Abstract
Organochlorine pesticides (OCPs) are pesticides with global scale ubiquity, persistence and bioaccumulation, which leave long-term residuals in the water body. OCPs’ high toxicity poses significant threats to human health and aquatic biodiversity, making assessment of OCPs’ impact on aquatic ecology and human health urgently necessary. In this research, the presence of 16 OCPs in surface water and groundwater along Shaying River, China, as well as OCPs concentration correlations, was investigated at 24 selected sampling sites. At the same time, the ecological risk and human carcinogenic risk were also analyzed by risk quotient method and USEPA’s Risk Assessment Guidance, respectively. Results showed that the total concentration of OCPs ranged from 21.0 to 61.4 ng L−1 in groundwater, and 12.3–77.5 ng L−1 in surface water. Hexachlorocyclohexane (HCHs) and heptachlor were the prominent contaminants in groundwater, which indicated their use in the recent past and confirmed their persistence. The α-HCH/γ-HCH ratios in groundwater confirmed that γ-HCH (lindane) was used as main substitute of technical HCH in the study area. The correlation analysis illustrated that δ-HCH and γ-HCH played a dominant role in HCHs residue. Heptachlor and α-HCH, as well as endosulfan and heptachlor epoxide, had a strongly significant positive correlation, suggesting an associated usage of the two pair OCPs. An extremely high ecological risk for aquatic organism was observed for γ-HCH, heptachlor and dieldrin, while the carcinogenic risks posed by the selected OCPs in surface water and groundwater were all acceptable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent organic pollutants (POPs) are ubiquitous, toxic, anthropogenic substances that have been widely used in agriculture and manufacturing industries since the 1930s (Buah-Kwofie and Humphries 2017). Organochlorine pesticides (OCPs), as one of the widely used POPs with persistence and bioaccumulation, left long-term residuals in the water body at global scale (Jayaraj et al. 2016). Five OCPs were firstly recorded in Stockholm Convention, saying dichlorodiphenyltrichloroethane (DDT), aldrin, dieldrin, toxaphene and HCH, which were also banded by Chinese government in 1983. Technical HCHs, as the most extensively concerned OCPs compounds, were produced and used in China from 1952 to 1983. Some other OCPs’ production and usage begins from the 1990s, like lindane and endosulfan, which may redistribute and change the composition of OCPs residuals in the environment. OCPs may reach the surface riverine water via agricultural runoff, direct applications, spray drift, aerial spraying and erosion (Chakraborty et al. 2016). The chemical properties of OCPs, such as low water solubility, stability to photo-oxidation and low vapor pressure, are the main reasons for their persistence in the environment.
Shaying River, the largest tributary of the Huaihe River, contributed large amounts of, was the main source of industry pollutants along the Huaihe River Basin, China. At the same time, rural area and farmland took over 80% of total basin area, on which pesticide applications were rising under increasing demand for grain production. From 1990 to 2008, the average pesticide application on farm land of Shaying River Basin increased from 7.43 to 22.63 kg km−2, although the efficient proportion on crop disease prevention is barely above 30% (Guzzella et al. 2006; Kolpin et al. 1998; Papastergiou and Papadopoulou-Mourkidou 2001). Investigation shows that most of the drinking and sanitary water were taken from shallow aquifer (over 90% well with depth above 15 m) in the rural area of Shaying River Basin (https://www.boxun.com), which was under severe safety risk from agriculture organic chemicals (Lacorte and Barcelo 1996). The problem of high cancer incidence in some parts of Shaying River Basin is very prominent, with up to 300/100,000 in some villages, which are mostly located along the Shaying River (Chen et al. 2016). A certain relationship between high incidence rate of cancer and water environment pollution in this area was reported (Gonghuan and Zhong 2013).
The available researches paid more attention to OCPs residues in soil (Chakraborty et al. 2015; Yu et al. 2014), surface water and the sediment (Agarwal et al. 1986; Turgut 2003). For example, it was reported that the levels of OCPs range from 1.01 to 791.0 ng L−1 in the surface water of the Yangtze River (Tang et al. 2008), Guanting Reservoir, China (Xue et al. 2006), and Gomti River, India (Malik et al. 2008). The OCPs concentration in sediment was reported as 0.4–813.6 ng g−1 in Guanting Reservoir (Xue et al. 2006), Huangpu River (Hu et al. 2005), Huanghe River, China (Sun et al. 2007), and Gomti River, India (Malik et al. 2008). Riverine runoff was reported as the major mode carrying OCPs from the River Basin (Guan et al. 2009). While the OCPs residues in groundwater, as well as the correlation between OCPs concentration in surface water and groundwater were neglected. OCPs’ high toxicity poses significant threats to human health and aquatic biodiversity (Qu et al. 2015), while there is no information on the ecological risk associated with the presence of pesticides including OCPs in various environmental compartments in Shaying River Basin, neither are there data available on the human carcinogenic risk associated with the consumption of pesticide-contaminated water in this area. Therefore, assessment of OCPs’ impact on aquatic ecology and human health in this area is urgently necessary.
The objective of this paper was to study the occurrence, sources, possible migration evidence and risk of OCPs in the aquatic environment of Shaying River Basin. The residue concentrations and distribution pattern of 16 OCPs were firstly investigated in this area, and the correlations of individual OCPs component in groundwater and surface water were analyzed. Besides, ecological and carcinogenic risks posted by OCPs were assessed based on a modified method, in order to depict the impact of these pesticide residues on aquatic ecology and human health in this area.
Materials and methods
Site description and sampling
Shaying River Basin is one of the largest tributary catchments of Huaihe River Basin, plain area of which is about 25,600 km2. Climate of this area is continental with an annual average temperature of 14–16 °C and annual average rainfall of 769.5 mm mostly distributed in high flow period (June to August). The main soil type of Shaying River Basin is cinnamon soil and lime concretion black soil, and main land use type is farmland, accounting for 87% of the total area.
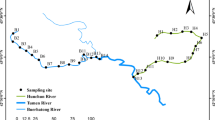
Sampling sites along Shaying River were selected equably to cover the research area. Fourteen surface water samples and 10 groundwater samples (0.5 m below water surface) were collected with pre-cleaned 1-L dark glass bottles using cylinder samplers in November 2013 (Fig. 1). All samples were placed in icebox after sampling and transported to the laboratory immediately for later analysis.
Used chemicals
The standard OCPs including α-HCH, β-HCH, γ-HCH, δ-HCH, p,p′-DDT, o,p′-DDT, p,p′-DDE, p,p′-DDD, aldrin, heptachlor, endosulfan I, endosulfan II, methoxychlor, dieldrin, endrin, heptachlor epoxide and internal standards (pentachloronitrobenzene) were purchased from Accustandard (USA). The solvent (petroleum ether) and concentrated sulfuric acid used for sample processing and analysis were analytical grade, and the former was re-distilled in a full-glass distilling appliance. Anhydrous sodium sulfate (analytical grade) was baked in a furnace oven at 500 °C for 8 h and kept in sealed desiccators prior to use.
Sample pretreatment
The pretreatment experimental process referred to gas chromatography determination method of HCHs and DDTs in Chinese water quality standard (China 1987). The sample pretreatment process was as follows. 0.6 L of water samples was taken for oscillation in 80 mL of petroleum ether for 10 min under liquid–liquid extraction method. Then, organic phase samples were purified with 10 mL liquid sulfuric acid and with 2% sodium sulfate solution acid washing. After liquid anhydrous, the organic phase was dehydrated with sodium sulfate (drying under 500 °C for 8 h) and then concentrated to 1 mL for later gas chromatography test.
OCPs quantification and quality control
An Agilent 7890 GC equipped with a micro-cell electron capture detector (μECD) was used for OCPs quantification. The capillary column used was HP-5MS (30 cm × 0.32 mm × 0.25 μm). The carrier gases were nitrogen for μECD at a flow of 2.0 mL min−1 under the constant flow mode. The inlet was heated to 250 °C, and the nitrogen purge time was 0.75 min. The GC column temperature was programmed as follows: initially at 80 °C (equilibrium time 2 min), increased to 180 °C at the rate of 15 °C min−1 and held for 2 min, before reaching at 280 °C at the rate of 5 °C min−1 and then held for 2 min. The temperature of μECD was held at 300 °C.
The linear correlation coefficient of standard calibration curves for target OCPs was all above 0.999, with a relatively high repeatability. The relative standard deviation (RSD) was between 3 and 20%, among which larger ones only appear in low-concentration samples (Guan et al. 2009).
All analytical operations were conducted under strict quality control guidelines. Procedural blanks and spiked samples with standard were used to monitor procedural performance and matrix effects. All experiments were carried out in duplicate. The instrument detection limits (IDLs) of all OCPs ranged between 0.062 and 0.858 ng mL−1, and the method detection limits (MDLs) ranged from 0.10 to 1.43 ng L−1. The surrogate recovery in this method was 89–97%. Values for OCPs lower than the method detection limits (< MDL) were substituted with zero prior to statistical analysis.
Statistical analysis
The Pearson correlation test (p < 0.01) of different OCP components in surface water and groundwater of Shaying River Basin, and the principal component analysis (PCA) of OCPs in surface water were conducted using the software SPSS version 21. ArcGIS version 10.2 was used for spatial distribution analysis and graph making.
Ecological risk assessment
The ecological risk of OCPs to aquatic organisms was assessed using the risk quotient (RQ) method (WHO 2001), which was performed by calculation of RQ for the detected OCPs.
where C is the measured concentration and PNEC is the predicted no-effect concentrations for a particular OCP, respectively. The PNEC data were obtained from ECOTOX database (http://www.epa.gov/ecotox) and some articles (Hu et al. 2015; Ogbeide et al. 2015; Vryzas et al. 2011), where the value was calculated by multiplying the laboratory toxicology data with an assessment factor (AF) which takes into account the uncertainty between laboratory toxicity tests with real environment. The classification of ecological risks was different according to various assessment standards and management purposes. Generally, RQ above 1 represents that the environmental concentration is higher than predicted no-effect concentrations. Subsequently, the ecological risks were classified into three levels based on RQ value in the prior literature: 0.01–0.1 represents relatively low risk, 0.1–1 represents a medium risk, and above 1 indicates high risk (Peng et al. 2014).
Carcinogenic risk assessment
The United States Environment Protection Agency (USEPA) recommended that toxicity data and exposure data are combined to assess chemical’s carcinogenic risk (USEPA 1989). In the present study, human carcinogenic risks associated with OCPs exposure were based on the approach from USEPA’s Risk Assessment Guidance for Superfund (RAGS) methodology (USEPA 1997). The potential exposure to OCPs was calculated by two exposure routes, ingestion and dermal contact, which represent water exposure for drinking and bathing, as described in the USEPA screening-level equations for preliminary remediation goals (USEPA 2010). For different exposure pathways, the increasing trend in cancer risk for OCPs is determined as follows: inhalation < dermal contact < ingestion, thus comparably, inhalation of OCP particulates is almost negligible (Qu et al. 2015) and is not taken in this research. Total carcinogenic risk (TLCR) was obtained by summing individual risks calculated for the two exposure routes.
In these equations, C is the detected concentrations of OCPs in the water (mg L−1); IR is the average daily ingestion of water (2 L day−1); CF is the conversion coefficient (1 × 10−6 kg mg−1); EF is the exposure frequency (365 day year−1); ED is the exposure duration (30 year); BW is the body weight (60 kg for Chinese people); AT is the average lifespan (70 years × 365 days year−1); TE is the contact time (0.4 h); τ is lag time for each OCP in the body (1 h); k is a skin permeability parameter (0.001 cm h−1); Asb is body surface area (16,600 cm2); FE is bathing frequency (0.3 times day−1); and f is the intestinal absorption ratio (1) (Schriks et al. 2010, Wei et al. 2015, WHO 2001).
In some studies, the carcinogenic risk was considered non-ignorable if the TLCR is above 1.0 × 10−6, and higher than 1 × 10−5 indicated a ‘not negligible’ risk (Butler-Jones 2010, Li et al. 2015a). USEPA (USEPA 1989) takes the range from 1.0 × 10−4 to 1.0 × 10−7 as acceptable carcinogenic risk. Qu et al. evaluated TLCR in five levels: Values lower than 10−6 represent very low risk; values between 10−6 and 10−4 represent low risk; values between 10−4 and 10−3 indicate moderate risk; values between 10−3 and 10−1 indicate high risk; and values higher than 10−1 represent very high risk (Qu et al. 2015). Generally, by integrating the above risk-rating standards, we took 1 × 10−6 as threshold value of potential carcinogenic risks for humans in this research.
Results and discussion
Residues of 16 OCPs were detected in surface water and groundwater along Jialu River and Shaying River (Table 1). The concentration of ∑OCPs ranged from 21.0 to 61.4 ng L−1 in groundwater, and from 12.3 to 77.5 ng L−1 in surface water, with the mean concentration of 44.6 and 30.6 ng L−1, respectively. The concentration of ∑HCHs ranged from 8.6 to 26.7 ng L−1 in groundwater, and from 2.5 to 23.9 ng L−1 in surface water, with the mean concentration of 18.7 and 14.3 ng L−1, respectively. Levels of HCHs and DDTs in surface water of upper reach of Huaihe River (Feng et al. 2011), Lake Baiyangdian (Guo and Feng 2014) and Yellow River Estuary (Li et al. 2015b) were 0.9–62.2 and 1.5–35.5 ng L−1, respectively, while in River Yamuna, India, the levels were with higher concentration of 12.8–593.5 and 66.2–723.0 ng L−1 (Kaushik et al. 2008). Concentration of total OCPs ranges from 1.0 to 46.5 ng L−1 in Yangtze River (Tang et al. 2008), Pearl River Delta (Guan et al. 2009), Chao River (Yu et al. 2014), and 0.8–64.5 ng L−1 in underground river of southwest China (Hu et al. 2011). OCPs concentration in surface water was with higher concentration of 2–1200 ng L−1 in other Asia countries such as River Brahmaputra and River Hooghly, India (Chakraborty et al. 2016), and River Chenab, Pakistan (Eqani et al. 2012). Individually, dieldrin, heptachlor, heptachlor epoxide were with high detection frequency in river water of Korea, whose concentration range was 3–5410 ng L−1 (Cho et al. 2014). Generally, the concentrations of OCPs were low in Shaying River Basin compared with other areas in China and Asia countries.
Occurrence of OCPs in Shaying River Basin
The results showed that the detection frequency of HCHs was the highest, indicating it as the prominent contaminants group of OCPs in Shaying River (Figs. 2a, 3a). Comparing with the centralized drinking water standards of China (China 2006), no residues were above the limits (0.005 mg L−1 for HCHs, 0.002 mg L−1 for lindane and 0.001 mg L−1 for DDTs). The concentration level of HCHs in Shaying River was higher than Sao Paulo State Rivers in Brazil (Rissato et al. 2006), surface water in Jiangsu China (Li et al. 2012) and Bosumtwi Lake in Ghana (Darko and Acquaah 2008), similar to Paranoa Lake in Brazil (Caldas et al. 1999), Yanzi River in China (Tang et al. 2008), but lower than most of other surface water (Kaushik et al. 2008; Singh et al. 2005; Turgut 2003; Wang et al. 2007; Zhang et al. 2003; Zhou et al. 2008).
All of the samples were detected with HCHs and heptachlor, indicating them as the prominent OCP contaminants in groundwater of Shaying River (Figs. 2b, 3b). No exceeding of concentration was observed based on centralized drinking water standards of China.
Principal component analysis (PCA) was performed to further determine the possible sources and composition in surface water of Shaying River Basin on four most frequently detected OCPs (Table 2-1). The contribution rate of each OCP to components can be taken as the main accordance of determination of main contaminant (Table 2-2).
The contribution rate of first main component F1 was 47.513%, which was highly associated with endosulfan I and heptachlor. Endosulfan I was firstly used in China from 1994, and the total consumption reached 25.7 thousand tons. On the 2011 ‘Stockholm Convention’ of United Nations fifth conference of the parties, 127 countries, including China, finally agreed to add the highly toxic pesticide endosulfan to the prohibited substance of the Convention, but because of the historical large usage and difficulty of degradation, endosulfan became the main component of organochlorine pesticide contaminant in the water of the research area. The contribution rate of the second main component F2 was 33.047%, with high positive load of HCHs and heptachlor, indicating a similar pollution source. HCHs and heptachlor have been prohibited in the 1980s, but are still highly detected in water body, which suggested that the historical inputs in surface water cannot be easily deciphered.
Chemical fingerprints including conventional isomer ratios and enantiomeric compositions were usually used for indicating the varied sources of OCPs in the environment. During the 1970s and early 1980s, China was the largest producer and consumer of technical HCH (60–70% α-HCH, 5–12% β-HCH, 10–12% γ-HCH and 6–10% δ-HCH) (Willett et al. 1998) in the world with an increasing production from zero (1952) to nearly 300 thousand tons per year (Li et al. 1998) because of the intense food production pressure, and was officially banned in China in the middle 1980s. As a substitute, lindane (> 99% γ-HCH) has been used in agriculture since 1990 (Li et al. 2001). Therefore, the α-HCH/γ-HCH (α/γ) ratio is in the range of 3–7 for technical HCH, whereas low α/γ ratio depicts the usage of lindane (Li et al. 1998; Vu Duc et al. 2009). In this study, α/γ ratios of groundwater samples along Jialu River ranged from 0.24 to 0.81 with an average of 0.46, which confirmed the use of lindane as the major source of HCHs contamination in the study area (Figs. 4, 5). Compared with other studies, the HCHs concentration is similar with the groundwater in karst area, Chongqing, China (Hu et al. 2011), and the γ-HCH, δ-HCH also took the major proportion of HCHs (Fig. 4). However, it was opposite to that of Qiantang River, Ganges River and Pearl River estuary (Yu et al. 2008, 2009; Zhou et al. 2006). The results may also due to the deduced γ-HCH (lindane) input in Shaying River Basin and its possible transformation to δ-HCH under anaerobic condition in groundwater aquifer (Li et al. 2006). Another possible explanation was that α-HCH has a higher vapor pressure and Henry’s law constant, which is approximately twice as that of γ-HCH (Walker et al. 1999) and 20 times higher than β-HCH (Li et al. 2004), indicating that α-HCH is more likely to partition to the air than to the water body (Li et al. 2014).
Correlation analysis of OCP components
Correlation analysis of the OCPs concentration in both surface water and groundwater was conducted to find out the relationship between the individual OCPs component in the research area and their possible migration evidence in the aquatic environment (Table 3). It can be seen that δ-HCH and γ-HCH showed a strongly significant positive correlation with ∑HCHs (P < 0.05), which illustrated their high residual in the water environment and their similarity of residual pattern with ∑HCHs. In addition, heptachlor and α-HCH, as well as endosulfan and heptachlor epoxide, have a strongly significant positive correlation, which may be an indication for the associated usage of these two pairs of OCPs.
Risk assessment of OCPs in Shaying River Basin
Ecological risk assessment
The results of ecological risk assessment of OCPs in surface water and groundwater in Shaying River Basin are shown in Table 4. RQ values between 0.01 and 0.1 were observed for α-HCH, β-HCH, δ-HCH, heptachlor epoxide, aldrin and endrin, suggesting that these OCP individuals post little ecological risk to selected aquatic organism in the research area. RQ value slightly above 0.1 was observed for β-HCH, while RQ values of dieldrin and endosulfan I were between 0.1 and 1.0 only in surface water, which indicated a generally moderate and potential ecological risk. Particularly, RQ value exceeding 1.0 was observed for γ-HCH (lindane) and heptachlor, revealing its extremely high ecological risk on aquatic organism in the research area which needs more concern. The average RQ value of γ-HCH (lindane) and heptachlor in surface water was 5.24 and 9.59, respectively, and that in groundwater was 5.72 and 13.97, respectively, resulting from the high detected concentration and their high toxicity on aquatic organism. Generally, OCPs post higher ecological risks to groundwater than surface water in the research area. Even though the selected target aquatic species may not appear in groundwater environment, but considering the possible groundwater discharging to surface water, it may provide reasonable reference to the ecological assessment and risk management practice.
The RQ value of 4 HCH isomers in surface water ranged from 1.79 to 9.52 with an average of 5.42, which was similar with that in groundwater. With regard to the contributions of the different OCPs to the relative concentrations of the mixture, γ-HCH played a dominant role in the comprehensive ecological risk. Guo and Feng (2014) found that α-HCH and β-HCH were the dominant factors for carcinogenic and non-carcinogenic risks in shallow freshwater lakes in China that are used as drinking water source. This difference might be a result of the divergence of residue concentration in these two regions. As the α/γ ratio of HCH in Guo’s research was above 1, while that of this paper is below 1, it can be deduced that there was a higher historical input of γ-HCH in Shaying River Basin, which could also result in the low proportion of risk posted by α-HCH and β-HCH.
Carcinogenic risk assessment
The calculated TLCRs using the RAGS method are shown in Table 5. All the TLCR values of OCPs in surface water and groundwater were between 10−6 and 10−4, suggesting that the carcinogenic risks posed by these contaminants were acceptable, but still needed considerably concern. Particularly, the TLCR value of γ-HCH and heptachlor was obviously higher that other OCPs both in surface water and groundwater, indicating their high carcinogenic risk potential. The result also indicated that the carcinogenic risk posted by OCPs in surface water and groundwater was low for dermal contact (bathing) and acceptable for drinking.
As from the literature, ingestion is evaluated as the main intake pathway for OCPs to the human body, the risks related to drinking water produced from surface water and groundwater are much higher than those of dermal contact, which can be seen in Table 5 and is accordance with other studies (Wei et al. 2015). The carcinogenic risk value of HCHs in this research was similar with that of edible fish from Wuhan, China (Cui et al. 2015), higher than that of drinking water sources from middle China (Guo and Feng 2014), underground river waters in Southeast China (Hu et al. 2011) and surface waters in Sichuan Basin China (Liu et al. 2015), which was around 10−7–10−9. In river water of Korea, there is a higher carcinogenic risk of 10−6 associated with OCPs (Lee et al. 2014). In this study, the OCPs in surface water and groundwater in Shaying River have much higher carcinogenic risks on human than those in tap water (with estimated carcinogenic risk of 10−8–10−10) (Li et al. 2012), which indicated drinking water treatment as an efficient and necessary procedure for water resource utilization.
The carcinogenic risk of OCPs in groundwater is higher than that of surface water, which is probably because of the direct leaching of irrigation and rainfall through the agriculture soil. However, the calculations may have over- or underestimated the risk because of the uncertainty of the residual concentration and intake rate. Besides, the research about assessing the carcinogenic risk of surface water and groundwater in the same region simultaneously was limited. Thus, more work should be done before accurate conclusion could be drawn.
Conclusions
-
In the research area, the concentration of ∑OCPs ranged from 21.0 to 61.4 ng L−1 in groundwater, and from 12.3 to 77.5 ng L−1 in surface water, with the mean concentration of 44.6 and 30.6 ng L−1, respectively. The concentration of ∑HCHs ranged from 8.6 to 26.7 ng L−1 in groundwater, and from 2.5 to 23.9 ng L−1 in surface water, with the mean concentration of 18.7 and 14.3 ng L−1, respectively.
-
According to the results of main components analysis, the prominent OCPs contaminant in surface water of Shaying River Basin was HCHs and endosulfan I. Among the HCH isomers, γ-HCH and δ-HCH were the dominant composition. HCHs and heptachlor were with the highest concentration in groundwater of Shaying River Basin.
-
The α-HCH/γ-HCH ratios were all less than 3, which confirmed the use of lindane as the major source of HCHs contamination in the research area. Heptachlor and α-HCH, as well as endosulfan and heptachlor epoxide, had a strongly significant positive correlation, suggesting an associated usage of the two pair OCPs.
-
An exceeding RQ value was observed for γ-HCH (lindane), heptachlor and dieldrin, revealing its extremely high ecological risk on aquatic organism in the research area. All the TLCR values of the selected 10 OCPs in surface water and groundwater were between 10−6 and 10−4, suggesting that the carcinogenic risks posed by these contaminants were acceptable based on the evaluation standard applied in this research.
Abbreviations
- OCPs:
-
Organochlorine pesticides
- HCHs:
-
Hexachlorocyclohexane
- DDT:
-
Dichlorodiphenyl trichloroethane
- RSD:
-
Relative standard deviation
- IDLs:
-
Instrument detection limits
- MDLs:
-
Method detection limits
- RQ:
-
Risk quotient
- C :
-
Measured concentration of OCPs
- PNEC:
-
Predicted no-effect concentrations for a particular OCP
- AF:
-
Assessment factor
- RAGS:
-
Risk Assessment Guidance for Superfund
- TLCR:
-
Total carcinogenic risk
- PCA:
-
Principal component analysis
- USEPA:
-
United States Environment Protection Agency
References
Agarwal, H. C., Mittal, P. K., Menon, K. B., & Pillai, M. K. K. (1986). DDT Residues in the river Jamuna in Delhi, India. Water Air and Soil Pollution, 28(1–2), 89–104.
Buah-Kwofie, A., & Humphries, M. S. (2017). The distribution of organochlorine pesticides in sediments from iSimangaliso Wetland Park: Ecological risks and implications for conservation in a biodiversity hotspot. Environmental Pollution, 229, 715–723.
Butler-Jones, D. D. (2010). The Chief Public Health Officer’s Report on the state of Public health in Canada.
Caldas, E. D., Coelho, R., Souza, L., & Silva, S. C. (1999). Organochlorine pesticides in water, sediment, and fish of Paranoa Lake of Brasilia, Brazil. Bulletin of Environmental Contamination and Toxicology, 62(2), 199–206.
Chakraborty, P., Khuman, S. N., Selvaraj, S., Sampath, S., Devi, N. L., Bang, J. J., et al. (2016). Polychlorinated biphenyls and organochlorine pesticides in River Brahmaputra from the outer Himalayan Range and River Hooghly emptying into the Bay of Bengal: Occurrence, sources and ecotoxicological risk assessment. Environmental Pollution, 219, 998–1006.
Chakraborty, P., Zhang, G., Li, J., Sivakumar, A., & Jones, K. C. (2015). Occurrence and sources of selected organochlorine pesticides in the soil of seven major Indian cities: Assessment of air–soil exchange. Environmental Pollution, 204, 74–80.
Chen, Y. Z., Chen, Z. F., & Ma, J. H. (2016). Effects of soil nitrate nitrogen on the nitrate accumulation in groundwater and vegetables in a typical high cancer area of Shaying River basin. Acta Scientiae Cercumstantiea, 36(3), 990–998.
China, E.P.A.o.P.R. (1987). Water quality. Determination of BHC and DDT. Gas chromatography.
China, N.H.a.F.P.C.o.t.P.R. (2006). Standards for drinking water quality.
Cho, E., Khim, J., Chung, S., Seo, D., & Son, Y. (2014). Occurrence of micropollutants in four major rivers in Korea. Science of the Total Environment, 491–492, 138–147.
Cui, L., Ge, J., Zhu, Y., Yang, Y., & Wang, J. (2015). Concentrations, bioaccumulation, and human health risk assessment of organochlorine pesticides and heavy metals in edible fish from Wuhan, China. Environmental Science and Pollution Research International, 22(20), 15866–15879.
Darko, G., & Acquaah, S. O. (2008). Levels of organochlorine pesticides residues in dairy products in Kumasi, Ghana. Chemosphere, 71(2), 294–298.
Eqani, S. A.-M.-A.-S., Malik, R. N., Katsoyiannis, A., Zhang, G., Chakraborty, P., Mohammad, A., et al. (2012). Distribution and risk assessment of organochlorine contaminants in surface water from River Chenab, Pakistan. Journal of Environmental Monitoring, 14(6), 1645.
Feng, J., Zhai, M., Liu, Q., Sun, J., & Guo, J. (2011). Residues of organochlorine pesticides (OCPs) in upper reach of the Huaihe River, East China. Ecotoxicology and Environmental Safety, 74(8), 2252–2259.
Gonghuan, Y., & Zhong, D. (2013). Huai River Basin water environment and digestive tract cancer death atlas. Beijing: China Map Publishing House.
Guan, Y.-F., Wang, J.-Z., Ni, H.-G., & Zeng, E. Y. (2009). Organochlorine pesticides and polychlorinated biphenyls in riverine runoff of the Pearl River Delta, China: Assessment of mass loading, input source and environmental fate. Environmental Pollution, 157(2), 618–624.
Guo, W., & Feng, Y. (2014). Health risk assessment of organochlorine pesticides in a shallow freshwater lake, China. Advanced Materials Research, 864–867, 871–875
Guzzella, L., Pozzoni, F., & Giuliano, G. (2006). Herbicide contamination of surficial groundwater in Northern Italy. Environmental Pollution, 142(2), 344–353.
Hu, X. X., Han, Z. H., Zhou, Y. K., Cheng, J. P., & Wang, W. H. (2005). Distribution of organochlorine pesticides in surface sediments from Huangpu River and its risk evaluation. Environmental Science (Chinese), 26(3), 44–48.
Hu, Y., Qi, S., Zhang, J., Tan, L., Zhang, J., Wang, Y., et al. (2011). Assessment of organochlorine pesticides contamination in underground rivers in Chongqing, Southwest China. Journal of Geochemical Exploration, 111(1–2), 47–55.
Hu, Y., Sun, S., Song, X., Ma, J., & Ru, S. (2015). Distribution and ecological risk assessment of HCHs and DDTs in surface seawater and sediment of the mariculture area of Jincheng Bay, China. Journal of Ocean University of China, 14(2), 301–308.
Jayaraj, R., Megha, P., & Sreedev, P. (2016). Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary Toxicology, 9(3–4), 90.
Kaushik, C. P., Sharma, H. R., Jain, S., Dawra, J., & Kaushik, A. (2008). Pesticide residues in river Yamuna and its canals in Haryana and Delhi, India. Environmental Monitoring and Assessment, 144(1–3), 329–340.
Kolpin, D. W., Barbash, J. E., & Gilliom, R. J. (1998). Occurrence of pesticides in shallow groundwater of the United States: Initial results from the National Water-Quality Assessment Program. Environmental Science and Technology, 32(5), 558–566.
Lacorte, S., & Barcelo, D. (1996). Improvements in the determination of organophosphorus pesticides in ground- and wastewater samples from interlaboratory studies by automated on-line liquid-solid extraction followed by liquid chromatography diode array detection. Journal of Chromatography A, 725(1), 85–92.
Lee, S.-H., Ra, J.-S., Choi, J.-W., Yim, B.-J., Jung, M.-S., & Kim, S.-D. (2014). Human health risks associated with dietary exposure to persistent organic pollutants (POPs) in river water in Korea. Science of the Total Environment, 470–471, 1362–1369.
Li, B., Qu, C., & Bi, J. (2012). Identification of trace organic pollutants in drinking water and the associated human health risks in Jiangsu Province, China. Bulletin of Environmental Contamination and Toxicology, 88(6), 880–884.
Li, J., Huang, Y., Ye, R., Yuan, G.-L., Wu, H.-Z., Han, P., et al. (2015a). Source identification and health risk assessment of Persistent Organic Pollutants (POPs) in the topsoils of typical petrochemical industrial area in Beijing, China. Journal of Geochemical Exploration, 158, 177–185.
Li, J., Li, F., & Liu, Q. (2015b). Sources, concentrations and risk factors of organochlorine pesticides in soil, water and sediment in the Yellow River estuary. Marine Pollution Bulletin, 100(1), 516–522.
Li, J., Zhang, G., Qi, S., Li, X., & Peng, X. (2006). Concentrations, enantiomeric compositions, and sources of HCH, DDT and chlordane in soils from the Pearl River Delta, South China. Science of the Total Environment, 372(1), 215–224.
Li, P., Wang, Y., Huang, W., Yao, H., Xue, B., & Xu, Y. (2014). Sixty-year sedimentary record of DDTs, HCHs, CHLs and endosulfan from emerging development gulfs: A case study in the Beibu Gulf, South China Sea. Bulletin of Environmental Contamination and Toxicology, 92(1), 23–29.
Li, X. D., Mai, B. X., Zhang, G., Sheng, G. Y., Fu, J. M., Pan, S. M., et al. (2001). Distribution of organochlorine pesticides in a sediment profile of the Pearl River Estuary. Bulletin of Environmental Contamination and Toxicology, 67(6), 871–880.
Li, Y. F., Cai, D. J., & Singh, A. (1998). Technical hexachlorocyclohexane use trends in China and their impact on the environment. Archives of Environmental Contamination and Toxicology, 35(4), 688–697.
Li, Y. F., Struger, J., Waite, D., & Ma, J. (2004). Gridded Canadian lindane usage inventories with 1/6 degrees x 1/4 degrees latitude and longitude resolution. Atmospheric Environment, 38(8), 1117–1121.
Liu, H., Hu, Y., Qi, S., Xing, X., Zhang, Y., Yang, D., et al. (2015). Organochlorine pesticide residues in surface water from Sichuan Basin to Aba Prefecture profile, east of the Tibetan Plateau. Frontiers of Earth Science, 9(2), 248–258.
Malik, A., Ojha, P., & Singh, K. P. (2008). Levels and distribution of persistent organochlorine pesticide residues in water and sediments of Gomti River (India)—A tributary of the Ganges River. Environmental Monitoring and Assessment, 148(1–4), 421–435.
Ogbeide, O., Tongo, I., & Ezemonye, L. (2015). Risk assessment of agricultural pesticides in water, sediment, and fish from Owan River, Edo State, Nigeria. Environmental Monitoring and Assessment, 187(10), 654.
Papastergiou, A., & Papadopoulou-Mourkidou, E. (2001). Occurrence and spatial and temporal distribution of pesticide residues in groundwater of major corn-growing areas of Greece (1996–1997). Environmental Science and Technology, 35(1), 63–69.
Peng, X., Ou, W., Wang, C., Wang, Z., Huang, Q., Jin, J., et al. (2014). Occurrence and ecological potential of pharmaceuticals and personal care products in groundwater and reservoirs in the vicinity of municipal landfills in China. Science of the Total Environment, 490, 889–898.
Qu, C., Qi, S., Yang, D., Huang, H., Zhang, J., Chen, W., et al. (2015). Risk assessment and influence factors of organochlorine pesticides (OCPs) in agricultural soils of the hill region: A case study from Ningde, Southeast China. Journal of Geochemical Exploration, 149, 43–51.
Rissato, S. R., Galhiane, M. S., Ximenes, V. F., de Andrade, R. M. B., Talamoni, J. L. B., Libanio, M., et al. (2006). Organochlorine pesticides and polychlorinated biphenyls in soil and water samples in the northeastern part of Sao Paulo State, Brazil. Chemosphere, 65(11), 1949–1958.
Schriks, M., Heringa, M. B., van der Kooi, M. M. E., de Voogt, P., & van Wezel, A. P. (2010). Toxicological relevance of emerging contaminants for drinking water quality. Water Research, 44(2), 461–476.
Singh, K. P., Malik, A., Mohan, D., & Takroo, R. (2005). Distribution of persistent organochlorine pesticide residues in Gomti River, India. Bulletin of Environmental Contamination and Toxicology, 74(1), 146–154.
Sun, J. H., Wang, G. L., Zhang, G., Li, J., Chai, Y., Wang, J. Z., et al. (2007). Distribution of organochlorine pesticides in surface sediments from the middle and lower reaches of the Yellow River. Environmental Science (Chinese), 28(6), 1332–1337.
Tang, Z., Yang, Z., Shen, Z., Niu, J., & Cai, Y. (2008). Residues of organochlorine pesticides in water and suspended particulate matter from the Yangtze River catchment of Wuhan, China. Environmental Monitoring and Assessment, 137(1–3), 427–439.
Turgut, C. (2003). The contamination with organochlorine pesticides and heavy metals in surface water in Kucuk Menderes River in Turkey, 2000–2002. Environment International, 29(1), 29–32.
USEPA. (1989). Risk assessment guidance for Superfund, Volume I, human health evaluation manual (Part A). Response, O.o.E.a.R. and Agency, U.S.E.P. (Eds.), Washington, DC.
USEPA. (1997). Ecological risk assessment guidance for Superfund: Process for designing and conducting ecological risk assessments. United States Environmental Protection Agency, E.R.T. (Ed.).
USEPA. (2010). Integrating ecological assessment and decision-making at EPA: A path forward, results of a Colloquium in Response to Science Advisory Board and National Research Council Recommendations. Risk Assessment Forum, U.S.E.P.A. (Ed.), Washington, DC.
Vryzas, Z., Alexoudis, C., Vassiliou, G., Galanis, K., & Papadopoulou-Mourkidou, E. (2011). Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in northeastern Greece. Ecotoxicology and Environmental Safety, 74(2), 174–181.
Vu Duc, T., Vu Duc, T., Walder, J., & Cao The, H. (2009). Residue, temporal trend and half-life time of selected organochlorine pesticides (OCPs) in surface soils from Bacninh, Vietnam. Bulletin of Environmental Contamination and Toxicology, 82(4), 516–521.
Walker, K., Vallero, D. A., & Lewis, R. G. (1999). Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environmental Science and Technology, 33(24), 4373–4378.
Wang, F., Jiang, X., Bian, Y.-R., Yao, F.-X., Gao, H.-J., Yu, G.-F., et al. (2007). Organochlorine pesticides in soils under different land usage in the Taihu Lake region, China. Journal of Environmental Sciences-China, 19(5), 584–590.
Wei, L., Yang, Y., Li, Q. X., & Wang, J. (2015). Composition, distribution, and risk assessment of organochlorine pesticides in drinking water sources in South China. Water Quality Exposure and Health, 7(1), 89–97.
WHO. (2001). Water quality: Guidelines, standards and health. London: IWA.
Willett, K. L., Ulrich, E. M., & Hites, R. A. (1998). Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environmental Science and Technology, 32(15), 2197–2207.
Xue, N., Zhang, D., & Xu, X. (2006). Organochlorinated pesticide multiresidues in surface sediments from Beijing Guanting reservoir. Water Research, 40(2), 183–194.
Yu, M., Luo, X., Chen, S., Mai, B., & Zeng, E. Y. (2008). Organochlorine pesticides in the surface water and sediments of the Pearl River Estuary, South China. Environmental Toxicology and Chemistry, 27(1), 10–17.
Yu, M., Luo, X.-J., Wu, J.-P., Chen, S.-J., & Mai, B.-X. (2009). Bioaccumulation and trophic transfer of polybrominated diphenyl ethers (PBDEs) in biota from the Pearl River Estuary, South China. Environment International, 35(7), 1090–1095.
Yu, Y., Li, Y., Shen, Z., Yang, Z., Mo, L., Kong, Y., et al. (2014). Occurrence and possible sources of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) along the Chao River, China. Chemosphere, 114, 136–143.
Zhang, Z., Hong, H., Cheng, W., Wang, X., Lin, J., & Yu, G. (2003). Contents of organochlorine pesticides in water, pore water and sediment in Minjiang River Estuary of China. Environment Science (Chinese), 24(1), 117–120.
Zhou, R., Zhu, L., & Chen, Y. (2008). Levels and source of organochlorine pesticides in surface waters of Qiantang River, China. Environmental Monitoring and Assessment, 136(1–3), 277–287.
Zhou, R., Zhu, L., Yang, K., & Chen, Y. (2006). Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. Journal of Hazardous Materials, 137(1), 68–75.
Acknowledgements
This study was funded by Key Program of National Natural Science Foundation of China (No. 41230640) and Major Science and Technology Program for Water Pollution Control and Treatment (Grant No. 2012ZX07204-003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Bai, Y., Ruan, X. & van der Hoek, J.P. Residues of organochlorine pesticides (OCPs) in aquatic environment and risk assessment along Shaying River, China. Environ Geochem Health 40, 2525–2538 (2018). https://doi.org/10.1007/s10653-018-0117-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0117-9