Abstract
Sub-surface water samples from the delta of Thamirabarani River of south India were evaluated for human health risks and seawater intrusion using the geochemical signatures. Electrical conductivity (EC), total dissolved solids (TDS), pH and the concentrations of major cations and anions in 40 samples collected during the winter (January) and summer (July) of 2018 show comparable values. Subsequently, the results were verified with respect to the international drinking water quality standards. The piper trilinear diagram shows mixed Ca–Mg–Cl, Na–Cl, Ca–HCO3 and mixed Ca–Na–HCO3 facies in the samples. Similarly, the plenteous of cations are sequenced as Na+ > Ca2+ > Mg2+ > K+ and the plenteous of anions are sequenced as Cl− > SO42− > HCO3−>Br− > NO3− > PO4−. Gibbs plots illustrate that rock–water interaction and evaporation control the geochemistry of sub-surface water. More than 40% of the samples are unsuitable for drinking, and their higher EC and TDS values reflected the seawater intrusion, in addition to the anthropogenic activities (salt panning). Interrelationship between ions of sub-surface water was used to get a better insight into the saline water intrusion in the study area. To mitigate the river water salinization and seawater incursion in the aquifers, engineering solution such as weir construction across the Thamirabarani River near Mukkani village has been proposed. After construction of the weir, freshwater in the river can be diverted to the salt-affected and seawater-intruded areas to improve the scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The quality and quantity of freshwater are continuously declining due to the overexploitation of water resources for various anthropogenic necessities such as irrigation and agriculture, industrial consumption and drinking water supply (Chidambaram et al. 2009; Li et al. 2017). In the southeast part of Asia, the population growth has inverse relation to the availability of freshwater resources, and the supply of freshwater comes mainly from the surface as well as sub-surface (Hema et al. 2010; Kim et al. 2003). The quality of water plays an important role in securing human health and its utility for several agricultural activities. Many researchers have studied the sub-surface water quality in various basins around the globe (Gorgij et al. 2019; Karunanidhi et al. 2019; Li et al. 2014a, b, 2016b, c, 2018; Wang et al. 2019; Wu et al. 2019; Wu and Sun 2016; Su et al. 2019; Subramani et al. 2010; Pazand et al. 2018; Prasanna et al. 2011). These studies indicate that sub-surface water is the prevailing drinking water source in many regions, and its worsening quality is mainly due to seawater intrusion, higher evaporation rates, interaction with the rock formation and saltpans (Capaccioni et al. 2005; Mondal et al. 2011; Satheeskumar and Subramani 2016; Park et al. 2005). Particularly, seawater intrusion is one of the routine phenomena that affect the coastal aquifers (Senthilkumar et al. 2017; Srinivasamoorthy et al. 2013). Among the other evaluation methods, the hydrochemical characteristic of sub-surface water is an effective way to discern the rock/saltwater reaction (Todd 1980; Kim et al. 2003; Selvam et al. 2013). Leaching of secondary salts in the coastal aquifers plays a vital role in sub-surface water quality (Chidambaram et al. 2009). Saltwater intrusion is the prime factor contributing to high salinity in the groundwater of coastal aquifers of the Damghan basin of Iran (Ebrahimi et al. 2016).
The water pollution deteriorates the quality of ecosystem and threatens the human health (Li and Wu 2019a, b). The Thamirabarani River is one of the perennial rivers of south India. Coastal aquifers are highly influenced by the saline water intrusion, probably due to the over-extraction of sub-surface water for the rapidly growing salt panning activities in this region. The salt panning contributes more revenue than agricultural farming in this coastal region. Thus, the agricultural lands are continuously transformed to saltpans, and it has escalated over the past two decades. The resultant saltwater ingression has highly deteriorated the sub-surface water quality in the southern side of the delta. The seawater intrusion is a prime threat in this deltaic region up to the village of Attur, located at 7 km from the coast of Bay of Bengal. Few researchers have explored and studied the geochemical characteristics in a part of this delta. Fluoride concentration in the sub-surface water of Thamirabarani River was studied by Magesh et al. (2016). Hydrogeochemistry of sub-surface water in coastal aquifers of Thoothukudi district was carried out by Singaraja et al. (2014) and Mondal et al. (2011). However, there has been no significant study on the geochemical signatures to characterize the quality of the sub-surface water for drinking purpose and demarcate the regions affected by the seawater intrusion in Thamirabarani delta. Therefore, this study is performed with the objectives of understanding the aptness of groundwater for human consumption as per the international standards and demarcating the seawater incursion zones using geospatial techniques based on the geochemical signatures.
Materials and methods
Thamirabarani delta
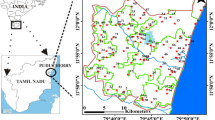
The study area is located at coastal part of the Thamirabarani River delta in the Thoothukudi district of Tamil Nadu state in the south India. It lies between latitudes 08° 35′ N to 08° 45′ N and longitudes 77° 55′ E to 78° 10′ E and spreads over an area of 400 km2 (Fig. 1). The water-bearing formations are Quaternary alluvium, Terri sands (sand dunes), Tertiary sediments and the weathered zones in gneisses and charnockite. In the coastal regions, the marine and fluviomarine deposits are the main litho units (Magesh et al. 2016; Narayanaswamy and Lakshmi 1967). The prime land-use types in this region include the agricultural lands, barren lands and saltpans apart from settlements and water bodies. The alluvial/Tertiary aquifers are linked with sea, and these aquifers are primarily affected by saline water incursions (CGWB 2009).
The Thamirabarani delta is underlain by rocks of Archean age, which is having gneisses, granites and charnockites (Narayanaswamy and Lakshmi 1967). The coastal region is underlain with marine, fluviomarine and eolian sediments. Hydrogeologically, the study area contains porous and fissured formations. The prime aquifer systems are weathered and fractured hard rock formations of Archean age and porous sedimentary formations of Tertiary and recent age (Magesh et al. 2016). The shallow aquifer in this region is under unconfined condition, and the deep aquifer is under semi-confined to confined conditions (Central Ground Water Board (CGWB) 2009).
Field and laboratory investigations
Settlements, surface water bodies, streams and roadways were digitized from the Survey of India (SOI) toposheets (58 H/10, 58 H/14, 58 L/2) on the scale of 1:50,000. The base map of the research area was prepared by using toposheets with the help of ArcGIS (v.10.2.1) software. Sub-surface water samples were collected in 1-L polyethylene bottles in two different seasons for a year, i.e., January 2018 (winter) and July 2018 (summer). A total of 40 sub-surface water samples were collected covering the entire research area. The values of pH, electrical conductivity (EC) and total dissolved solids (TDS) of the collected samples were measured in situ using field instruments (HANNA-HI98192). Water analyses were carried out as per the American Public Health Association standards (American Public Health Association 1995). Bicarbonate, magnesium, chloride and calcium ions were analyzed by titrimetric method. Potassium and sodium ion concentrations were analyzed by the flame photometer (Elico CL-220). Sulfate ions were detected using a spectrophotometer (Elico SL-164), and the concentrations of nitrate and bromide ions were detected using the ion-sensitive electrodes (Eutech Ion meter: ION 6+). The chemical analysis results were verified for ion-balancing errors (< 10%).
Results and discussion
Hydrogeochemistry of groundwater
Water quality is an eminent factor to evaluate fitness of sub-surface water for consumption (Subramani et al. 2005). The variables of sub-surface water collected during the months of January (winter) and July (summer) are listed in Table 1. Table 2 represents sub-surface water quality variables surpassing the maximum allowable values as suggested by the World Health Organization (WHO) for drinking water quality. In the study area, the pH varied from 6.6 to 9.0 during the winter and 6.54–9.15 during the summer, indicating the acidic to alkaline natures of the sub-surface water. High pH values were acquired in the samples collected in the sites with saltwater intrusions and along the regions with saltpan. EC indicates the potential of water to carry current; thus, the occurrence of salts is higher in water with greater EC values (Jalali 2005). EC varied from 296 to 79,650 µS/cm in sub-surface samples collected during the winter, and it fluctuated between 224 and 81,160 µS/cm in samples collected during the summer. Both the pH and EC values of samples collected in both the seasons were comparable.
Total dissolved solids (TDS) are again similar, and it varied from 105 to 36,800 mg/L in the winter samples and remained 113 to 40,650 mg/L in the summer samples. Figures 2 and 3 show the spatial dispersals of TDS for the winter and summer samples in the study area, and they indicate about 48% samples in this region are unsuitable for ingestion. Based on the classifications of Freeze and Cherry (1979), about 62.5% winter and 60% summer season sub-surface water samples are freshwater (TDS < 1000 mg/L). Nearly 25% of winter and 27.5% of summer season samples are brackish water (TDS: 1000–10,000 mg/L). Similarly, about 12.5% of samples collected in both the seasons are saline water (TDS: 10,000–100,000 mg/L). This study further reveals more than 40% of the samples are unfit for ingestion, and these samples with higher TDS values were acquired from the region that is subjected to seawater pass.
The plenteous of cations of these samples are sequenced as Na+ > Ca2+ > Mg2+ > K+. Concentrations of the cations are compared with the drinking water standards set by World Health Organization (WHO 2011), and these ions surpass by 37.5% (Na+), 12.5% (Ca2+) and 12.5% (Mg2+) respectively. Figures 4 and 5 show the spatial dispersion of Na+ during the winter and summer season samples, respectively. Higher Na+ in sub-surface water is primarily due to sodium liberated from silicate weathering and also due to seawater intrusion. The origin of Ca2+ and Mg2+ ions in sub-surface water might be derived from leaching of Ca and Mg-rich rock-forming silicates and gypsum dissolution (Subramani et al. 2013; Krishna Kumar et al. 2014). The plenteous of anions of these samples are sequenced as Cl− > SO42− > HCO3− > Br− > NO3− > PO4−. The concentrations of Cl− and SO42− surpassed the WHO standards by about 30% and 12.5% respectively. Figures 6 and 7 show the spatial dispersal of Cl− in the winter and summer season sub-surface water samples, respectively.
Agricultural return flow, seawater incursion and weathering of salt deposits are the prime factors of the presence of Cl− in sub-surface water. The higher amount of SO42− in sub-surface water might be derived from leaching of domestic wastes and marine sources. The presence of high amount of SO42− in the coastal part of the study area may be from the marine sources (Krishna Kumar et al. 2014). Among all the ions, the concentrations of Mg2+ and Cl− surpass the permissible values and Na+ and Ca2+ succeed them. This is an indication that the saline water ingression is a principal mechanism of higher salinity along with the involvement of anthropogenic activities like the salt panning as suggested by Singaraja et al. (2014). Nitrate and phosphate ions are within the permissible limits. About 42.5% of the samples exceed Br− value as 1 mg/L, indicating the sub-surface water is affected by seawater incursion (Sridharan and Nathan 2017).
Implications to human health
More than one-fourth of these samples have TDS > 1000 mg/L, and about 12.5% them have TDS > 10,000 mg/L. Excessive total dissolved solids (TDS) are associated with coronary and cardiovascular diseases, gallbladder inflammation and gallstones (Burton and Cornhill 1977; Duraisamy et al. 2018). Higher concentrations of chlorine in about 30% of the drinking water could also lead to bladder cancer (Cantor et al. 1987). Similarly, the presence of excessive sulfate in 12.5% of the drinking water could lead to cathartic effect in adults and diarrhea in infants (Backer et al. 2001). Concentration of Na+ above the permissible limit of WHO in 37.5% of the samples might cause health hazards such as heart diseases, kidney malfunction and hypertension. Calcium and magnesium are essential nutrients, and both of them are required for the survival of vegetation and animals. Excessive presence of them in drinking water, however, could lead to bone breakage and issues in peripheral nerve system (Tiwari et al. 2017). About 12.5% of the sub-surface samples in the study area have Ca and Mg above the permissible limit of WHO.
Geochemical facies
Concentrations of the cations and anions were plotted in a piper diagram using the AquaChem (v.2014.2) software. Geochemistry of sub-surface water is inferred by using the trilinear plotting systems (Piper 1944) in Fig. 8. The samples accumulate toward sodium side in cation triangle. It can be concluded that bicarbonate and chloride are present, but sulfate ions are absent from the anion triangle. Among the geochemical facies, the mixed Ca–Mg–Cl group and Na–Cl group are the prime water groups. This indicates the role of seawater intrusion in Cl enrichment (Richter and Kreitler 1993). The increasing trend of salinity in sub-surface water is due to mixing of freshwater with the seawater. Mixed Ca–Mg–Cl > Na–Cl > Ca–HCO3 > mixed Ca–Na–HCO3 is the order of presence of geochemical facies in the studied area for the sub-surface water.
Gibbs plot
Gibbs (1970) suggests a simple diagram of TDS versus (Na + K)/(Na + K + Ca) and Cl/(Cl + HCO3) to illustrate the effect of rock–water interaction, evaporation and precipitation on sub-surface water geochemistry (Fig. 9). The plot suggests water–rock/sediment interaction and some extent of evaporation (during summer) are the prime factors controlling the groundwater chemistry in the study area. The higher amount of evaporation or anthropogenic activities enhances the TDS, and water samples tend to shift from rock predominant to the evaporation zone (Krishna Kumar et al. 2014; Li et al. 2016a). The shift of sampling points approaching the evaporation zone from the rock–water interaction zone illustrates an increase of Na+ and Cl− ions, and further, higher TDS might be due to seawater intrusion (Krishna Kumar et al. 2014).
Demarcation of seawater intrusion
Cl/Br ratio is used here as a tracer to demarcate saline water intrusion in this coastal aquifer system (Fig. 10). This ratio varied from 288 to 292 for the Standard Mean Ocean Water (SMOW) (Sridharan and Nathan 2017; McArthur et al. 2012). It is considered that the samples with values near to the SMOW ratio are affected by seawater intrusion. The values of Cl/Br higher than the SMOW are associated with the samples collected in the regions with saltpans. Samples located below the SMOW line may have the influence of agricultural activities (Alcalá and Custodio 2008).
This geochemical signature helped to demarcate the regions with seawater intrusion and the regions affected by saltpan activities (Fig. 11). Both TDS and Cl are the prime water quality variables, and they are subtilized for identification of the intrusion zones. Additionally, the interrelationship between Na and Cl, TDS and Cl, Ca and Na, Cl and Cl/Br ratio also assisted in the demarcation of seawater incursion regions. The Thamirabarani River has five major distributaries in the delta through which the seawater enters into the river during the high-tide period and summer season. This effect has been observed up to the village of Attur, situated 7 km away from the coast of the Bay of Bengal along the upstream. We observed this effect within 3.5–5 km from the coast (south to north) in the sub-surface samples. The restricted influence of seawater intrusion during the winter season shows more samples in the field of Ca–HCO3 and Ca–Na–HCO3 facies (Fig. 8).
Remedial measures
The earthen bunds are created across the distributaries of the Thamirabarani River to minimize the seawater pass in the upstream side. These bunds are totally washed out during the monsoonal rainfall and surface water flow. We spotted a site near Mukkani for construction of weir considering the surface water discharge, tidal variation and sub-surface lithological data (Satheeskumar and Subramani 2016), and the work has been initiated by the Public Works Department of the Tamil Nadu state to mitigate the effects of seawater pass into the river and aquifer (Fig. 12). Construction of this weir will help to shift the freshwater and seawater interface toward downstream side and mitigate the effect of seawater intrusion into the sub-surface water in adjacent villages that get recharged with the freshwater. Canals can also be constructed from the weir to divert the freshwater flow to the saltwater-affected areas for recharging the sub-surface water in order to reduce the salinity of these water resources.
Conclusions
-
The geochemical characteristics of sub-surface water from aquifers present within the Thamirabarani delta of south India helped to assess the drinking water quality as well as the possible health risks. About 40% of the groundwater samples surpass the values suggested by the international water quality standards (WHO 2011). Cations such as Na+, Ca2+ and Mg2+ and anions such as Cl−, SO42− and Br− regulate its geochemistry.
-
The sub-surface water chemistry is dominated by seawater intrusion, ion exchange process, dissolution and anthropogenic activities. The Ca2+ and Mg2+ ions in sub-surface water are derived from leaching of Ca2+ and Mg2+ bearing rock-forming silicates and gypsum dissolution. It also illustrates that Na+ and Cl− are mainly derived from anthropogenic activities inland and seawater entry in the coastal region.
-
Samples with higher EC and TDS are acquired from coastal part of the research area, indicating the influence of seawater intrusion and salt panning on ion exchange process. Geochemical facies of these sub-surface samples are characterized by mixed Ca–Mg–Cl, Na–Cl, Ca–HCO3 and Ca–Na–HCO3 groups. The Cl/Br ratio demarcated the saline-water-intruded regions within these study areas.
-
Engineering solution of mitigation like weir construction at Mukkani village is suggested in this study to control seawater entry into the surface water. Additionally, the construction of canals could also provide remedial measure to reduce seawater ingression in the saltwater-affected areas. Exploitation of sub-surface water for salt panning can be stopped to control the seawater intrusion.
References
Alcalá, F. J., & Custodio, E. (2008). Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. Journal of Hydrology, 359(1–2), 189–207.
American Public Health Association (APHA). (1995). Standard methods for the examination of water and wastewater (Vol. 21). Washington, DC: American Public Health Association.
Backer, L. C., Esteban, E., Rubin, C. H., Kieszak, S., & McGeehin, M. A. (2001). Assessing acute diarrhea from sulfate in drinking water. Journal-American Water Works Association, 93(9), 76–84.
Burton, A. C., & Cornhill, J. F. (1977). Correlation of cancer death rates with altitude and with the quality of water supply of the 100 largest cities in the United States. Journal of Toxicology and Environmental Health, Part A Current Issues, 3(3), 465–478.
Cantor, K. P., Hoover, R., Hartge, P., Mason, T. J., Silverman, D. T., Altman, R., et al. (1987). Bladder cancer, drinking water source, and tap water consumption: A case-control study. JNCI Journal of the National Cancer Institute, 79(6), 1269–1279.
Capaccioni, B., Didero, M., Paletta, C., & Didero, L. (2005). Saline intrusion and refreshening in a multilayer coastal aquifer in the Catania Plain (Sicily, Southern Italy): Dynamics of degradation processes according to the hydrochemical characteristics of groundwaters. Journal of Hydrology, 307(1–4), 1–16.
Central Ground Water Board (CGWB). (2009). District subsurface water brochure, Tirunelveli district, Tamil Nadu. Technical Report Series
Chidambaram, S., Kumar, G. S., Prasanna, M. V., Peter, A. J., Ramanthan, A. L., & Srinivasamoorthy, K. (2009). A study on the hydrogeology and hydrogeochemistry of groundwater from different depths in a coastal aquifer: Annamalai Nagar, Tamilnadu, India. Environmental geology, 57(1), 59–73.
Duraisamy, S., Govindhaswamy, V., Duraisamy, K., Krishinaraj, S., Balasubramanian, A., & Thirumalaisamy, S. (2018). Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-018-0183-z.
Ebrahimi, M., Kazemi, H., Ehtashemi, M., & Rockaway, T. D. (2016). Assessment of groundwater quantity and quality and saltwater intrusion in the Damghan basin, Iran. Chemie der Erde, 76(2), 227–241.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater. Englewood Cliffs: Prentice-Hall.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 17, 1088–1090.
Gorgij, A. D., Wu, J., & Moghadam, A. A. (2019). Groundwater quality ranking using the improved entropy TOPSIS method: A case study in Azarshahr Plain aquifer, east Azerbaijan, Iran. Human and Ecological Risk Assessment, 25(1–2), 176–190. https://doi.org/10.1080/10807039.2018.1564235.
Hema, S., Subramani, T., & Elango, L. (2010). GIS study on vulnerability assessment of water quality in a part of Cauvery River. International Journal of Environmental Sciences, 1(1), 1–17.
Jalali, M. (2005). Major ion chemistry of groundwaters in the Bahar area, Hamadan, Western Iran. Journal of Environmental Geography, 47, 763–772.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Wu, J., & Srinivasamoorthy, K. (2019). Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River basin, South India. Human and Ecological Risk Assessment, 25(1–2), 250–270. https://doi.org/10.1080/10807039.2019.1568859.
Kim, J. H., Kim, R. H., Lee, J., & Chang, H. W. (2003). Hydrogeochemical characterization of major factors affecting the quality of shallow groundwater in the coastal area at Kimje in South Korea. Environmental Geology, 44(4), 478–489.
Krishna Kumar, S., Bharani, R., Magesh, N. S., Godson, P. S., & Chandrasekar, N. (2014). Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Applied Water Sciences, 4, 341–350.
Li, P., Li, X., Meng, X., Li, M., & Zhang, Y. (2016a). Appraising groundwater quality and health risks from contamination in a semiarid region of Northwest China. Exposure and Health, 8(3), 361–379. https://doi.org/10.1007/s12403-016-0205-y.
Li, P., Qian, H., & Wu, J. (2018). Conjunctive use of groundwater and surface water to reduce soil salinization in the Yinchuan Plain, North-West China. International Journal of Water Resources Development, 34(3), 337–353. https://doi.org/10.1080/07900627.2018.1443059.
Li, P., Qian, H., Wu, J., Chen, J., Zhang, Y., & Zhang, H. (2014a). Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River. China. Environmental Earth Sciences, 71(7), 3133–3145. https://doi.org/10.1007/s12665-013-2691-6.
Li, P., Tian, R., Xue, C., & Wu, J. (2017). Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environmental Science and Pollution Research, 24(15), 13224–13234. https://doi.org/10.1007/s11356-017-8753-7.
Li, P., & Wu, J. (2019a). Sustainable living with risks: Meeting the challenges. Human and Ecological Risk Assessment, 25(1–2), 1–10. https://doi.org/10.1080/10807039.2019.1584030.
Li, P., & Wu, J. (2019b). Drinking water quality and public health. Exposure and Health, 11(2), 73–79. https://doi.org/10.1007/s12403-019-00299-8.
Li, P., Wu, J., & Qian, H. (2014b). Hydrogeochemistry and quality assessment of shallow groundwater in the southern part of the yellow river alluvial plain (Zhongwei section) China. Earth Science Research Journal, 18(1), 27–38. https://doi.org/10.15446/esrj.v18n1.34048.
Li, P., Wu, J., & Qian, H. (2016b). Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County, China. Arabian Journal of Geosciences, 9(1), 15. https://doi.org/10.1007/s12517-015-2059-1.
Li, P., Wu, J., Qian, H., Zhang, Y., Yang, N., Jing, L., et al. (2016c). Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Exposure and Health, 8(3), 331–348. https://doi.org/10.1007/s12403-016-0193-y.
Magesh, N. S., Chandrasekar, N., & Elango, L. (2016). Occurrence and distribution of fluoride in the groundwater of the Tamiraparani River basin, South India: A geostatistical modeling approach. Environmental Earth Sciences, 75(23), 1483.
McArthur, J. M., Sikdar, P. K., Hoque, M. A., & Ghosal, U. (2012). Waste-water impacts on groundwater: Cl/Br ratios and implications for arsenic pollution of groundwater in the Bengal Basin and Red River Basin, Vietnam. Science of the Total Environment, 437, 390–402.
Mondal, N. C., Singh, V. P., Singh, S., & Singh, V. S. (2011). Hydrochemical characteristic of coastal aquifer from Tuticorin, Tamil Nadu, India. Environmental monitoring and assessment, 175(1–4), 531–550.
Narayanaswamy, S., & Lakshmi, P. (1967). Charnockitic rocks of Tirunelvelli district, Madras. Geological Society of India, 8, 38–50.
Park, S. C., Yun, S. T., Chae, G. T., Yoo, I. S., Shin, K. S., Heo, C. H., et al. (2005). Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. Journal of Hydrology, 313(3–4), 182–194.
Pazand, K., Khosravi, D., Ghaderi, M. R., & Rezvanianzadeh, M. R. (2018). Identification of the hydrogeochemical processes and assessment of groundwater in a semi-arid region using major ion chemistry: A case study of Ardestan basin in Central Iran. Groundwater for Sustainable Development, 6, 245–254.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water-analyses. Eos, Transactions American Geophysical Union, 25(6), 914–928.
Prasanna, M. V., Chidambaram, S., Hameed, A. S., & Srinivasamoorthy, K. (2011). Hydrogeochemical analysis and evaluation of groundwater quality in the Gadilam river basin, Tamil Nadu, India. Journal of earth system science, 120(1), 85–98.
Richter, B. C., & Kreitler, C. W. (1993). Geochemical techniques for identifying sources of ground-water salinization. Boca Raton: CRC Press.
Satheeskumar, V., & Subramani, T. (2016). Preliminary investigation on environmental degradation due to salinization of river and groundwater in Thamirabarani Delta, South India. Indian Journal of Geo-Marine Sciences, 45(9), 1148–1153.
Selvam, S., Manimaran, G., & Sivasubramanian, P. (2013). Hydrochemical characteristics and GIS-based assessment of groundwater quality in the coastal aquifers of Tuticorin corporation, Tamilnadu, India. Applied Water Science, 3(1), 145–159.
Senthilkumar, S., Balasubramanian, N., Gowtham, B., & Lawrence, J. F. (2017). Geochemical signatures of groundwater in the coastal aquifers of Thiruvallur district, south India. Applied Water Science, 7(1), 263–274.
Singaraja, C., Chidambaram, S., Prasanna, M. V., Thivya, C., & Thilagavathi, R. (2014). Statistical analysis of the hydrogeochemical evolution of groundwater in hard rock coastal aquifers of Thoothukudi district in Tamil Nadu, India. Environmental earth sciences, 71(1), 451–464.
Sridharan, M., & Nathan, D. S. (2017). Hydrochemical facies and ionic exchange in coastal aquifers of Puducherry region, India: Implications for seawater intrusion. Earth Systems and Environment, 1(1), 5.
Srinivasamoorthy, K., Vasanthavigar, M., Vijayaraghavan, K., Sarathidasan, R., & Gopinath, S. (2013). Hydrochemistry of groundwater in a coastal region of Cuddalore district, Tamilnadu, India: Implication for quality assessment. Arabian Journal of Geosciences, 6(2), 441–454.
Su, F., Wu, J., & He, S. (2019). Set pair analysis-Markov chain model for groundwater quality assessment and prediction: A case study of Xi’an City, China. Human and Ecological Risk Assessment, 25(1–2), 158–175. https://doi.org/10.1080/10807039.2019.1568860.
Subramani, T., Anandakumar, S., Kannan, R., & Elango, L. (2013). Identification of major hydrogeochemical processes in a hard rock terrain by NETPATH modeling. Book on Earth Resources and Environment, 29, 365–370.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environmental Geology, 47(8), 1099–1110.
Subramani, T., Rajmohan, N., & Elango, L. (2010). Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environmental monitoring and assessment, 162(1–4), 123–137.
Tiwari, A. K., Singh, A. K., Singh, A. K., & Singh, M. P. (2017). Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh district, Uttar Pradesh, India. Applied Water Science, 7(4), 1609–1623.
Todd, D. K. (1980). Groundwater hydrology (2nd ed.). New York: Wiley.
Wang, D., Wu, J., Wang, Y., & Ji, Y. (2019). Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: Analysis, assessment, and management. Exposure and Health. https://doi.org/10.1007/s12403-019-00314-y.
World Health Organization (WHO). (2011). Guidelines for drinking-water quality. WHO Chronicle, 38(4), 104–108.
Wu, J., & Sun, Z. (2016). Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Exposure and Health, 8(3), 311–329. https://doi.org/10.1007/s12403-015-0170-x.
Wu, J., Zhou, H., He, S., & Zhang, Y. (2019). Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environmental Earth Sciences, 78(15), 446. https://doi.org/10.1007/s12665-019-8471-1.
Acknowledgements
The authors are grateful to the Department of Civil Engineering, Government College of Technology, Coimbatore, for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Satheeskumar, V., Subramani, T., Lakshumanan, C. et al. Groundwater chemistry and demarcation of seawater intrusion zones in the Thamirabarani delta of south India based on geochemical signatures. Environ Geochem Health 43, 757–770 (2021). https://doi.org/10.1007/s10653-020-00536-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00536-z