination of vegetables when grown in areas with high atmospheric contamination of heavy metal(loid)s. Therefore, air quality needs to be considered and monitored continuously for the human health risk assessment and quality of vegetables cultivated on polluted soils (roadside and industrial vicinity).
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi N, Khan M, Amjad M, Hussain M, Natasha (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bakhat HF, Zia Z, Fahad S, Abbas S, Hammad HM, Shahzad AN, Abbas F, Alharby H, Shahid M (2017) Arsenic uptake, accumulation and toxicity in rice plants: possible remedies for its detoxification: a review. Environ Sci Pollut Res 24:9142–9158
Bondada BR, Tu S, Ma LQ (2004) Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.). Sci Total Environ 332:61–70
Carey A-M, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA (2010) Grain unloading of arsenic species in rice. Plant Physiol 152:309–319
Colle C, Madoz-Escande C, Leclerc E (2009) Foliar transfer into the biosphere: review of translocation factors to cereal grains. J Environ Radioact 100:683–689
Corrêa AX, Testolin RC, Torres MM, Cotelle S, Schwartz J-J, Millet M, Radetski CM (2017) Ecotoxicity assessment of particulate matter emitted from heavy-duty diesel-powered vehicles: influence of leaching conditions. Environ Sci Pollut Res 24:9399–9406
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Di Vaio P, Magli E, Caliendo G, Corvino A, Fiorino F, Frecentese F, Saccone I, Santagada V, Severino B, Onorati G (2018) Heavy metals size distribution in PM10 and environmental-sanitary risk analysis in Acerra (Italy). Atmosphere 9:58
Dollard G (1986) Glasshouse experiments on the uptake of foliar applied lead. Environmental Pollution Series A, Ecological and Biological 40:109–119
Douay F, Pruvot C, Waterlot C, Fritsch C, Fourrier H, Loriette A, Bidar G, Grand C, De Vaufleury A, Scheifler R (2009) Contamination of woody habitat soils around a former lead smelter in the North of France. Sci Total Environ 407:5564–5577
Facts, G., 2008. Facts on health and the environment. Obtained of Facts on health and the environment: http://www. greenfacts. org/es/cambio-climatico-ie5-base-cienc ia/. Accessed on. 2.
Fritsch C, Giraudoux P, Cœurdassier M, Douay F, Raoul F, Pruvot C, Waterlot C, De Vaufleury A, Scheifler R (2010) Spatial distribution of metals in smelter-impacted soils of woody habitats: influence of landscape and soil properties, and risk for wildlife. Chemosphere 81:141–155
Goix S, Lévêque T, Xiong T-T, Schreck E, Baeza-Squiban A, Geret F, Uzu G, Austruy A, Dumat C (2014) Environmental and health impacts of fine and ultrafine metallic particles: assessment of threat scores. Environ Res 133:185–194
Golob A, Kavčič J, Stibilj V, Gaberščik A, Vogel-Mikuš K, Germ M (2017) The effect of selenium and UV radiation on leaf traits and biomass production in Triticum aestivum L. Ecotoxicol Environ Saf 136:142–149
Hemeda HM, Klein B (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Kermani M, Farzadkia M, Kalantari RR, Bahmani Z (2018) Fine particulate matter (PM 2.5) in a compost facility: heavy metal contaminations and health risk assessment, Tehran, Iran. Environ Sci Pollut Res:1–11
Khalid S, Shahid M, Dumat C, Niazi NK, Bibi I, Gul Bakhat HFS, Abbas G, Murtaza B, Javeed HMR (2017a) Influence of groundwater and wastewater irrigation on lead accumulation in soil and vegetables: implications for health risk assessment and phytoremediation. International Journal of Phytoremediation 19:1037–1046
Khalid, S., Shahid, M., Niazi, N. K., Rafiq, M., Bakhat, H. F., Imran, M., Abbas, T., Bibi, I., Dumat, C., 2017b Arsenic behaviour in soil-plant system: biogeochemical reactions and chemical speciation influences. Enhancing Cleanup of Environmental Pollutants: Non Biological Approaches. Springer, N. Anjum et al. (eds.), .
Kováčik J, Klejdus B, Štork F, Hedbavny J (2012) Physiological responses of Tillandsia albida (Bromeliaceae) to long-term foliar metal application. J Hazard Mater 239:175–182
Lerner JEC, Elordi ML, Orte MA, Giuliani D, de los Angeles Gutierrez M, Sanchez E, Sambeth JE, Porta AA (2018) Exposure and risk analysis to particulate matter, metals, and polycyclic aromatic hydrocarbon at different workplaces in Argentina. Environ Sci Pollut Res:1–10
Lichtenthaler, H. K., 1987 [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Plant Cell Membranes. Academic Press, , pp. 350-382.
Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB (2010) Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4:319–330
Liu K, Shang Q, Wan C, Song P, Ma C, Cao L (2017) Characteristics and sources of heavy metals in PM2. 5 during a typical haze episode in rural and urban areas in Taiyuan, China. Atmosphere 9:2
Manaf HH (2016) Beneficial effects of exogenous selenium, glycine betaine and seaweed extract on salt stressed cowpea plant. Ann Agric Sci 61:41–48
Mohan, T. C., Castrillo, G., Navarro, C., Zarco-Fernández, S., Ramireddy, E., Mateo, C., Zamarreño, A. M., Paz-Ares, J., Muñoz, R., Garcia-Mina, J. M., 2016. Cytokinin determines thiol-mediated arsenic tolerance and accumulation in Arabidopsis thaliana. Plant physiology. pp. 00372.2016.
Mukherjee, A., Agrawal, M., 2017 A global perspective of fine particulate matter pollution and its health effects. Reviews of Environmental Contamination and Toxicology Volume 244. Springer, pp. 5-51.
Niazi NK, Bibi I, Shahid M, Ok YS, Burton ED, Wang H, Shaheen SM, Rinklebe J, Lüttge A (2018a) Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: an integrated spectroscopic and microscopic examination. Environ Pollut 232:31–41
Niazi NK, Bibi I, Shahid M, Ok YS, Shaheen SM, Rinklebe J, Wang H, Murtaza B, Islam E, Nawaz MF (2018b) Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci Total Environ 621:1642–1651
Pal A, Saha S, Maji SK, Kundu M, Kundu A (2012) Wet-chemical synthesis of spherical arsenic nanoparticles by a simple reduction method and its characterization. Adv Mater Lett 3:177–180
Rafiq M, Shahid M, Abbas G, Shamshad S, Khalid S, Niazi NK, Dumat C (2017a) Comparative effect of calcium and EDTA on arsenic uptake and physiological attributes of Pisum sativum. International Journal of Phytoremediation. 19:662–669
Rafiq M, Shahid M, Shamshad S, Khalid S, Niazi NK, Abbas G, Saeed MF, Ali M, Murtaza B (2017b) A comparative study to evaluate efficiency of EDTA and calcium in alleviating arsenic toxicity to germinating and young Vicia faba L. seedlings. Journal of Soils and Sediments:1–11
Rehman ZU, Khan S, Qin K, Brusseau ML, Shah MT, Din I (2016) Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan. Sci Total Environ 550:321–329
Sachs R, Michael J (1971) Comparative phytotoxicity among four arsenical herbicides. Weed Sci:558–564
Schreck E, Foucault Y, Sarret G, Sobanska S, Cécillon L, Castrec-Rouelle M, Uzu G, Dumat C (2012) Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: mechanisms involved for lead. Sci Total Environ 427-428:253–262
Schreck, E., Laplanche, C., Le Guédard, M., Bessoule, J.-J., Austruy, A., Xiong, T., Foucault, Y., Dumat, C., 2013. Influence of fine process particles enriched with metals and metalloids on Lactuca sativa L. leaf fatty acid composition following air and/or soil-plant field exposure. Environmental pollution (Barking, Essex: 1987). 179, 242-249.
Schreck E, Dappe V, Sarret G, Sobanska S, Nowak D, Nowak J, Stefaniak EA, Magnin V, Ranieri V, Dumat C (2014) Foliar or root exposures to smelter particles: consequences for lead compartmentalization and speciation in plant leaves. Sci Total Environ 476-477:667–676
Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, Niazi NK (2017a) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazard Mater 325:36–58
Shahid, M., Khalid, M., Dumat, C., Khalid, S., Niazi, N. K., Imran, M., Bibi, I., Ahmad, I., Hammad, H. M., Tabassum, R. A., 2017b. Arsenic level and risk assessment of groundwater in Vehari, Punjab Province, Pakistan. Exposure and Health. 1-11.
Shahid, M., Rafiq, M., Niazi, N. K., Dumat, C., Shamshad, S., Khalid, S., Bibi, I., 2017c. Arsenic accumulation and physiological attributes of spinach in the presence of amendments: an implication to reduce health risk. Environ Sci Pollut Res 24, 16,097-16,106.
Shahid M, Niazi NK, Dumat C, Naidu R, Khalid S, Rahman MM, Bibi I (2018) A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ Pollut
Shakoor MB, Bibi I, Niazi NK, Shahid M, Nawaz MF, Farooqi A, Naidu R, Rahman MM, Murtaza G, Lüttge A (2018a) The evaluation of arsenic contamination potential, speciation and hydrogeochemical behaviour in aquifers of Punjab, Pakistan. Chemosphere 199:737–746
Shakoor MB, Niazi NK, Bibi I, Shahid M, Sharif F, Bashir S, Shaheen SM, Wang H, Tsang DC, Ok YS (2018b) Arsenic removal by natural and chemically modified water melon rind in aqueous solutions and groundwater. Sci Total Environ 645:1444–1455
Shamshad S, Shahid M, Rafiq M, Khalid S, Dumat C, Sabir M, Murtaza B, Farooq ABU, Shah NS (2018) Effect of organic amendments on cadmium stress to pea: a multivariate comparison of germinating vs young seedlings and younger vs older leaves. Ecotoxicol Environ Saf 151:91–97
Shigeta T (2000) Environmental investigation in Pakistan. Pak-EPA/JICA, Islamabad
Suriyagoda LD, Dittert K, Lambers H (2018) Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric Ecosyst Environ 253:23–37
Tabassum RA, Shahid M, Dumat C, Niazi NK, Khalid S, Shah NS, Imran M, Khalid S (2018) Health risk assessment of drinking arsenic-containing groundwater in Hasilpur, Pakistan: effect of sampling area, depth, and source. Environ Sci Pollut Res:1–12
Tiwari S, Sarangi BK (2017) Comparative analysis of antioxidant response by Pteris vittata and Vetiveria zizanioides towards arsenic stress. Ecol Eng 100:211–218
Uzu G, Sobanska S, Aliouane Y, Pradere P, Dumat C (2009) Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environ Pollut 157:1178–1185
Uzu G, Sobanska S, Sarret G, Muñoz M, Dumat C (2010) Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ Sci Technol 44:1036–1042
Uzu G, Sauvain JJ, Baeza-Squiban A, Riediker M, Hohl MS, Val S, Tack K, Denys S, Pradere P, Dumat C (2011) In vitro assessment of the pulmonary toxicity and gastric availability of lead-rich particles from a lead recycling plant. Environ Sci Technol 45:7888–7895
Wang J, Liu G, Wu H, Zhang T, Liu X, Li W (2018) Temporal-spatial variation and partitioning of dissolved and particulate heavy metal (loid) s in a river affected by mining activities in Southern China. Environ Sci Pollut Res:1–12
Xiong T, Leveque T, Shahid M, Foucault Y, Mombo S, Dumat C (2014) Lead and cadmium phytoavailability and human bioaccessibility for vegetables exposed to soil or atmospheric pollution by process ultrafine particles. J Environ Qual 43:1593–1600
Xiong T, Austruy A, Pierart A, Shahid M, Schreck E, Mombo S, Dumat C (2016a) Kinetic study of phytotoxicity induced by foliar lead uptake for vegetables exposed to fine particles and implications for sustainable urban agriculture. J Environ Sci 46:16–27
Xiong T, Dumat C, Pierart A, Shahid M, Kang Y, Li N, Bertoni G, Laplanche C (2016b) Measurement of metal bioaccessibility in vegetables to improve human exposure assessments: field study of soil–plant–atmosphere transfers in urban areas, South China. Environmental Geochemistry and Health 38:1283–1301
Xiong T, Dumat C, Dappe V, Vezin H, Schreck E, Shahid M, Pierart A, Sobanska S (2017) Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agriculture. Environ Sci Technol 51:5242–5251
Xiong, T., Zhang, T., Dumat, C., Sobanska, S., Dappe, V., Shahid, M., Xian, Y., Li, X., Li, S., 2018. Airborne foliar transfer of particular metals in Lactuca sativa L.: translocation, phytotoxicity, and bioaccessibility. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-018-3084-x.
Xu H, Han S, Bi X, Zhao Z, Zhang L, Yang W, Zhang M, Chen J, Wu J, Zhang Y (2016) Atmospheric metallic and arsenic pollution at an offshore drilling platform in the Bo Sea: a health risk assessment for the workers. J Hazard Mater 304:93–102
Zandstra B, De Kryger T (2007) Arsenic and lead residues in carrots from foliar applications of monosodium methanearsonate (MSMA): a comparison between mineral and organic soils, or from soil residues. Food Addit Contam 24:34–42
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yi-ping Chen
Electronic supplementary material
ESM 1
(DOCX 56 kb)
Rights and permissions
About this article
Cite this article
Natasha, Shahid, M., Dumat, C. et al. Foliar uptake of arsenic nanoparticles by spinach: an assessment of physiological and human health risk implications. Environ Sci Pollut Res 26, 20121–20131 (2019). https://doi.org/10.1007/s11356-018-3867-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3867-0