Abstract
Organochlorine pesticides (OCPs) were widely used in agriculture and disease control, which causes potential harm to eco-environment and human health, and the OCP pollution is closely related to past or current human activities. A total of 27 surface water samples were collected from two rivers with different human interference intensity, and 13 OCPs were analyzed. The study results showed that the mean concentrations of OCPs in Buerhatong River (BR) and Hunchun River (HR) were 780.45 ng L−1and 900.69 ng L−1, respectively. On the basis of environmental quality standards of China, the concentrations of DDTs, heptachlor epoxide, and γ-HCH were lower than standard values, but the maximum concentration of γ-HCH in HR (1923.26 ng L−1) is close to the standard limit, showing a potential risk to the aquatic environment. And there are significant differences in residues of OCPs between two tributaries due to the different human activities. DDTs and HCHs are the main components of OCPs, and the DDTs in BR were mostly derived from historical use residues, whereas HR might have recent DDT use input. And BR had a good water environment with high dissolved oxygen, but there are some anaerobic sections in HR. The HCHs in BR are mainly the historical residues, except for one site, which might have lindane input in the environment, and there also might be recent imports of lindane in the HR basin. The health risk assessment results showed that there was no adverse health effects for non-carcinogenic risk. But carcinogenic risk caused by dermal contact for adults had moderate risk in two sites of the studied rivers. The study of the impact of human activities on the residues of organochlorines in surface water is of great significance to the prevention and control of organochlorine pesticide pollution in the water environment and the protection of the ecological environment and the health of the population, especially for adults.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
OCPs are the persistent organic pollutants (POPs) and have persistence, high toxicity, refractory, and bioaccumulativity, which can be long-term remained in the environmental mediums at global scale (Ravindran et al., 2016). Because of their high pesticide efficiency and low cost, OCPs were widely used in agriculture, industry, and for malaria control (Buah-Kwofie & Humphries, 2017; Luz et al., 2014). Therefore, a large amount of OCPs have been remained in the environmental media, which can enter the human body by consuming contaminated water and aquatic products (Zhou et al., 2008b). In the process of applying OCPs, the OCPs can enter the soil or atmosphere by spray drift, and the residual OCPs in the soil will volatilize into the atmosphere and then fall out into the rivers through dry and wet deposition (Agarwal et al., 1987; Kaushik, 1989, 1991; Kaushik et al., 1987, 1991). OCPs can cause a variety of diseases, such as cancer, reproductive defects, endocrine, and immunological toxicities, so they have been widely banned all over the world (Mrema et al., 2013). But because OCPs are harmful and resistant to degradation and have low water solubility, so they have persistence in the environment and still have a high detection rate in different environment media (Antary et al., 2020; Chakraborty et al., 2019; Fernandes et al., 2020; Tang et al., 2020; Tham et al., 2019). OCP residues in the environment media have a significant impact on the ecological environment and human health. The study results of Ravi River and its three northern tributaries in Pakistan showed that all the studied streams for endosulfan (α-endosulfan) and endrin had a high ecological risk, and the carcinogenic risk assessment indicated that the water of study area is possessing carcinogenic risk to local population and is unsafe for human bathing purposes (Baqar et al., 2018).
In China, OCPs are also ubiquitous and have been produced in a large amount, such as dichloro-diphenyl-trichloroethanes (DDTs), hexachlorocyclohexanes (HCHs), hexachlorobenzene (HCB), and mirex (Hua, 1996). Approximately 4.5 million tons of technical HCHs and 0.27 million tons of DDTs were produced in China after 1952 until being banned in 1983 (Liu et al., 2016). And about 11,400 t of lindane was still reportedly being produced after 1983 (Li et al., 2001), and DDTs have been continuously produced for approximately 20 years owing to export demands, malaria control, and dicofol production (Yang et al., 2008). OCPs may enter the river via agricultural runoff, direct applications, spray drift, aerial spraying, and erosion (Chakraborty et al., 2016), so the OCP pollution in estuaries and rivers also has attracted general attention in China (Luo et al., 2004; Tang et al., 2008; Wang et al., 2010; Wei et al., 2014). Moreover, OCPs have long-distance migration ability through the food chain and even can reach high latitudes through atmospheric deposition (dry and wet) (Ribes et al., 2002). Previous research showed that OCPs could be detected in Tuotuo River (the origin of Yangtze River), but OCPs had never been used in this area because of its 4,540-m height above sea level (Liu et al., 2011).
Buerhatong River and Hunchun River are the main tributaries of Tumen River, which have an important role in the sustainable development of the Tumen River basin. Tumen River basin is an important area for China’s open development along the border and an important gateway to Northeast Asia. There are significant differences in soil land use patterns in the two river basins, and the proportion of agricultural land and urban land in the Buerhatong River basin is significantly higher than Hunchun River. Although the two rivers are in the Tumen River basin, the degree of human disturbance in history shows significant differences. And affected by natural conditions, industrial structure, economic level, etc., the ecological environment of BR and HR has been contaminated to varying degrees. Therefore, the research on the pollution and risks of organochlorine pesticide residues in rivers under different disturbance intensity of human activities will provide important theoretical and data support for the in-depth exploration of the impact of human activities on the distribution of organochlorine pollution. The objectives of the current study were to firstly investigate the residue concentrations and distribution pattern of 13 OCPs (α-HCH, β-HCH, γ-HCH, δ-HCH, PP′-DDE, PP′-DDD, OP′-DDT, PP′-DDT, HCB, heptachlor, heptachlor epoxide, dieldrin, and endrin) in the surface water of BR and HR; to identify the sources of OCPs and indicate the effect of human activities on the residues of OCPs in the study area; and to assess the non-carcinogenic risk and carcinogenic risk of OCPs for children and adults through different exposure pathways.
2 Materials and Methods
2.1 Site Description
The Buerhatong River and Hunchun River are the main tributaries of the Tumen River basin. The BR flows about 242 km and originates in Haerba mountain ridge with an approximately tributary area of 7141km2, and HR, about 200 km long, flowing from Pan mountain ridge to Tumen River. Climate of the study area is continental monsoon climate with an annual average temperature of 2.6–5.4℃, and annual rainfall varied from 400 to 800 mm mostly distributed in high flow period (June to September). The area proportion of different land use types in the different watersheds of Tumen River is listed in Table 1 (Li et al., 2013). The population density in BR basin is high, and the industry and agriculture are relatively developed, and the industry is dominated by coal, food, tobacco, textile and clothing, electricity, wood processing, etc. The HR basin is less affected by the human activities relative to Buerhatong River.
2.2 Sampling
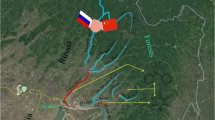
Sampling sites along BR and HR were selected equably to cover the research area. Fourteen and thirteen surface water samples were collected with pre-cleaned 1-L dark glass bottles using cylinder samplers from Buerhatong River and Hunchun River, respectively, and the sampling locations were marked as B1 to B14 for Buerhatong River and H1 to H13 for Hunchun River, respectively (Fig. 1), and the distance between two adjacent sampling points is about 10–15 km. Water samples were collected within 1 day, and the pH of samples were adjusted to < 2 with hydrochloric acid solution after sampling and stored at 4 °C. The extraction was completed within 7 days, and the analysis was completed within 40 days.
2.3 Chemical Analysis
The chemical analysis method of this study mainly refers to the environmental protection standard of the People’s Republic of China (HJ 921–2017). First, 10 mL of methanol and 20.0μL standard solution of substitute (tetrachloro-m-xylene, decachlorobiphenyl) were added in 200.0 mL of water sample and mixed well. In turn, 5 mL of ethyl acetate, 5 mL of methanol, and 10 mL of water were used to activate the solid-phase extraction cartridge at a flow rate of about 5 mL/min. The water sample was passed through the solid-phase extraction cartridge at a flow rate of 10 mL/min. After loading, the solid-phase extraction cartridge was rinsed with 10 mL of water and the cartridge was drained. Then the solid-phase extraction cartridge was eluted with 2.5 mL of ethyl acetate and 5 mL of dichloromethane in sequence, with a flow rate of about 5 mL/min, and the eluent was collected into a concentration tube. The eluent was passed through the drying column, and the concentration tube was washed with a small amount of dichloromethane 2–3 times, and the washing solution was dehydrated through the drying column. All the dehydrated eluate was collected into a concentration tube and was concentrated to about 3 mL. Then purification process was proceed, Flory diatomaceous earth was infiltrated with 8 mL of n-hexane, the extract was transferred to the small column before the liquid level disappeared, the concentration tube was washed with 1–2 mL of n-hexane, the washing solution was put on the column together (note: always keep a liquid surface above the packing), then the column was eluted with 10 mL of acetone/n-hexane, and all the eluent was collected. The eluent was concentrated to less than 1 mL, 5.0μL of internal standard use solution was added, was diluted to 1.0 mL with n-hexane, was mixed well, and was transferred to the autosampler vial for testing. Analytical reagents and distilled water that met national standards were used for residue analysis.
2.4 Quantitative Analysis and Quality Control
A Shimadzu GC-2010Plus equipped with a micro-cell electron capture detector (ECD) was used for OCP quantification in the current study, and the capillary column used was Rxi-5 ms (30 cm × 0.25 mm × 0.25 μm), which is a low polarity 5% diphenyl/95% dimethyl polysiloxane stationary phase. The inlet temperature is 250 °C, and the injection is splitless. The GC column temperature was programmed as follows: initially at 80 ℃ (equilibrium time 1 min), increased to 150 ℃ at the rate of 20 ℃ min−1 and held for 2 min, before reaching at 300 ℃ at the rate of 5 ℃ min−1, and then held for 5 min. The column flow rate is 1.0 mL/min. While analyzing the sample, take the same volume of pure water, prepare a blank sample according to the preparation of the sample, and measure the blank sample with the same instrument analysis conditions as the calibration curve. The relative standard deviation was 1.50–5.54%, and the surrogate recovery in this method was 80–110%, and the method detection limits (MDLs) ranged from 0.022 to 0.069 ng L−1 (HJ 921–2017).
2.5 Statistical Analysis
The analysis of variance for different OCP components in surface water of BR and HR was conducted using the software of SPSS version 21, and Origin Pro 8.0 was used for graph making.
The hazard quotient (HQ) is used to assess non-carcinogenic risks exposure to OCPs in surface water. The exposure dose through the pathways of water ingestion and dermal contact were calculated with Eqs. (1) and (2) (Chen et al., 2020; Kerr et al., 1998):
where C is the arithmetic mean for OCP concentrations of water. Exposure was expressed in terms of a daily dose (mg/kg/day) and calculated separately for each OCP. ADDing (mg/kg/day) is daily exposure amount of OCPs through ingestion, and ADDder (mg/kg/day) is daily exposure amount of OCPs through dermal contact. The exposure factors for these models are shown in Table 2.
After ADDing and ADDder were calculated, a hazard quotient (HQ) based on non-cancer toxic risk can then be calculated by dividing daily dose to a specific reference dose (RfD):
where HQ is the hazard quotient, indicating the non-carcinogenic risk of single contamination; the reference dose (RfD) is an estimate of maximum permissible risk to a human population through daily exposure during a lifetime. When HQ ≤ 1 indicates no adverse health effects and HQ > 1 indicates likely adverse health effects, and the hazard index (HI) was equal to the sum of HQs and was used to estimate the health risk, HI values of ≤ 1 indicate no adverse health effects, and HI values > 1 indicate possible adverse health effects (USEPA, 2001). Toxicological characteristics of the investigated OCPs used for health risk assessments are presented in Table 2.
For carcinogens, the carcinogenic health risk can be calculated by multiplying ADD by cancer slope factor (SF):
where CRing is the carcinogenic risk for the exposure pathway of ingestion and CRder is the carcinogenic risk for the exposure pathway of dermal contact. SFi is the corresponding slope factor for the specific exposure pathway of the ith OCP. For carcinogenic risk (CR), risks surpassing 1 × 10−1 are viewed as very high risk, risk values between 1 × 10−3 and 1 × 10−1 are considered to pose high risk, risks ranging from 1 × 10−4 to 1 × 10−3 represent moderate risk, values between 1 × 10−6 and 1 × 10−4 indicate low risk, and values smaller than 1 × 10−6 indicate very low risk (Qu et al., 2015).
3 Results and Discussion
3.1 Content and Distribution Characteristics of Organochlorine Pesticides in BR and HR
Residues of 13 OCPs were detected in surface water along BR and HR, and the concentration and detection rate of OCPs are shown in Table 3. The current study has revealed that a significant number of pesticide residues were detected in the BR and HR, a total of 12 OCPs were detected in surface water of BR, and the detection rates of β-HCH, γ-HCH, δ-HCH, and HCB are over 90%, of which HCB achieved 100%, but Dieldrin had not been detected. And except for α-HCH and PP′-DDT, 11 OCPs were also detected in Hunchun River, and γ-HCH (92.3%), δ-HCH (92.3%), and OP′-DDT (100%) have high detection rate. The concentration of ∑OCPs in BR ranged from 111.56 to 2211.49 ng L−1 with the mean concentration of 780.45 ng L−1 and varied from 12.92 to 3521.54 ng L−1 in HR with the mean concentration of 900.69 ng L−1. Among all the sampling sites, the B9 station and H6 station had the highest ΣOCPs.
By comparing and analyzing the residual characteristics of OCPs in different rivers, it is found that there are significant differences in OCP pollution levels in many rivers at home and abroad, but the pollution of HCHs and DDTs is prominent. In the current study, the HCHs and DDTs in the two rivers are also the hot pollutants. In China, technical HCH and DDT were widely used in agriculture before they were banned in 1983 (Gan et al., 2002; Tao et al., 2005), and the other OCPs, such as HCB, heptachlor, heptachlor epoxide, dieldrin, and endrin, have also been produced and applied in China in the past. But during nearly 40 years, research shows that many rivers in China still have the OCP residues, which are most likely caused by the historical residues and the continued use of OCPs in other fields. For example, DDT is still allowed to be used for controlling the reproduction of mosquitoes and prevent diseases such as malaria (World Health Organization, WHO). OCPs were monitored along the Yangtze River from headstream to estuary, and the results showed that the concentrations of ∑HCHs and ∑DDTs ranging from 0.11 to 13.68 and from 0 to 23.31 ng L−1 (Liu et al., 2011), respectively, were lower than the current study. Moreover, the mean concentration of ∑HCHs and ∑DDTs in BR and HR was also higher than Pearl River (Tang et al., 2018; Yang et al., 2004), Huaihe River (Feng et al., 2011; Wang et al., 2009), Peacock River in Xinjiang (Chen et al., 2011), Qiantang River (Zhou et al., 2008a), and Shayi River (Bai et al., 2018). But the OCPs in the current study were also lower than the other rivers, such as the concentrations of ∑DDTs and dieldrin in the surface river from BR and HR were lower than Tonghui River in Beijing (Zhang et al., 2004), ∑HCHs, heptachlor, dieldrin, and endrin were significantly lower than the surface water from Lagos lagoon complex in Nigeria (Adeboyejo et al., 2011), and the ∑DDT content was also lower than river Yamuna in Haryana and Delhi, India (Kaushik et al., 2008).
Affected by the way and degree of disturbance of human activities, there are significant differences in the residues of OCPs in different sections of the river from upstream to downstream, and the high residues were mainly present in the middle reaches in the current study (Fig. 2A and B). For BR, the maximum residual concentrations of β-HCH, δ-HCH, ∑HCHs, OP′-DDT, ∑DDTs, HCB, and ∑OCPs were present in the sampling site of B9, and the maximum concentrations of PP′-DDE and heptachlor were in B2, γ-HCH and PP′-DDD were in B14, and α-HCH, heptachlor epoxide, and endrin were present in B5, B3, and B13, respectively, except for PP′-DDT and dieldrin which had no residues (Fig. 2A). For HR, the sampling site of H6 had the maximum residual concentrations for γ-HCH, δ-HCH, ∑HCHs, HCB, and ∑OCPs, and H2 had the maximum concentrations for PP′-DDD, OP′-DDT, and ∑DDTs, and H5 had the maximum concentrations for heptachlor, heptachlor epoxide, and dieldrin; the high residues of β-HCH, PP′-DDE, and endrin were present in H9, H7, and H4, respectively, except for α-HCH and PP′-DDT which had no residues (Fig. 2B). So the sites of B9 (the entrance of BR into Yanji city) and H6 (near the village of Pear ditch) are the pollution hotspot for OCPs in BR and HR, respectively, indicating that the regional environment of B9 and H6 is easy to cause the accumulation of OCPs and is not conducive to the degradation, and it might be caused by the specific physical and chemical properties of river sediments in this area, which could provide anaerobic conditions where the metabolites may be different. But in order to ensure the safety of water use for local residents, effective control measures should be taken against high-pollution areas to improve the quality of the water environment.
According to Chinese guideline (GB 3838–2002), the concentration of DDTs should be less than 1000 ng L−1 (same to the critical value for DDT in USEPA guideline), and the concentration of lindane and heptachlor epoxide should be less than 2000 ng L−1 and 200 ng L−1, respectively. The maximum concentrations of DDTs for BR (153.25 ng L−1) and HR (180.52 ng L−1) are significantly lower than the value of standard, and the maximum concentrations of heptachlor epoxide for BR (37.15 ng L−1) and HR (8.93 ng L−1) are also significantly less than the value of 200 ng L−1. Meanwhile, the average concentrations of γ-HCH (account for 99% of lindane (Li et al., 2018) for BR (303.91 ng L−1) and HR (334.59 ng L−1) are significantly lower than 2000 ng L−1, so the quality of surface water of BR and HR are reasonably good. But compared to other regions (Bai et al., 2018; Chen et al., 2011; Feng et al., 2011; Liu et al., 2011; Tang et al., 2018; Wang et al., 2009; Yang et al., 2004; Zhou et al., 2008a), the values of ∑HCHs are relatively high for both rivers, and the maximum concentration of γ-HCH in HR (1923.26 ng L−1) is close to the standard limit, which is a potential risk to the aquatic environment and human health.
3.2 Effect of Human Activities on the OCP Pollution
OCPs are a class of synthetic insecticides with a broad spectrum of insecticides, low toxicity, and long residual effect. However, because they pose a serious threat to the ecological environment and human health, they are now banned by many countries, including China (Gan et al., 2002; Tao et al., 2005). The OCP pollution is closely related to history or current human activities, and OCP residues in the current environment may come from historical residues or new imports. In the current study, BR and HR had different disturbed intensities by human activities, and the proportion of land use area for farmland, grassland, canal, cities, and town in BR basin is significantly higher than HR (Table 1), indicating that BR basin had strong human being activities compared to HR. The results show that the concentrations of α-HCH, δ-HCH, PP′-DDE, OP′-DDT, ∑DDTs, heptachlor, and dieldrin are significantly different between the two rivers (Fig. 3). BR had higher residue levels of α-HCH, β-HCH, PP′-DDE, and heptachlor than HR, but the concentrations of δ-HCH and OP′-DDT were lower, indicating that although the historical activities of human beings have a greater interference with the river environment, the degree of change in the residual amount of OCPs between different rivers will be significantly different that may be due to the environmental conditions of dissolved oxygen, temperature, altitude, later input, etc. (Chakraborty et al., 2016; Hitch & Day, 1992; Liu et al., 2011). In order to further analyze the reasons of OCP residue in BR and HR, the sources of the main OCP residues were analyzed.
Among the OCPs, DDTs and HCHs are the most widely used OCPs with high content and detection rate (Bai et al., 2018; Chen et al., 2011; Feng et al., 2011; Wang et al., 2009; Zhou et al., 2008a), and in the current study, the proportion of DDTs and HCHs in OCPs averaged 95.7%. DDTs are mainly derived from the direct use of industry and the use of dicofol. Due to the different isomer composition of DDT in industrial DDT and dicofol, the source of DDT can be analyzed according to the content characteristics of OP′-DDT and PP′-DDT remaining in the environment (Jaward et al., 2005). In addition, DDTs will degrade into different products of DDD and DDE after entering the environment, so the ratio of (DDE + DDD)/DDTs is commonly used to track the degree of degradation of DDTs and used to determine the source of DDTs in the environment (historical use residues or recently used DDTs input), and (DDE + DDD)/DDTs > 0.5 indicates that it is mainly derived from historical use residues; on the contrary, (DDE + DDD)/DDTs < 0.5 indicates that there are recent DDT use input; meanwhile, it can also be used to trace the degradation conditions by analyzing the ratio characteristics between DDD and DDE (Hitch & Day, 1992; Hong et al., 1999). The values of (DDE + DDD)/DDTs for BR and HR are presented in Fig. 4. The ratios of (DDE + DDD)/DDTs in 57.14% of the sampling sites of BR were higher than 0.5, indicating that the DDTs were mainly derived from historical use residues in these area, whereas the ratios of HR were all lower than 0.5, indicating that there were recent DDT use input (Hitch & Day, 1992). The detection rate of DDE in surface water of BR reached at 71.4%, and only one site had DDD, showing that BR had a good water environment with high dissolved oxygen. However, compared with DDE, DDD had a higher detection rate for HR, showing that there are some anaerobic sections in the river, which causes DDTs to be mainly anaerobic degradation. Therefore, in order to improve the water environment, the water quality should be regulated for these anaerobic sections, which can promote the degradation of OCPs.
Compositional differences of HCHs also can indicate different contamination sources. Technical HCHs contain 60–70% α-HCH, 5–12% β-HCH, 10–12% γ-HCH, and 6–10% δ-HCH, and lindane contains more than 99% of γ-HCH (Li et al., 2018). Technical HCH application is prohibited, but lindane has been widely used until 2019 in China (Ministry of Ecology and Environment et al. 2019). The β-HCH is the most stable isomer of HCHs, and α-HCH and γ-HCH in the environment can be converted to β-HCH; therefore, if there is no new HCH input, the proportion of β-HCH isomer will gradually increase (Walker et al., 1999). Figure 5 shows the composition distribution of the isomers of HCHs in BR and HR. It can be seen from the figure that the β-HCH component of most sampling points in BR is the highest, and the average proportion of β-HCH is 58%, which is much higher than the initial proportion value (5–12%) of β-HCH in industrial HCH, indicating that the HCHs in the study area have been degraded for a long period of time, mainly based on historical residues. However, the composition of the isomer γ-HCH in the water of B13 is the highest, reaching 98%, indicating that there is lindane input in the environment. Meanwhile, the detection rates of α-HCH and β-HCH in HR are low, but γ-HCH and δ-HCH all have a high detection rate, indicating that there may be recent imports of lindane in the basin, and an in-depth investigation should be conducted to protect ecological safety of HR basin. Moreover, due to the less proportion of δ-HCH in technical HCHs, the δ-HCH in surface water of HR may derive from the interconversion between different components of HCHs, and the transformation mechanism needs to be further studied.
3.3 Health Risk Assessment
The OCPs contained in river water could enter human bodies and endanger human’s health through dermal contact and mistaken oral intake (Chen et al., 2020). In the current study, dermal contact was the main exposure pathway for the OCPs, and ingestion contributed the lower exposure doses through drinking raw water or accidental ingestion. Values of hazard quotient (HQ), hazard index (HI), and carcinogenic risk (CR) for the studied elements in surface water from BR and HR are listed in Table 4. The HQ and HI of OCPs through two pathways were all below 1 in two rivers, indicating no adverse health effects for non-carcinogenic risk. The health risk assessment results showed that the carcinogenic risks for dermal contact and mistaken oral intake were generally low, except for adults, whose CR caused by dermal contact was moderate risk in one sampling site for BR and HR, respectively. Adults had the highest carcinogenic risk in both exposure pathways, because the adults have larger body weight, exposed skin area, and exposure duration than children, which would make them have the highest exposure doses (Chen et al., 2020). Besides that, the carcinogenic and non-carcinogenic risks caused by dermal contact were much higher than that of ingestion. Thus, the carcinogenic and non-carcinogenic risks of OCPs in rivers to the adults should be of great concern in the current study area, especially for the exposure pathway of dermal contact.
4 Conclusions
The current study exposed residual levels of 13 OCPs in the surface water of two main tributaries of Tumen River, Northeast China. In the research area, the concentration of ∑OCPs in the surface water of BR ranged from 111.56 to 2211.49 ng L−1 with the mean concentration of 780.45 ng L−1 and varied from 12.92 to 3551.50 ng L−1 in HR with the mean concentration of 900.69 ng L−1. According to Chinese guideline (GB 3838–2002), the maximum concentrations of DDTs and heptachlor epoxide for BR and HR are significantly lower than the value of standard, and the average concentrations of γ-HCH are significantly lower than 2000 ng L−1, so the quality of surface water of BR and HR are reasonably good. But compared to other regions, the values of ∑HCHs are relatively high for both rivers, and the maximum concentration of γ-HCH in HR (1923.26 ng L−1) is close to the standard limit, which is a potential risk to the aquatic environment and human health. Moreover, the entrance of BR into Yanji city and the site near the village of Pear ditch are the pollution hotspot for OCPs in BR and HR, respectively. Although BR and HR are all the main tributaries of the Tumen River, there are significant differences in residues of OCPs due to the different intensity of human activities. BR had higher residue levels of α-HCH, PP′-DDE, and heptachlor than HR, but the concentrations of δ-HCH, OP′-DDT, ∑DDTs, and dieldrin were lower, indicating that although the historical activities of human beings have a greater interference with the river environment, the degree of change in the residual amount of OCPs between different rivers will be significantly different due to the environmental conditions of dissolved oxygen, later input, etc. Among the OCPs in the current study, DDTs and HCHs have high content and detection rate, and the proportion of DDTs and HCHs in OCPs averaged 95.7%. The source analysis results showed that DDTs in BR were mostly derived from historical use residues, whereas HR might have recent DDT use input. And BR had a good water environment with high dissolved oxygen, but there are some anaerobic sections in HR, which causes a relatively poor degradation environment for HR compared with BR. The HCHs in BR have been degraded for a long period of time, mainly based on historical residues, except for the site of B13, which might have lindane input in the environment. Meanwhile, there also might be recent imports of lindane and pesticides containing δ-HCH in the HR basin. In order to further protect the eco-environmental health of the study area, relevant environmental protection departments should conduct in-depth investigations on the OCP pollution in hotspots, especially the problems of poor degradation conditions or the possibility of recent OCP use input, and take immediate steps in reducing the potential contamination of the study area. The OCPs contained in river water could enter human bodies and endanger human’s health through dermal contact and ingestion. The health risk assessment results showed that the HQ and HI of OCPs through two pathways were all below 1 in two rivers, indicating no adverse health effects for non-carcinogenic risk. But carcinogenic risk caused by dermal contact for adults had moderate risk in two sampling sites in the current study. The adults had the higher non-carcinogenic risk and carcinogenic risk in both exposure pathways than children. Thus, the carcinogenic and non-carcinogenic risks of OCPs in rivers to the adults should be of great concern in the current study area, especially for the exposure pathway of dermal contact.
Data Availability
The data that support the findings of this study are not openly available due to the requirements of support project and are available from the corresponding author upon reasonable request.
References
Adeboyejo, O., Clarke, E. O., & Olarinmoye, M. O. (2011). Organochlorine pesticides residues in water, sediments, fin and shell-fish samples from Lagos lagoon complex Nigeria. Researcher, 3(3), 38–45.
Agarwal, H. C., Kaushik, C. P., & Pillai, M. K. K. (1987). Organochlorine insecticide residues in the rain water in Delhi, India. Water, Air, and Soil Pollution, 32(3), 293–302.
Antary, T. M. A., Alawi, M. A., Keewan, R., & Haddad, N. A. (2020). Evaluation of organochlorine pesticides in foodstuff of animal origin from middle governorates of Jordan in 2018 and 2019 using GC- ECD. Toxin Reviews, 38(4), 1–7.
Bai, Y., Ruan, X., & van der Hoek, J. P. (2018). Residues of organochlorine pesticides (OCPs) in aquatic environment and risk assessment along Shaying River China. Environmental Geochemistry and Health, 40(6), 2525–2538.
Baqar, M., Sadef, Y., Ahmad, S. R., AdeelMahmood, L. J., & Zhang, G. (2018). Organochlorine pesticides across the tributaries of River Ravi, Pakistan: Human health risk assessment through dermal exposure, ecological risks, source fingerprints and spatio-temporal distribution. Science of the Total Environment, 618, 291–305.
Buah-Kwofie, A., & Humphries, M. S. (2017). The distribution of organochlorine pesticides in sediments from iSimangaliso Wetland Park: Ecological risks and implications for conservation in a biodiversity hotspot. Environmental Pollution, 229(10), 715–723.
Chakraborty, P., Khuman, S. N., Selvaraj, S., Sampath, S., Devi, N. L., Bang, J. J., & Katsoyiannis, A. (2016). Polychlorinated biphenyls and organochlorine pesticides in River Brahmaputra from the outer Himalayan Range and River Hooghly emptying into the Bay of Bengal: Occurrence, sources and ecotoxicological risk assessment. Environmental Pollution, 219(12), 998–1006.
Chakraborty, P., Zhang, G., Li, J., Sampathkumar, P., Balasubramanian, T., Kathiresan, K., Takahashi, S., Subramanian, A., Tanabe, S., & Jones, K. C. (2019). Seasonal variation of atmospheric organochlorine pesticides and polybrominated diphenyl ethers in Parangipettai, Tamil Nadu, India: Implication for atmospheric transport. Science of the Total Environment, 649, 1653–1660.
Chen, C., Zou, W., Chen, S., Zhang, K., & Ma, L. (2020). Ecological and health risk assessment of organochlorine pesticides in an urbanized river network of Shanghai China. Environmental Sciences Europe, 32(42), 1–14.
Chen, W., Jing, M., Bu, J., Ellis, B. J., Qi, S., Song, Q., Ke, Y., Miao, J., Liu, M., & Yang, C. (2011). Organochlorine pesticides in the surface water and sediments from the Peacock River Drainage Basin in Xinjiang, China: A study of an arid zone in Central Asia. Environmental Monitoring & Assessment, 177(1–4), 1–21.
Feng, J., Zhai, M., Liu, Q., Sun, J., & Guo, J. (2011). Residues of organochlorine pesticides (OCPs) in upper reach of the Huaihe River East China. Ecotoxicology & Environmental Safety, 74(8), 2252–2259.
Fernandes, C. L. F., Volco, L. M., Ramires, P. F., Moura, R. R. D., & Júnior, F. M. R. D. S. (2020). Distribution of pesticides in agricultural and urban soils of Brazil: A critical review. Environmental Science: Processes & Impacts, 22, 256–270.
Gan, Z., Parker, A., House, A., Mai, B., & Wang, Z. (2002). Sedimentary records of DDT and HCH in the Pearl River Delta, South China. Environmental Science & Technology, 36(17), 3671–3677.
Hitch, R. K., & Day, H. R. (1992). Unusual persistence of DDT in some Western USA soils. Bulletin of Environmental Contamination & Toxicology, 48(2), 259–264.
Hong, H., Chen, W., Xu, L., Wang, X., & Zhang, L. (1999). Distribution and fate of organochlorine pollutants in the Pearl River estuary. Marine Pollution Bulletin, 39(1–12), 376–382.
Hua, X. (1996). The producticn and application of pestidices and factor analysis of their pollution in environment in China. Advances in Environmental Science, 4(2), 35–47.
Jaward, F. M., Zhang, G., Nam, J. J., Sweetman, A. J., Obbard, J. P., Kobara, Y., & Jones, K. C. (2005). Passive air sampling of polychlorinated biphenyls, organochlorine compounds, and polybrominated diphenyl ethers across Asia. Environmental Science & Technology, 39(22), 8638–8645.
Kaushik, C. P., Agarwal, H. C., & Pillai, M. K. K. (1991). Dry aerial fallout of organochlorine insecticide residues in Delhi India. Environmental Pollution, 71(1), 83–86.
Kaushik, C. P. (1991). Persistence and metabolism of HCH and DDT in soil under subtropical conditions. Soil Biology and Biochemistry, 23(2), 131–134.
Kaushik, C. P. (1989). Loss of HCH from surface soil layers under subtropical conditions. Environmental Pollution, 59(3), 253–264.
Kaushik, C. P., Pillai, M., Raman, A., & Agarwal, H. C. (1987). Organochloride insecticide residues in air in Delhi India. Water Air & Soil Pollution, 32(1), 63–76.
Kaushik, C. P., Sharma, H. R., Jain, S., Davvra, J., & Kaushik, A. (2008). Pesticide residues in river Yamuna and its canals in Haryana and Delhi India. Environmental Monitoring & Assessment, 144(1–3), 329–40.
Kerr, S. B., Bonczek, R. R., McGinn, C. W, Land, M. L., Bloom, L. D., Sample, B. E., & Dolislager, F. G. (1998). The risk assessment information system, Office of scientific & technical information technical reports, http://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/201903/t20190312_695462.html. Accessed 31 Aug 2021.
Li, L. I., Zheng, J. P., Fan, C. N., Liu, S. L., Wang, C., & Guo, Z. L. (2013). Analyzing on landscape patterns of land-use in Tumenjiang watershed. Journal of Beihua University, 14(2), 95–100.
Li, Q., Lu, Y., Wang, P., Wang, T., Zhang, Y., Suriyanarayanan, S., Liang, R., Baninla, Y., & Khan, K. (2018). Distribution, source, and risk of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in urban and rural soils around the Yellow and Bohai Seas China. Environmental Pollution, 239(AUG), 233–4.
Li, Y. F., Cai, D. J., Shan, Z. J., & Zhu, Z. L. (2001). Gridded usage inventories of technical hexachlorocyclohexane and lindane for china with 1/6° latitude by 1/4° longitude resolution. Archives of Environmental Contamination & Toxicology, 41(3), 261–266.
Li, Z., Ma, Z., Kuijp, T., Yuan, Z., & Lei, H. (2014). A review of soil heavy metal pollution from mines in china: Pollution and health risk assessment. Science of the Total Environment, 468–469, 843–853.
Liu, C., Yuan, G. L., Yang, Z. F., Yu, T., Xia, X. Q., Hou, Q. Y., & Chen, L. (2011). Levels of organochlorine pesticides in natural water along the Yangtze River, from headstream to estuary, and factors determining these levels. Environmental Earth Sciences, 62(5), 953–960.
Liu, J., Qi, S., Yao, J., Yang, D., Xing, X., Liu, H., & Qu, C. (2016). Contamination characteristics of organochlorine pesticides in multimatrix sampling of the Hanjiang River Basin, southeast China. Chemosphere, 163, 35–43.
Luo, X., Mai, B., Yang, Q., Fu, J., Sheng, G., & Wang, Z. (2004). Polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides in water columns from the Pearl River and the Macao harbor in the Pearl River Delta in South China. Marine Pollution Bulletin, 48(11/12), 1102–1115.
Luz, R. S., Ricardo, C. C., Norma, R. P., Antonio, T. A., Griselda, G. N., Violette, G., & Ricardo, B. M. (2014). Levels of organochlorine pesticides in blood plasma from residents of malaria-endemic communities in Chiapas, Mexico. International Journal of Environmental Research & Public Health, 11(10), 10444–10460.
Ministry of Ecology and Environment, Ministry of Foreign Affairs, Development and Reform Commission, Ministry of Science and Technology, Ministry of Agriculture and Rural Affairs, Ministry of Commerce, Health Commission, Emergency department, General Administration of Customs, General Administration of Market Supervision. (2019). Announcement on prohibition of production, circulation, use and import and export of persistent organic pollutants such as lindane. http://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/201903/t20190312_695462.html. Accessed 31 Aug 2021.
Mrema, E. J., Rubino, F. M., Brambilla, G., Moretto, A., Tsatsakis, A. M., & Colosio, C. (2013). Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology, 307, 74–88.
Qu, C., Qi, S., Dan, Y., Huang, H., & Xing, X. (2015). Risk assessment and influence factors of organochlorine pesticides (OCPs) in agricultural soils of the hill region: A case study from Ningde, southeast China. Journal of Geochemical Exploration, 149, 43–51.
Ravindran, Jayaraj, Pankajshan, Megha, Puthur & Sreedev (2016). Review article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary Toxicology, 9(3–4), 90–100.
Ribes, A., Grimalt, J. O., Torres Garcia, C. G., & Cuevas, E. (2002). Temperature and organic matter dependence of the distribution of organochlorine compounds in mountain soils from the subtropical Atlantic (Teide, Tenerife Island). Environmental Science & Technology, 36(9), 1879–1885.
Tang, D., Liu, X., He, H., Cui, Z., Gan, H., & Xia, Z. (2020). Distribution, sources and ecological risks of organochlorine compounds (DDTs, HCHs and PCBs) in surface sediments from the Pearl River Estuary. China. Marine Pollution Bulletin, 152, 110942.
Tang, J., An, T., Li, G., & Wei, C. (2018). Spatial distributions, source apportionment and ecological risk of SVOCs in water and sediment from Xijiang River, Pearl River Delta. Environmental Geochemistry & Health, 40(5), 1853–1865.
Tang, Z., Yang, Z., Shen, Z., Niu, J., & Cai, Y. (2008). Residues of organochlorine pesticides in water and suspended particulate matter from the Yangtze River catchment of Wuhan China. Environmental Monitoring & Assessment, 137(1–3), 427–439.
Tao, S., Xu, F. L., Wang, X. J., Liu, W. X., & Luo, Y. M. (2005). Organochlorine pesticides in agricultural soil and vegetables from Tianjin China. Environmental Science & Technology, 39(8), 2494–2499.
Tham, T. T., Anh, H. Q., Trinh, L. T., Lan, V. M., Truong, N. X., Yen, N. T. H., Anh, N. L., Tri, T. M., & Minh, T. B. (2019). Distributions and seasonal variations of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in surface sediment from coastal areas of central Vietnam. Marine Pollution Bulletin, 144, 28–35.
USEPA (2001). Supplemental guidance for developing soil screening levels for Superfund sites. Office of Solid Waste and Emergency Response, 9355, 4–24.
Walker, K., Vallero, D. A., & Lewis, R. G. (1999). Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environmental Science & Technology, 33(24), 4373–4378.
Wang, B., Yu, G., Huang, J., Yu, Y., Hu, H., & Wang, L. (2009). Tiered aquatic ecological risk assessment of organochlorine pesticides and their mixture in Jiangsu reach of Huaihe River China. Environmental Monitoring & Assessment, 157(1–4), 29–42.
Wang, G. L., Ma, L. M., Sun, J. H., & Gan, Z. (2010). Occurrence and distribution of organochlorine pesticides (DDT and HCH) in sediments from the middle and lower reaches of the Yellow River, China. Environmental Monitoring & Assessment, 168(1–4), 511–521.
Wei, L., Yang, Y., Li, Q. X., & Wang, J. (2014). Composition, distribution, and risk assessment of organochlorine pesticides in drinking water sources in South China. Water Quality, Exposure and Health, 7(1), 89–97.
Yang, Q. S., Mai, B. X., Fu, J. M., Sheng, G. Y., & Wang, J. X. (2004). Spatial and temporal distribution of organochlorine pesticides (OCPs) in surface water from the Pearl River Artery estuary. Huan Jing Ke Xue, 25(2), 150–156.
Yang, X., Wang, S., Bian, Y., Chen, F., Yu, G., Gu, C., & Jiang, X. (2008). Dicofol application resulted in high DDTs residue in cotton fields from northern Jiangsu province, China. Journal of Hazardous Materials, 150(1), 92–98.
Zhang, Z., Huang, J., Yu, G., & Hong, H. (2004). Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environmental Pollution, 130(2), 249–261.
Zhou, R., Zhu, L., & Chen, Y. (2008a). Levels and source of organochlorine pesticides in surface waters of Qiantang River, China. Environmental Monitoring & Assessment, 136(1–3), 277–287.
Zhou, R., Zhu, L., Chen, Y., & Kong, Q. (2008b). Concentrations and characteristics of organochlorine pesticides in aquatic biota from Qiantang River in China. Environmental Pollution, 151(1), 190–199.
Acknowledgements
The authors acknowledge the support of the Education Department of Jilin Province (Study on Habitat Characteristics and Survival Strategies of Three Early Spring Plants in Summer Green Broad-leaved Forest, JJKH20180350KJ), Scientific Investigation Report Project of Three Lakes National Nature Reserve of Songhua River of Jilin (ZCGJ-2020-FW006), and the Doctoral Scientific Research Foundation of Beihua University (Characteristics of Heavy Metal Pollution and Accumulation in Vegetable Soils in the Suburbs of Typical Industrial Cities).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qiang, L., Jinping, Z., Zhongling, G. et al. Sources and Health Risk of Organochlorine Pesticides in Surface Water from Buerhatong River and Hunchun River in Northeast China. Water Air Soil Pollut 232, 407 (2021). https://doi.org/10.1007/s11270-021-05329-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05329-3