Abstract
Purpose
Salinization of groundwater by seawater intrusion is a major concern for the coastal aquifers worldwide. Seawater intrusion occurs mainly due to overpumping of freshwater and sea-level rise which causes lateral and vertical movements of seawater into the coastal aquifers.
Methods
There are several methods to identify and assess the extent of seawater ingress towards land. In the present study, a hydrochemical approach is adopted to understand the status of intrusion. The ions such as Na+, K+, Ca2+, Mg2+, Cl−, SO4 2−, HCO 2−3 , and in-situ parameters namely TDS, pH, and EC were determined for 174 water samples collected both in pre- and post-monsoon in Puducherry region, India.
Results
Ionic ratios such as HCO3 2−/Cl−, Na+/Cl−, Ca2+/Cl−, Ca2+/Na+, Mg2+/Cl−, K+/Cl−, SO4 2−/Cl−, and Cl−/Br− were calculated and correlated with total dissolved solids (TDS) to evaluate seawater intrusion. The ionic ratios such as HCO3 2−/Cl−, Ca2+/Cl−, Mg2+/Cl−, K+/Cl−, and SO4 2−/Cl− vs. TDS shows negative correlation indicating salinization of groundwater. Cl−/Br− ratio is used to distinguish the causes of salinity in groundwater. The Hydrochemical Facies Evolution diagram (HFE-diagram) and heat maps generated out of it have been very well used to understand evolution of seawater intrusion and freshening process in the coastal aquifer to time. The majority of samples in pre-monsoon fall under the facies Na–HCO3/SO4, followed by Na–mixHCO3/mix SO4, Mix Na–HCO3/Mix SO4, and MixNa–MixHCO3/mix SO4 facies indicating direct cations exchange process, whereas, in post-monsoon, Na–Cl, Mix Na–Cl, and Mix Ca–Cl facies are dominant indicating reverse ion exchange process. In the study area, five locations, viz. Ariyankuppam, Kariambattur, Kalapet, Mutialpet, and Parikalpet, fall under Na–Cl and Ca–Cl facies in pre- and post-monsoon which indicates consistent seawater intrusion. The hydrochemical changes that take place during seawater freshwater interaction along coastal aquifer are determined by ionic exchange.
Conclusion
About 24.2% of samples in pre-monsoon and 13.5% of samples in post-monsoon show mixing of seawater. The highly negative ionic exchange values of sodium during pre-monsoon indicate increased amount of seawater fraction in groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coast is a landform where land and sea meet each other. Throughout the world, coasts are the regions thickly populated with large-scale development. In the coastal region, groundwater is major source of water supply. Coastal aquifers which hold groundwater are said to be most virgin. Its virginity is always tested by deterioration of groundwater quality. The major problem affecting quality of coastal groundwater is salinization. Salinization of groundwater is controlled by factors like seawater intrusion, rock–water interaction, over exploitation, rise in sea level, climatic changes, hydrogeological setting of the study area, salt panning, improper sewage disposal, use of chemical fertilizers and pesticides, marine formations, etc. (Nair et al. 2015; Batayneh et al. 2014; Anders et al. 2013; Amiri et al. 2015). Among all these factors, seawater incursion is considered to be a major threat to coastal aquifers globally.
Seawater intrusion is a phenomenon caused by movement of seawater into the fresh water coastal aquifer either by natural or anthropogenic means (Christina et al. 2014). Freshwater floats over the seawater due to less density without any distinct boundary called transition zone. Due to overpumping, the decrease in freshwater level results in invasion of seawater. Overexploitation of bore wells at transition zone or at deeper depths along coast leads to salinization of groundwater (Ayolabi et al. 2013). Tsunamis, and Tidal and estuarine activities cause infiltration of seawater into coastal aquifers of shallow depth (Mondal et al. 2010a; Longe et al. 1987; Saxena et al. 2003; Lee and Song 2006; Mondal et al. 2007; Kim et al. 2008; Todd 1953). Thus, groundwater and seawater together play an important role in the hydrological system of coastal aquifers (Mondal et al. 2010a, b).
To assure safe drinking water to all living community and to save our prosperous environment, it is necessary to manage water resources. There are several methods that can be adopted for studying salinization of groundwater like hydrochemical facies evolution method (Giménez-Forcada 2014); freshwater–seawater interaction (Mondal et al. 2010a, b); isotopic techniques (Anders et al. 2013); ionic ratios (Batayneh et al. 2014; Chen and Jiao 2007); and geophysical surveys (Ayolabi et al. 2013). The main purpose of this study is (1) to understand the evolution of hydrochemical facies during intrusion and freshening phase using hydrochemical facies evolution—diagram and (2) to determine the amount of seawater incurred into groundwater using empirical hydrochemical data, by which seawater ingress into the coastal aquifers of Puducherry region can be traced out spatially and temporally.
2 Materials and Methods
2.1 Study Area
Puducherry, a union territory, lies along Coromandel coast of India between the latitudes 11°45′ and 12°03′N, and longitudes 79°37′ and 79°53′ (CGWB 2007). Enclaves of study area are bounded by Cuddalore and Villupuram districts of Tamil Nadu on north, west, and south, with Bay of Bengal on the east. It covers an area of about 293 sq. km geographically. The annual rainfall is about 1272.70 mm on an average. Topographically study area is a flat terrain with mean elevation of 15 m above mean sea level (MSL). River Gingee and River Ponnaiyar are the two major rivers run across the study area and drain into Bay of Bengal.
2.2 Geology and Hydrogeology
The study area is geologically covered entirely by sedimentary formations of marine and non-marine origin, whose ages range from Cretaceous to Recent with Archaean charnockite as basement (CGWB 2007; Table 1.). Here, the major aquifers that hold groundwater are Cuddalore sandstone, Vanur sandstone, and Ramanathapuram formation, under confined condition (Fig. 1; CGWB 2007). Groundwater is the main source of water supply for domestic, agricultural, and industrial purposes in Puducherry and is extracted by bore wells (Thilagavathi et al. 2012).
2.3 Methodology
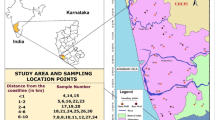
For the current study, 175 groundwater samples were collected from bore wells covering all the lithology of Puducherry region. Groundwater samples were collected in and around Puducherry region (Fig. 2) and analyzed using standard procedures (APHA 2005). Bore wells were pumped out for 10–15 min to remove the stagnated water. Prior to sampling, polyethylene bottles were acid washed and rinsed with water to be sampled. The hydrogen ion concentrations (pH), electrical conductivity (EC), total dissolved solids (TDS), and salinity were measured in situ condition using Hanna portable water analyzer. Ions such as sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), sulphate (SO4 2−), and bromide (Br−) were analyzed using ion chromatography (ICS DIONEX 1100) and bicarbonate using volumetric titration (sulphuric acid method). The ionic balance error was found to be ±10%. Locations of sampling sites were identified using Garmin GPS device. The analytical results of hydrochemistry are tabulated in Table 2.
2.4 Hydrochemical Facies Evolution during Freshening and Intrusion Process
There are several types of graphical representation to understand hydrochemical characteristics and facies of water system. To understand freshening and intrusion phases than happens for a period of time in coastal aquifer, Hydrochemical Facies Evolution Diagram has been proposed by Giménez -Forcada (2010). Based on the distribution of anion and cation percentages, the process which interrupt in coastal aquifer and the stages involved during ingress/regress of sea water into the coastal aquifers can be understood. In the HFE-diagram, the abscissae indicate evolution of %Na+ + %K+ and %Ca2+ + %Mg2+ in meq/L and ordinates indicate evolution of %Cl−, %HCO3 − and %SO4 2−. As freshening and intrusion in coastal aquifers are more complex, some considerations are made, which includes ignorance of ions such as Mg2+, K+, and SO4 2− because they occupy secondary position in freshwater and groundwater. As the concentration of K+ is lesser, it is incorporated/substituted by Na+, and hence, Na+ is added with K+. The concentration of bicarbonate and sulphate ions is not added up, because both the ions are present in larger concentration in freshwater.
In HFE-diagram, based on the percentage of ions, there are four major heterotopic facies, namely Ca–HCO3 (freshwater), Ca–Cl (salinizing water indicating reverse cation exchange reaction), Na–HCO3 (salinized water undergoing direct cation exchange reactions), and Na–Cl (seawater). The two major phases are freshening (FW) and intrusion (SW). When seawater ingress takes place in the coastal aquifers, hydrochemical facies evolve from Ca–HCO3 facies to Na–Cl facies via intermediate Ca–Cl facies and other sub-facies characterizing the intrusion. This intrusion phase is associated with reverse cation exchange process stimulated by sodium rich seawater. When aquifer is recharged by freshwater, the hydrochemical facies evolves from facies Na–Cl to Ca–HCO3 via intermediate facies Na-HCO3, and this phase is said to be Freshening phase. Freshening phase is associated with direct cation exchange stimulated by infiltration of freshwater through rainfall. The line which separates freshening and intrusion phase is designated as conservative mixing line. Freshening phase lies above the conservative mixing line, whereas intrusion phase lie below conservative mixing line.
Groundwater samples are grouped into particular hydrochemical facies based on highest percentage (greater than 50%) of both cation and anion. In addition, based on the above consideration, each of nine major heterotopic facies, viz., Ca-HCO3, Ca–SO4, Ca–Cl, Mg–HCO3, Mg–SO4, Mg–Cl, Na–HCO3, Na–SO4, and Na–Cl facies, are further classified into four isotopic facies. For example, Ca-HCO3 encompasses four isotopic facies such as MixCa–MixHCO3, Ca–mixHCO3, MixCa–HCO3, and Ca–HCO3. If the ion does not exceed 50%, such type of facies is defined with prefix ‘mix’. Freshening and intrusion fields of HFE-diagram have been classified into sub-stages for freshening and are designated as f1, f2, f3, and f4, and FW and intrusion sub-stages are designated as i1, i2, i3, i4, and SW.
During the freshening phase, the salinized aquifer gets freshened from inland towards coast by recharge. This freshening process continues until there exist equilibrium between salinized Na–Cl water and recharging freshwater. The initial sub-stage f1 is characterized by Mix Na–Cl facies followed by Mix Na–Mix Cl facies as f2 sub-stage, Mix Ca–Mix HCO3 and Ca–Mix HCO3 as f3 sub-stage, and Ca-HCO3 as f4 sub-stage. Ultimate freshening phase is characterized by heterotopic facies Ca-HCO3. Freshening hydrochemical facies evolution series indicates direct cation exchange process. An aquifer which holds completely freshwater (Ca-HCO3) is salinized by intrusion of seawater through sub-stages from i1—Ca–MixHCO3 and then to sub-stage i2—MixCa–Mix Cl, Ca–Mix Cl followed by sub-stage i3—Mix Na–Cl, Mix Ca–Cl, Ca–Cl, and sub-stage i4—Mix Na–Cl. Finally, complete salinization is characterized by facies Na–Cl which indicates reverse cation exchange process. Each sub-stages are quantified assigning values from 1(lowest value to freshwater) to 10 (highest value to saline water). Values from 2 to 5 indicate sub-stages of freshening phase f1, f2, f3, and f4, respectively. Similarly, values from 6 to 9 indicate sub-stages of intrusion phase i1, i2, i3, and i4. Value 5.5 indicates boundary separating freshening and intrusion phases.
2.5 Ionic Changes
The groundwater along the coastal aquifers often shows fluctuations in its chemistry due to mixing of fresh groundwater and saline seawater (Mondal et al. 2010a, b; Giménez-Forcada 2014; Pulido-Lebeuf 2004; Appelo and Postma 2005). Such fluctuations can be calculated using hydrochemical ionic changes (∆) by comparing theoretical conservative mixing values of freshwater and seawater with measured concentration of ions in the sampled groundwater as follows:
where ∆C i is the changes in concentration of ion i, C i sample is the measured concentration of ion i in the collected sample, and C i mixed is the theoretical concentration of ion in the mixing zone of freshwater and groundwater. The theoretical mixing concentrations of ions are determined by considering seawater contribution based on concentration of chloride ion in freshwater, seawater, and sample. Following is the expression which aids us to determine the contribution of seawater, i.e., seawater fraction in groundwater of coastal aquifers:
where f sea is the seawater contribution and C cl, sample, C cl, fresh, and Ccl, sea are the concentration of chloride ions in sample, freshwater, and seawater, respectively. Using Eqs. 1 and 2, the theoretical concentration of individual ion in the groundwater samples is determined as follows:
While determining the theoretical concentration of ions and seawater fraction in groundwater, Cl− ion is considered as a conservative tracer element as its concentration remains stable for longer time and also its high solubility nature. The sources for Cl− ion in groundwater may be either seawater ingress or aquifer materials or both. Here, the concentrations of ions are expressed in meq/L.
3 Results and Discussion
3.1 Ionic Ratios
For comprehensive study of seawater ingress along the coastal aquifers, ionic ratios are widely used (Batayneh et al. 2014; Barbecot et al. 2000; El Moujabber et al. 2006; Kim et al. 2003; Sanchez Martos et al. 1999). Apart from chemistry of groundwater, mineralogy of rocks can also be utilised to some extent (Nwankwoala and Udom 2011). In the current study, ionic ratios such as HCO3 2−/Cl−, Na+/Cl−, Ca2+/Cl−, Ca2+/Na+, Mg2+/Cl−, K+/Cl−, and SO2− 4/Cl− were calculated and plotted. Total dissolved solids (TDS) are very good proxy for chloride (Fig. 3c) which can be very well used to study ingress of seawater (Batayneh et al. 2014; Nwankwoala and Udom 2011; Lee and Song 2006). The ratio of HCO3 2−/Cl− in almost all the samples analyzed was greater than that of seawater ratio 0.0069. However, this ratio of samples is gradually approaching seawater ratio with increasing TDS level (Fig. 3b) indicating intrusion of seawater. Ca2+/Na+ ratio shows similar trend as HCO3 2−/Cl−, i.e., ratio shows decreasing trend with increasing TDS (Fig. 3d). It indicates that high TDS is associated with high Cl− and higher Na+ concentration (Saxena et al. 2003). Na+/Cl− ratio does not show any correlation with TDS indicating co-existence of both the ions during event of intrusion phase (Fig. 3e; Batayneh et al. 2014). Similar to HCO3 2−/Cl− ratio, other ionic ratios such as Ca2+/Cl−, Mg2+/Cl−, K+/Cl−, and SO2− 4/Cl− show negative trend indicating enhancement in concentration of chloride, i.e., increase in TDS with decrease in ratio (Fig. 3f–i).
The plot Na+ +K+ vs. TDS shows very good positive correlation in pre-monsoon (PRM) when compared to post-monsoon (POM) indicating signature of salinization of groundwater (Fig. 3j; Saxena et al. 2003). Ca2+ + Mg 2+ vs. TDS exhibits moderate correlation in post-monsoon, whereas in pre-monsoon, it does not show any relationship (Fig. 3k). This may be due to recharge of groundwater during post-monsoon by which groundwater gets freshened.
Groundwater may acquire salinity by various means like agriculture, industries, salt panning, improper sewage disposal, domestic wastewater, ocean marine sediments, seawater intrusion, etc. Being the most conservative element, bromide (Br) and Chloride (Cl) ions are used widely as a tracer for seawater intrusion in coastal aquifers and to distinguish other modes of salinization from seawater intrusion (Fig. 3a; Table 1; Morris and Riley 1966; McCaffrey et al. 1987; Davis et al. 1998; Nair et al. 2015). These two ions do not take part in redox or adsorption–desorption reaction. The Standard Mean Ocean Water (SMOW) value of Cl/Br ratio ranges from 288 to 292. The locations having Cl/Br ratio falling closer to the SMOW line in Fig. 3a are affected by seawater intrusion. If Cl/Br ratio is more than SMOW value, groundwater salinity is considered to be influenced by domestic waste water and below SMOW value suggests the influence of agricultural waste. In the study area, 23.2 and 16.4% of water samples show signature of intrusion during pre- and post-monsoon, respectively.
3.2 Hydrochemical Facies Evolution for identifying Seawater Intrusion Process
To evaluate the series of hydrochemical facies changes during intrusion and freshening processes, Hydrochemical Facies Evolution-Diagram (HFE-D) can be used to extract detailed information (Giménez -Forcada 2010).
In HFE-diagram (Fig. 4a, b), a field below the conservative mixing line (Intrusion phase) shows increase in salinity with gradual exchange of Ca by Na. Evolution of hydrochemical facies starts from Ca–HCO3 facies (freshwater) to Na–Cl (seawater) facies via characteristic Ca–Cl (intermediate salinizing) facies (Giménez-Forcada 2010). Here, the exchange of Na by Ca indicates occurrence of reverse cation exchange reaction. Ca–Mix HCO3/Mix SO4, Mix Ca–Mix Cl, Mix Ca–Cl, and Mix Na–Cl are the hydrochemical facies of evolution during pre-monsoon. The evolution of hydrochemical facies in post-monsoon is Mix Ca–Cl, Ca–Cl, and Mix Na–Cl. About 24.1% of water in pre-monsoon and 18.8% of water samples in post-monsoon falls under intrusion field indicating reverse cation exchange process (Table 3) (Appelo and Postma 2005).
In freshening stage, above conservative mixing line in HFE-diagram (Fig. 4a, b), there will be freshening of groundwater slowly from Na–Cl facies to Ca-HCO3 facies (Giménez-Forcada 2010). Na–Mix Cl, Na–Mix HCO3/Mix SO4, Mix Na–Mix HCO3/Mix SO4, Na–HCO3/SO4, Mix Na–HCO3/SO4, and Mix Ca–HCO3/SO4 are the facies achieved during pre-monsoon. Na–Mix Cl, Na–Mix HCO3/Mix SO4, and Na–HCO3/SO4 is the dominant facies in post-monsoon. About 75.9 and 76.4% of samples fall under freshening field indicating replacement of Na by Ca indicating direct cation exchange process in pre- and post-monsoon, respectively. Samples of both pre- and post-monsoon which fall along the conservative mixing line depict that there is no base exchange reactions; instead, there occurs mixing of freshwater and seawater (Table 4) (Fig. 4a, b; Giménez-Forcada 2010).
Freshening and intrusion events of the study area during pre- and post-monsoon are brought to focus by heat maps using Arc-GIS software. Kriging interpolation method was used in Arc-GIS tool to prepare spatial distribution maps (Fig. 5a, b). Kriging method is an interpolation technique which weighs values of neighbor location and anticipates value for location where measurement is not done. Figure 5a, b shows the spatial distribution maps for seawater intrusion at Puducherry region during pre- and post-monsoon.
Spatial distribution heat map (Fig. 5b) displays the progression of seawater from sub-stage i4 (navy blue) to i1 (olive green) via sub-stages i3 (sky blue); i2 (bluish green) during pre-monsoon, but there is a small patch of area with less intense intrusion. It is due to continuous fresh water recharge by major River Sankarabarani in the study area where several check dams lie across the river and vegetative cover with less dense concrete structures facilitates the infiltration. During the post-monsoon, there is a significant regression of seawater due to precipitation and recharge, especially in the southern part of study area, while in northern and eastern part, it shows constant influx of seawater. Highly developed city, lesser recharge, industrialization, overpumping, etc., are the major factors responsible for intrusion. Though there is a significant decrease in area of intrusion in southern part during post-monsoon, complete freshening of groundwater is lacking. In addition, the groundwater quality of this region is still identified to be influenced by ingress of seawater. Along the northwestern part of the study area, we can find hydrochemical facies showing signature of seawater intrusion but are actually due to cretaceous marine formations namely Ottai claystone and Turuvai formation. Apart from it, some chemical industries release their waste products and other effluents into the ground without treatment at Sedarapet, Karasoor, Tondamanatam, Madagadipet, etc. Apart from it, garbage dumping sites such as nearer to locations Lawspet and Perambai also shows deterioration in quality of groundwater.
3.3 Hydrochemical Ionic Changes
When freshwater (groundwater) and saline water (seawater) interact each other, there occur certain chemical reactions. Such chemical reactions can be figured out explicitly by comparing theoretical mixing values of freshwater and seawater with concentration of ions determined from collected water samples. Based on the expressions 1, 2, and 3 (given in methodology), the fraction of seawater in water samples was estimated. Ionic changes were calculated for ions such as Na+, Ca2+, Mg2+, K+, HCO3 −, and SO4 2− (Table 5). The average mixing rate of freshwater and seawater during pre-monsoon is 24.2%, whereas it is 13.5% in post-monsoon. The ionic change values clearly display the hydrochemical process that takes place along the seawater and freshwater mixing zone in coastal aquifers (Mondal et al. 2010a, 2010b). The hydrochemistry of groundwater along such coastal aquifers is very much intricate to study and it varies with respect to space and time (Mondal et al. 2010a).
Figures 6a–g, and 7a–g show calculated ionic change values for ions Na+, Ca2+, Mg2+, K+, HCO3 −, and SO4 2− during pre- and post-monsoon. Among all the ions, sodium and calcium show a distinct pattern when compared to other ions (Figs. 6a, b, 7a, b). Such pattern is due to infuse of seawater into freshwater. Usually, the concentration of Na+ in seawater and Ca2+ in freshwater is higher and so ionic change value is positive for sodium in groundwater and negative in seawater (Mondal et al. 2010a, b). However, some locations show negative Na+ ionic exchange values for both pre- and post-monsoon. Such negative values indicate ingress of seawater. Higher sodium concentration denotes direct cation exchange process along mixing zone.
Fig. 6a, b Ionic changes plot of Na+ and Ca2+ groundwater samples during pre-monsoon (PRM). c–g Ionic changes plot of K+, Mg2+, Cl−, and SO4 2− groundwater samples during pre-monsoon (PRM). Fig. 7a, b Ionic changes plot of Na+ and Ca2+ groundwater samples during post-monsoon (POM). c–g Ionic changes plot of K+, Mg2+, Cl−, and SO4 2− groundwater samples during post-monsoon (POM)
In general, the freshwater possesses higher concentration of calcium and bicarbonates due to dissolution of carbonates along the coastal aquifer (Mondal et al. 2010a). The dominant ions constituting seawater are Na+ and Cl− ions and the coastal aquifers showing signature of seawater ingress have Na+ ions in aquifer materials (Appelo and Postma 2005). Seawater intrusion can be understood more clearly by the relationship between Na+ and Ca2+ (Figs. 6a, b, 7a, b). The negative ionic exchange values of Na+, K+, and positive ionic exchange values of Mg2+ in samples of study area indicate seawater intrusion (Figs. 6a, c d, 7a, b, d; Mondal et al. 2010a). The ionic exchange values of sodium and potassium are highly negative and sulphate is highly positive (Figs. 6g, 7g; Mondal et al. 2010a) indicating seawater intrusion along the coast of Puducherry region.
4 Conclusion
Seawater intrusion is a major problem that exists along coastal aquifers. Puducherry is one of the coastal cities situated in the Southern East Coast of India. In the present investigation, hydrochemical techniques have been used to study seawater intrusion in coastal aquifers of Puducherry region. The groundwater samples were collected and analyzed to understand the evolution of hydrochemical facies and ion exchange in response to seawater intrusion.
Ionic ratios are excellent tool to demarcate the areas affected by seawater intrusion. The relationship between TDS and ionic ratios, viz., HCO3 2−/Cl−, Na+/Cl−, Ca2+/Cl−, Ca2+/Na+, Mg2+/Cl−, K+/Cl−, SO2− 4/Cl−, and Cl−/Br−, showed the signature of seawater influx along the coastal aquifers of study area.
HFE-diagram reveals that 24.1 and 18.8% of samples were affected by seawater intrusion in pre- and post-monsoon. Na–HCO3/SO4, Na–mixhco3/MixSO4, MixNa–HCO3/MixSO4, and MixNa–MixHCO3/Mixso4 are the major facies indicating direct cations exchange process in pre-monsoon. Na–Cl, Mix Na–Cl, and Mix Ca–Cl facies are dominant facies indicating reverse ion exchange process during post-monsoon.
Heat maps generated from HFE-diagram display that there is continuous intrusion of seawater in northern and southern parts of study area both in pre- and post-monsoon. It is also identified that locations such as Ariyankuppam, Kariambattur, Muthialpet, Kalapet, and Parikalpet are intensely affected by seawater ingress. Because of urbanization, industrialization, overpumping, and lack of infiltration, the northeastern and eastern parts of the study area are intensely affected by seawater intrusion. However, along the coastal area, there are patchy zones which do not fall under intrusion facies due to freshening by river and check dams constructed across it as well as vegetal cover. Apart from coastal zones, there are locations at inland which fall under intrusion facies due to the aquifers containing sediments of marine origin, improper disposal of industrial and hospital wastes, and improper maintenance of garbage dumping sites.
Hydrochemical ionic exchange study reveals that the average mixing rate of seawater intrusion is 24.2 and 13.5% in both pre and post-monsoon. The ionic exchange values of sodium in groundwater samples were found to be highly negative in pre-monsoon when compared to post-monsoon. Similarly, negative ionic exchange values of potassium and positive ionic exchange values of magnesium and sulphate imply intrusion of seawater. Overall, the study area is found to be vulnerable to seawater ingression. To avoid major threat to life forms and other activities, regular monitoring of groundwater remedial measures is essential. Remedial measures include rainwater harvesting, afforestation, reduction in pumping, proper monitoring of waste disposal, modern agricultural practices, i.e., high productivity with less water usage, and most importantly individual awareness about the valuable asset “WATER”.
References
Amiri V, Nakhaei M, Lak R, Kholghi M (2015) Assessment of seasonal groundwater quality and potential saltwater intrusion: a study case in Urmia coastal aquifer (NW Iran) using the groundwater quality index (GQI) and hydrochemical facies evolution diagram (HFE-D). Stoch Environ Res Risk Assess 30(5):1473–1484. doi:10.1007/s00477-015-1108-3
Anders R, Mendez GO, Futa K, Danskin WR (2013) A Geochemical approach to determine sources and movement of saline groundwater in a coastal aquifer. Groundwater 52(5):756–768. doi:10.1111/gwat.12108
APHA (2005) Standards methods for the examination of water and wastewater, 21st edn. APHA, Washington DC
Appelo C, Postma D (2005) Geochemistry, groundwater and pollution, Second Edn. doi:10.1201/9781439833544
Ayolabi EA, Folorunso AF, Odukoya AM, Adeniran AE (2013) Mapping saline water intrusion into the coastal aquifer with geophysical and geochemical techniques: the University of Lagos campus case (Nigeria). Springer Plus 2(1):433. doi:10.1186/2193-1801-2-433
Barbecot F, Marlin C, Gibert E, Dever L (2000) Hydrochemical and isotopic characterisation of the Bathonian and Bajocian coastal aquifer of the Caen area (northern France). Appl Geochem 15(6):791–805. doi:10.1016/s0883-2927(99)00088-8
Batayneh A, Zaman H, Zumlot T, Ghrefat H, Mogren S, Nazzal Y, Al-Taani A et al (2014) Hydrochemical facies and ionic ratios of the coastal groundwater aquifer of Saudi Gulf of Aqaba: implication for seawater intrusion. J Coastal Res 293:75–87. doi:10.2112/jcoastres-d-13-00021.1
Central Ground Water Board (CGWB) (2007) Groundwater brochure of Puducherry region U.T of Puducherry, pp 1–27. http://www.cgwb.gov.in/District_Profile/Puduchery/Puducherry.pdf
Chen KP, Jiao JJ (2007) Seawater intrusion and aquifer freshening near reclaimed coastal area of Shenzhen. Water Sci Technol Water Suppl 7(2):137. doi:10.2166/ws.2007.048
Christina G, Konstantinos S, Alexandros G, Dimitrios K, Aikaterini K (2014) Seawater intrusion and nitrate pollution in coastal aquifer of almyros–Nea Anchialos Basin, Central Greece
Davis SN, Whittemore DO, Fabryka-Martin J (1998) Uses of chloride/bromide ratios in studies of potable water. Ground Water 36(2):338–350. doi:10.1111/j.1745-6584.1998.tb01099.x
Giménez -Forcada E (2010) Dynamic of sea water interface using hydrochemical facies evolution diagram. Ground Water 48(2):212–216. doi:10.1111/j.1745-6584.2009.00649.x
Giménez-Forcada E (2014) Space/time development of seawater intrusion: a study case in Vinaroz coastal plain (Eastern Spain) using HFE-diagram, and spatial distribution of hydrochemical facies. J Hydrol 517:617–627. doi:10.1016/j.jhydrol.2014.05.056
Gurumoorthy C, Sasidhar P, Arunugham V, Nathur RK (2004) Sub-surface investigations on deep saline ground water of charnokite rock formation, Kalpakkam, India. Environ Monit Assess 91:211–22
Kim J, Kim R, Chang H (2003) Hydrogeochemical characterization of major factors affecting the quality of shallow groundwater in the coastal area at Kimje in South Korea. Environ Geol 44(4):478–489
Kim K-Y, Park Y-S, Kim G-P, Park K-H (2008) Dynamic freshwater–saline water interaction in the coastal zone of Jeju Island, South Korea. Hydrogeol J 17(3):617–629. doi:10.1007/s10040-008-0372-4
Lee J-Y, Song S-H (2006) Evaluation of groundwater quality in coastal areas: implications for sustainable agriculture. Environ Geol 52(7):1231–1242. doi:10.1007/s00254-006-0560-2
Longe EO, Malomo S, Olorunniwo MA (1987) Hydrogeology of Lagos metropolis. J African Earth Sci (1983) 6(2):163–174. doi:10.1016/0899-5362(87)90058-3
McCaffrey MA, Lazar B, Ho HD (1987) The evaporation path of seawater and the coprecipitation of Br− and K+ with Halite. SEPM JSR. doi:10.1306/212f8cab-2b24-11d7-8648000102c1865d
Mondal NC, Singh VS, Saxena VK, Prasad RK (2007) Improvement of groundwater quality due to fresh water ingress in Potharlanka Island, Krishna delta, India. Environ Geol 55(3):595–603. doi:10.1007/s00254-007-1010-5
Mondal NC, Singh VP, Singh VS, Saxena VK (2010a) Determining the interaction between groundwater and saline water through groundwater major ions chemistry. J Hydrol 388(1–2):100–111. doi:10.1016/j.jhydrol.2010.04.032
Mondal NC, Singh VS, Saxena VK, Singh VP (2010b) Assessment of seawater impact using major hydrochemical ions: a case study from Sadras, Tamilnadu, India. Environ Monit Assess 177(1–4):315–335. doi:10.1007/s10661-010-1636-8
Morris AW, Riley JP (1966) The bromide/chlorinity and sulphate/chlorinity ratio in sea water. Deep Sea Res Oceanogr Abstr 13(4):699–705. doi:10.1016/0011-7471(66)90601-2
Moujabber ME, Samra BB, Darwish T, Atallah T (2006) Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese Coast. Water Resour Manage 20(2):161–180. doi:10.1007/s11269-006-7376-4
Nair IS, Rajaveni SP, Schneider M, Elango L (2015) Geochemical and isotopic signatures for the identification of seawater intrusion in an alluvial aquifer. J Earth Syst Sci 124(6):1281–1291. doi:10.1007/s12040-015-0600-y
Nwankwoala H, Udom G (2011) Hydrochemical faciès and ionic ratios of groundwater in Port Harcourt, Southern Nigeria. Res J Chem Sci 1(3):87–101
Pulido-Lebeuf P (2004) Seawater intrusion and associated processes in a small coastal complex aquifer (Castell de Ferro, Spain). Appl Geochem 19:1517–1527
Sanchez Martos F, Bosch AP, Calaforra J (1999) Hydrogeochemical processes in an arid region of Europe (Almeria, SE Spain). Appl Geochem 14(6):735–745. doi:10.1016/s0883-2927(98)00094-8
Saxena VK, Singh VS, Mondal NC, Jain SC (2003) Use of hydrochemical parameters for the identification of fresh groundwater resources, Potharlanka Island, India. Environ Geol 44(5):516–521. doi:10.1007/s00254-003-0807-0
Thilagavathi R, Chidambaram S, Prasanna MV, Thivya C, Singaraja C (2012) A study on groundwater geochemistry and water quality in layered aquifers system of pondicherry region, southeast India. Appl Water Sci 2(4):253–269. doi:10.1007/s13201-012-0045-2
Todd DK (1953) Sea-water intrusion in coastal aquifers. Trans Am Geophys Union 34(5):749. doi:10.1029/tr034i005p00749
Acknowledgements
The current work has been supported financially by University Grants Commission, New Delhi [F.No. 41-1035/2012 (SR); Date: 23.07.2012] through major research project. We extend our sincere thanks to Department of Earth Sciences, Department of Ecology and Environmental Sciences and Central Instrumentation Facility of Pondicherry University for providing facilities for analysis. We also extend our thanks to the editor and anonymous reviewer for their valuable suggestions for bringing this research paper to the present level.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sridharan, M., Senthil Nathan, D. Hydrochemical Facies and Ionic Exchange in Coastal Aquifers of Puducherry Region, India: Implications for Seawater Intrusion. Earth Syst Environ 1, 5 (2017). https://doi.org/10.1007/s41748-017-0006-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41748-017-0006-x