Abstract
This study, based on a greenhouse pot culture experiment conducted with 15-day-old rapeseed (Brassica campestris L. cv. Pusa Gold; family Brassicaceae) and moong bean (Vigna radiata L. Wilczek cv. Pusa Ratna; family Fabaceae) plants treated with cadmium (Cd) concentrations (0, 50, and 100 mg kg−1 soil), investigates their potential for Cd accumulation and tolerance, and dissects the underlying basic physiological/biochemical mechanisms. In both species, plant dry mass decreased, while Cd concentration of both root and shoot increased with increase in soil Cd. Roots harbored a higher amount of Cd (vs. shoot) in B. campestris, while the reverse applied to V. radiata. By comparison, root Cd concentration was higher in B. campestris than in V. radiata. The high Cd concentrations in B. campestris roots and V. radiata shoots led to significant elevation in oxidative indices, as measured in terms of electrolyte leakage, H2O2 content, and lipid peroxidation. Both plants displayed differential adaptation strategies to counteract the Cd burden-caused anomalies in their roots and shoots. In B. campestris, increasing Cd burden led to a significantly decreased reduced glutathione (GSH) content but a significant increase in activities of GSH reductase (GR), GSH peroxidase (GPX), and GSH sulfotransferase (GST). However, in V. radiata, increasing Cd burden caused significant increase in GSH content and GR activity, but a significant decline in activities of GPX and GST. Cross talks on Cd burden of tissues and the adapted Cd tolerance strategies against Cd burden-accrued toxicity indicated that B. campestris and V. radiata are good Cd stabilizer and Cd extractor, respectively, wherein a fine tuning among the major components (GR, GPX, GST, GSH) of the GSH redox system helped the plants to counteract differentially the Cd load-induced anomalies in tissues. On the whole, the physiological/biochemical characterization of the B. campestris and V. radiata responses to varying Cd concentrations can be of great help in elaborating the innovative plant-based remediation technologies for metal/metalloid-contaminated sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), ranked as # 7 among the top 20 toxins (Yang et al. 2004), is classified as a human carcinogen; food crops constitute the main source of Cd intake by humans (IARC 1993; Satarug et al. 2002; UNEP 2008). At global scale, Cd content in surface soils lies in the range of 0.07 to 1.1 mg kg−1 soil, whereas values above 0.5 mg kg−1 usually reflect the anthropogenic Cd inputs (WHO 2007). Although 100 mg Cd kg−1 soil is considered as the regulatory limit of Cd in agricultural soils (Salt et al. 1995), this threshold is rising gradually due to agricultural application of phosphate fertilizers and municipal sewage sludge for soil amendment (Sanita di Toppi and Gabbrielli 1999; Polle and Schützendübel 2003; DalCorso et al. 2010; Gill et al. 2012). Despite its physiological function being unknown, Cd is readily taken up by crop plants to toxic levels. Given this, Cd-contaminated agricultural soil as well as the subsequent food chain deserves special attention (Satarug et al. 2002; Gill and Tuteja 2011).
Development of strategies to clean sustainably the metal-contaminated agricultural soils has long been a challenge before the global scientific community. In this perspective, the plant- and associated microbe-mediated phytoremediation/bioremediation has been accepted as a preferable, effective, environment-friendly, and low-cost cleanup option (Ali et al. 2013). Phytoextraction and phytostabilization are among the most useful phytoremediation techniques for metal/metalloid removal and stabilization in polluted soils. These are controlled by several plant-associated factors, including (a) high metal accumulation and its subsequent translocation from roots to harvestable organs (such as shoots), and (b) high metal accumulation by roots but least translocation of the accumulated metals to the above ground plant parts, which modulate the efficiency of plants for metal/metalloid extraction and stabilization, respectively. In turn, the plant capacity to tolerate high metal/metalloid burdens caused by phytoextraction or phytostabilization decides the success of the metal/metalloid remediation system (Clemens et al. 2002; Ali et al. 2013).
Most plants are sensitive to Cd concentrations in the range of 5–10 μg g−1 leaf dry weight (White and Brown 2010), but Cd hyperaccumulators can tolerate Cd concentrations of even 100 μg Cd g−1 leaf dry weight (Verbruggen et al. 2009). In order to tolerate the potential impact of metals/metalloids in roots or in the aerial organs, plants undergo several physiological/biochemical changes so as to manage a balance between metal/metalloid-accrued elevated generation of oxidants (reactive oxygen species, ROS) and their antioxidants-assisted metabolism (Gill and Tuteja 2010; Anjum et al. 2012ab). The ROS, if not completely metabolized, may cause membrane integrity weakening, elevated electrolyte leakage (EL), oxidation of proteins (producing reactive carbonyls, RCs) and membrane lipids (lipid peroxidation, LPO; producing thiobarbituric acid reactive substances, TBARS), and cell/plant death (Anjum et al. 2008a, b; 2013a). However, plants are endowed with an efficient antioxidant defense system, comprising both enzymatic (glutathione reductase, GR; glutathione peroxidase, GPX; glutathione sulfotransferase, GST; catalase, CAT; ascorbate peroxidase, APX) and non-enzymatic (reduced glutathione, GSH, and ascorbate, AsA pools) components, which control, directly or indirectly, the ROS-accrued potential anomalies (Polle and Schützendübel 2003; Anjum et al. 2010, 2012ab, 2013a; Gill and Tuteja 2010; Gill et al. 2013). Thus, the choice of plant species with a capacity to manage successfully a fine tuning between the metal/metalloid-led enhanced ROS and their metabolism through enzymatic and non-enzymatic antioxidants is of prime importance in a metal/metalloid phytoextraction/stabilization program.
Field-crop plants offer a reliable alternative to hyperaccumulators and can be used in remediating the metal/metalloid-contaminated soils in order to manage the risk of a long-term pollutant dispersion (Vamerali et al. 2010). In this perspective, a number of members of Brassicaceae are well known to hyperaccumulate/remediate metals/metalloids, including Cd (Anjum et al. 2012c), whereas those of Fabaceae (Leguminosae) are least explored for their large-scale use in phytoremediation technology (Zaidi et al. 2012). Considering the previous scenario, it was hypothesized that the difference in Cd tolerance and stabilization/extraction potential of plants from different families (such as Brassica campestris L., family Brassicaceae; Vigna radiata L. Wilczek, family Fabaceae/Leguminosae) can be modulated differently by physiological/biochemical mechanisms prevalent in these plants under Cd stress.
Based on B. campestris (cv. Pusa Gold) and V. radiata (cv. Pusa Ratna), the present study was undertaken (i) to determine the shoot and root Cd burden and analyze the oxidative stress (measured as H2O2, LPO, and EL); and (ii) to cross talk the organ-specific Cd burden and oxidative stress status with the modulation of GSH-based (GSH pool, GSSG, GPX, GST, and GR) defense components.
Materials and methods
Plant material, growth conditions, and treatments
Seeds of B. campestris cv. Pusa Gold and V. radiata cv. Pusa Ratna were sown in 40-cm-diameter earthen pots filled with 10 kg soil (texture, sandy loam; pH, 7.8; electrical conductivity, 3.8; organic carbon, 0.43 %; available K and S, 70 and 5 mg kg−1 soil, respectively). N, P, and K were applied at the rate of 120, 30, and 80 mg kg−1 soil, respectively, in the form of urea, single super phosphate, and muriate of potash. Subsequently, the soil was thoroughly mixed with Cd (50 and 100 mg Cd kg−1 soil; in the form of CdCl2). The soil with nutrients and Cd was left to stabilize for 24 h before seeds were sown. The treatments were arranged in a randomized block design and each treatment was replicated three times. After seedling emergence, three plants per pot were maintained and irrigated when needed. The pots were kept in naturally illuminated greenhouse {photosynthetically active radiation (PAR) of 960 μmol/m2/s; day/night temperature of 25/20 ± 4 °C; relative humidity of 70 ± 5 %}. All measurements were obtained from 15-day-old seedlings.
Plant dry mass
The seedlings were carefully uprooted and separated into root and shoot, which were subsequently weighed and dried in a hot air oven at 65 °C for 48 h. The dry mass was determined, using a digital balance, by unitary method and expressed in gram dry weight.
Cd determination in root and shoot
Cadmium concentration in root and shoot was determined as per the method described previously (Anjum et al. 2008a), using the atomic absorption spectrophotometer (AAS, ZEEnit 65, Analytik Jena, Germany).
Oxidative stress indices
The root and shoot H2O2, LPO, and EL levels were considered as indices of oxidative stress. H2O2 content was determined following the method of Loreto and Velikova (2001). EL was assessed as described by Anjum et al. (2013a). TBARS content, showing the status of membrane-lipid peroxidation in fresh roots and shoots, was determined as per the method adopted and described by Anjum et al. (2013a).
Antioxidant assays
Supernatants were obtained by homogenizing the fresh root and shoot tissues for antioxidant (enzymatic and non-enzymatic) assays, following the method as adopted and described by Anjum et al. (2013a). The method based on GSH-dependent oxidation of NADPH was followed for the determination of GR activity (Foyer and Halliwell 1976). Oxidation of NADPH was monitored at 340 nm for 3 min, using H2O2 as substrate, for GPX activity estimation, whereas GST activity was determined by measuring, at 340 nm for 3 min, the increase in absorbance due to the formation of conjugate 1-chloro-2,4-dinitrobenzene (CDNB), following the methods adopted and described by Anjum et al. (2013a). As to the non-enzymatic antioxidant assay, the reduced (GSH) and oxidized glutathione (GSSG) contents in root and shoot were determined by the method of Anderson (1985).
Results
Significant changes in plant dry mass and Cd burden and in the response of oxidative stress indices and antioxidant (enzymatic and non-enzymatic) components in roots and shoots of B. campestris and V. radiate are described below. Additionally, significant differences in these parameters between the two model plants have been highlighted.
Plant dry mass and Cd accumulation
Irrespective of the plant species, Cd application led to a significant decrease in plant dry mass (vs. control). With 50 mg Cd kg−1 soil, the dry mass of both B. campestris and V. radiata exhibited ≈ 1.4-fold decrease. Approximately 2.7- and 2.2-fold decreases were perceptible in B. campestris and V. radiate, respectively, with 100 mg Cd kg−1 soil (Fig. 1). Cd-exposed plant roots and shoots accumulated higher levels of Cd (vs. control) in both the plants. The B. campestris roots displayed a significantly higher Cd accumulation with both 50 and 100 mg Cd kg−1 in comparison to V. radiata roots. With 50 and 100 mg Cd kg−1 soil, it was 2.7- and 2.3-fold higher, respectively. On the contrary, B. campestris shoots displayed a significantly lower (about 2½- and 2.0-fold lower) Cd accumulation with both 50 and 100 mg Cd kg−1, as compared with V. radiata shoots (Table 1).
Plant dry mass (mg per ten plants) of B. campestris cv. Pusa Gold and V. radiata cv. Pusa Ratna exposed to cadmium levels (0, 50, and 100 mg kg−1 soil). Values represent the means of five replicates (±standard deviation) from each of three independent experiments. Significant differences are as follows: superscript a, vs. 0 and superscript b, vs. 100 (within the plant); superscript c, vs. B. campestris (between plants)
Oxidative stress indices
A significant increase in the extent of oxidative stress was perceptible in roots and shoot of both the test plants with increase in Cd concentration applied (vs. control). The percent EL and the contents of H2O2 and TBARS displayed significant increase in comparison with the control; the maxima occurred with 100 mg Cd kg−1, followed by 50 mg Cd kg−1. In B. campestris, the EL, H2O2, and TBARS levels in roots were 2.2-, 2.3-, and 1.6-fold higher, respectively, than in the shoot, with 100 mg Cd kg−1 soil treatment. In V. radiata, on the contrary, the roots exhibited 1.6-, 1.3-, and 1.3-fold lower levels of EL, H2O2, and TBARS, respectively, than in the shoot. By comparison, with the highest level of Cd treatment, B. campestris roots exhibited 2.3-, 1.7-, and 1.5-fold higher elevation in EL, H2O2, and TBARS, respectively, than in V. radiata roots. Likewise, B. campestris shoot evinced a 1.6-, 1.8-, and 1.4-fold higher enhancement of EL, H2O2, and TBARS levels, respectively, than in V. radiata shoot, with the highest Cd stress applied (Fig. 2a–f).
Membrane permeability (a, b), H2O2 content (c, d), and lipid peroxidation (e, f) in roots and shoot of B. campestris and V. radiata exposed to cadmium levels (0, 50, and 100 mg kg−1 soil). Values represent the means of five replicates (±standard deviation) from each of three independent experiments. Significant differences are as follows: superscript a, vs. 0 and superscript b, vs. 100 (within the plant); superscript c, vs. B. campestris (between plants). f.w. = fresh weight
Enzymatic and non-enzymatic antioxidants
The enzymatic antioxidants, namely GR, GPX, and GST, exhibited differential response to Cd treatments in roots and shoot of the test plants. In B. campestris, increase in Cd level significantly increased the GR, GPX, and GST activity in both the plant parts, with reference to the control. Compared to roots, the shoot displayed 2.6- and ≈ 3.0-fold higher activity of GPX and GST, respectively, and a 2-fold lower activity of GR, with 100 mg Cd kg−1 soil. In V. radiata roots and shoot, on the contrary, increase in Cd level decreased the GPX and GST activity, whereas significantly elevated the GR activity with reference to the control. The impact was significantly greater with 100 mg than with 50 mg Cd kg−1 soil. With 100 mg Cd kg−1soil, V. radiata shoot displayed a 1.5-fold enhancement in the activities of GR and GPX, whereas GST activity displayed ≈ 0.8-fold decrease in comparison to roots. By comparison, B. campestris roots had a 2.2-fold lower GR activity than V. radiata roots, whereas GPX and GST activities were 6.8- and 24.0-fold higher, with the highest level of Cd treatment. In B. compestris shoots, activities of GPX and GST were 1.8- and 11.5-fold higher, respectively, while GR activity was 1.6-fold lower than in V. radiata shoot, with the same level of Cd application (Fig. 3a–f).
Activity of glutathione reductase (a, b), glutathione peroxidase (c, d), and glutathione sulfotransferase (e, f) in roots and shoot of B. campestris and V. radiata exposed to cadmium levels (0, 50, and 100 mg kg−1 soil). Values represent the means of five replicates (±standard deviation) from each of three independent experiments. Significant differences are: superscript a, vs. 0 and superscript b, vs. 100 (within the plant); superscript c, vs. B. campestris (between plants). f.w. = fresh weight
As to the non-enzymatic antioxidant, with increase in Cd level, B. campestris roots and shoot exhibited significant decrease in the reduced GSH content; whereas in V. radiata roots and shoot, it increased significantly, with reference to the control. Regarding the GSH oxidation, the roots and shoot of both the plants displayed significant increases in GSSG content (vs. control) under the influence of Cd. In B. campestris, the GSH and GSSG contents of roots revealed a 1.5-fold decline and 2.0-fold increase (vs. shoot), respectively. Similar comparison in V. radiata displayed 1.0-fold increase and 2.0-fold decline in the root GSH and GSSG contents (vs. shoot), respectively. Comparison between the 100 mg Cd-exposed B. campestris and V. radiata indicated that B. campestris roots carried 6-fold lower GSH content (vs. V. radiata root), whereas the shoot exhibited 3.5-fold decline vs. V. radiata shoot. Similarly, the GSSG content depicted 1.8-fold decline in roots and 2.2-fold increase in shoot, as compared with that in V. radiata, under the impact of 100 mg Cd kg−1 soil (Fig. 4a–d).
Content of reduced (a, b) and oxidized (c, d) glutathione in roots and shoot of B. campestris and V. radiata exposed to cadmium levels (0, 50, and 100 mg kg−1 soil). Values represent the means of five replicates (±standard deviation) from each of three independent experiments. Significant differences are: superscript a, vs. 0 and superscript b, vs. 100 (within the plant); superscript c, vs. B. campestris (between plants). f.w. = fresh weight
Discussion
This is the first report giving a comparative account of accumulation, allocation, and impact of Cd on modulation of the toxicity and tolerance indices in plants from Brassicaceae (B. campestris) and Fabaceae (V. radiata). Cd-contaminated growth conditions have been reported to retard plant growth significantly in terms of fresh or dry plant mass (reviewed by Irfan et al. 2013). Our observations on the Cd treatment (100 > 50 mg Cd kg−1 soil), showing a significant decrease in plant dry mass of both B. campestris and V. radiata, endorse some earlier works on B. campestris (Anjum et al. 2008ab), Brassica juncea (Mohamed et al. 2012), and V. radiata (Wahid et al. 2007; Anjum et al. 2008c). Despite its non-essential physiological role, Cd is taken up by plants to varying extent. Exhibition of a 3-fold higher Cd accumulation capacity of B. campestris roots (vs. V. radiata roots) is indicative of a unique Cd stabilization potential of this plant. Nevertheless, compared to B. campestris shoot, a 2-fold higher Cd allocation in V. radiata shoot has displayed the Cd extraction potential of this species. Plants have many endogenous biochemical/physiological properties that render them capable to tolerate/counteract impacts of metal/metalloid burden, thus making them ideal agents for cleanup of contaminated sites (reviewed by Maestri et al. 2010; Irfan et al. 2013). Despite this fact, a comparative assessment of the physiology/biochemistry of Cd tolerance of B. campestris and V. radiata has not yet been done.
The Cd load in plant organs can impair important physiological/biochemical processes by inducing oxidative stress due to imbalance between generation and metabolism of ROS in these organs (Anjum et al. 2010, 2012a, b; 2013a). Although Cd is a non-redox active metal, its phytotoxicity as a result of Cd-accrued induction of oxidative stress is extensively reported (Gill and Tuteja 2010; Cuypers et al. 2012; Anjum et al. 2012a, b; 2013a). Thus, a higher oxidative stress (in terms of H2O2 and its consequences (such as EL, H2O2, and TBARS) in B. campestris roots and V. radiata shoot are a natural outcome of high Cd burdens. We studied the status of homeostasis between ROS and its metabolism in the Cd-burdened roots and shoot of both B. campestris and V. radiata and found a differential tuning among the major components of their GSH redox system (i.e., GR, GPX, GST, and GSH), which control the enhanced H2O2 and its consequences (Gill and Tuteja 2010; Anjum et al. 2012a, b, 2013a, b, 2014). The maintenance of low oxidative stress and damage to membrane and its lipids in the highest Cd-exhibiting B. campestris roots was possible as a result of the balanced tuning between the GSH pool and its regenerating (GR) as well as consuming (GPX and GST) enzymes. The roots adopted the strategy of accelerating such enzymes as GPX and GST for efficiently metabolizing high Cd-mediated H2O2 levels and controlling the H2O2-accrued consequences like EL and LPO. Therefore, the decreased GSH pool and increased GSSG level can be considered as an outcome of the previous processes, where GR activity in B. campestris roots (despite being 2.2-fold lower than in V. radiata roots) seems to be efficient for counteracting the Cd-mediated enhanced H2O2 and its consequences (such as EL and LPO). In contrast, the highest Cd burden-exhibiting V. radiata shoot displayed a failure of the GPX- and GST-mediated H2O2-scavenging system despite the occurrence of 1.6-fold higher GSH-regenerating enzyme (GR) and 3.5-fold higher content of GSH (vs. B. campestris shoot) with 100 mg Cd kg−1 soil (Fig. 5). Thus, the possible role of H2O2-scavenging system other than GSH-based system is envisaged in high Cd burden-exhibiting V. radiata shoot. Our observations on the efficiency of the GSH redox system components for counteracting the Cd-caused anomalies (H2O2, EL, LPO) substantiate a number of earlier studies on metal/metalloid-exposed plants (reviewed by Gill and Tuteja 2010; Maestri et al. 2010; Anjum et al. 2012a, b; Anjum et al. 2013a).
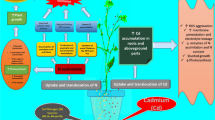
Schematic representation of the basic biochemical mechanisms underlying cadmium (Cd; 50 and 100 mg kg−1 soil)-mediated modulation of components of the glutathione (GSH)-based antioxidant defense system in B. campestris and V. radiata and their cumulative significance for the control of H2O2 metabolism, its anomalies (i.e., lipid peroxidation, LPO; electrolyte leakage, EL), plant health, and the execution of Cd stabilization (B. campestris) and Cd extraction (V. radiata). [GR, glutathione reductase; GST, glutathione sulfotransferase; GPX, glutathione peroxidase; GSH, reduced glutathione; GSSG, oxidized glutathione]
Conclusions
The organ-wise analysis of Cd burdens led us to conclude that B. campestris and V. radiata are good stabilizer and extractor of Cd, respectively. In addition, the cross talks on Cd burdens of organs and the adapted Cd tolerance strategies against the consequent toxicity indicate that the major components (GR, GPX, GST, and GSH) of the GSH redox system differentially modulate the Cd accumulation, toxicity, and tolerance in the test plants. Nevertheless, despite a higher Cd burden in roots of B. campestris and shoots of V. radiata, a better tuning among GR, GST, and GPX activities presumably allowed these plants to tolerate the Cd burden-caused anomalies at plant level. In short, the outcome of the physiological/biochemical characterization of the B. campestris and V. radiata responses to Cd stress reveals the adaptive potential of these plants to Cd-contaminated conditions, which in turn can be significant in elaborating the innovative plant-based remediation technologies for metal/metalloid-contaminated sites.
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Anderson ME (1985) Determination of glutathione and glutathione disulphides in biological samples. Method Enzymol 113:548–570
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008a) Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul 54:271–279
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008b) Ontogenic variation in response of Brassica campestris L. to cadmium toxicity. J Plant Interac 3:189–198
Anjum NA, Umar S, Ahmad A, Iqbal M (2008c) Responses of components of antioxidant system in moongbean [Vigna radiata (L.) Wilczek] genotypes to cadmium stress. Commun Soil Sci Plant Anal 39:2469–2483
Anjum NA, Umar S, Chan MT (2010) Ascorbate-glutathione pathway and stress tolerance in plants, 1st edn. Springer, Dordrecht
Anjum NA, Ahamd I, Mohmood I, Pacheco M, Duarte AC et al (2012a) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—a review. Environ Exp Bot 75:307–324
Anjum NA, Umar S, Ahmad A (2012b) Oxidative stress in plants: causes, consequences and tolerance. IK International, New Delhi
Anjum NA, Ahmad I, Pereira ME, Duarte AC et al (2012c) The plant family Brassicaceae: contribution towards phytoremediation, 1st edn. Springer (Science + Business Media), Dordrecht
Anjum NA, Ahmad I, Rodrigues SM, Henriques B et al (2013a) Eriophorum angustifolium and Lolium perenne metabolic adaptations to metals- and metalloids-induced anomalies in the vicinity of a chemical industrial complex. Environ Sci Pollut Res 20:568–581
Anjum NA, Singh N, Singh MK, Shah ZA et al (2013b) Single-bilayer graphene oxide sheet tolerance and glutathione redox system significance assessment in faba bean (Vicia faba L.). J Nanopart Res 15:1770
Anjum NA, Duarte AC, Pereira E, Ahmad I (2014) Oxidative stress status, antioxidant metabolism and polypeptide patterns in Juncus maritimus shoots exhibiting differential mercury-burdens in Ria de Aveiro coastal lagoon (Portugal). Environ Sci Pollut Res. doi:10.1007/s11356-014
Clemens S, Palmgren MG, Kraemer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trend Plant Sci 7:309–315
Cuypers A, Keunen E, Bohler S, Jozefczak M et al (2012) Cadmium and copper stress induce a cellular oxidative challenge leading to damage versus signalling. In: Gupta DKG, Sandalio LM (eds) Metal toxicity in plants: perception, signaling and remediation. Springer, Berlin, pp 65–90
DalCorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gill SS, Tuteja N (2011) Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signal Behav 6:215–222
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120
Gill SS, Anjum NA, Hasanuzzaman M, Gill R et al (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212
International Agency for Research on Cancer (IARC) (1993) IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 58. Lyon: International Agency for Research on Cancer; 1993. Beryllium, cadmium, mercury and exposures in the glass manufacturing industry; pp. 41-117
Irfan M, Hayat S, Ahmad A, Alyemeni MN (2013) Soil cadmium enrichment: allocation and plant physiological manifestations. Saudi J Biol Sci 20:1–10
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quences ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68:1–13
Mohamed AA, Castagna A, Ranieri A, Sanita di Toppi L (2012) Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol Biochem 57:15–22
Polle A, Schützendübel A (2003) Heavy metal signalling in plants: linking cellular and organismic responses. In: Hirt H, Shinozaki K (eds) Plant responses to abiotic stress. Springer, Berlin, pp 187–215
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Satarug S, Baker JR, Reilly PEB, Moore MR, Williams DJ (2002) Cadmium levels in the lung, liver, kidney cortex and urine samples from Australians without occupational exposure to metals. Arch Environ Health 57:69–77
United Nations Environment Programme (2008) Draft final review of scientific information on cadmium. http://www.chem.unep.ch/pb_and_cd/SR/Draft_final_reviews_Nov2008.htm (accessed on 4 March 2014)
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land—a review. Environ Chem Lett 8:1–17
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Wahid A, Ghani A, Ali I, Ashraf MY (2007) Effect of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J Agron Crop Sci 193:357–365
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080
WHO (2007) Health risks of heavy metals from long-range transboundary air pollution. World Health Organization 2007. WHO Regional Office for Europe, Copenhagen, Denmark
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Zaidi A, Wani PA, Khan MS (2012) Toxicity of heavy metals to legumes and bioremediation. Springer, Dordrecht
Acknowledgements
Financial assistance received from Hamdard National Foundation (HNF), New Delhi, India (DSW/HNF-18/2006) is gratefully acknowledged. NAA also thanks the FCT (Govt. of Portugal) for providing post-doctoral grants (FRH/BPD/64690/2009; SFRH/BPD/84671/2012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Anjum, N.A., Umar, S. & Iqbal, M. Assessment of cadmium accumulation, toxicity, and tolerance in Brassicaceae and Fabaceae plants—implications for phytoremediation. Environ Sci Pollut Res 21, 10286–10293 (2014). https://doi.org/10.1007/s11356-014-2889-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2889-5