Abstract
Polycyclic aromatic hydrocarbons are of great concern due to their persistence, bioaccumulation and toxic properties. The occurrence, source and ecological risk assessment of 26 polycyclic aromatic hydrocarbons in industrial and agricultural effluents affecting the Wadi El Bey watershed were investigated by means of gas chromatographic/mass spectrometric analysis (GC/MS). Total PAHs (∑ 26 PAH) ranged from 1.21 to 91.7 µg/L. The 4- and 5-ring compounds were the principal PAHs detected in most of 5 sites examined. Diagnostic concentration ratios and molecular indices were performed to identify the PAH sources. Results show that PAHs could originate from petrogenic, pyrolytic and mixed sources. According to the ecotoxicological assessment, the potential risk associated with PAHs affecting agricultural and industrial effluents ranged from moderate to high for both aquatic ecosystem and human health. The toxic equivalency factor (TEF) approach indicated that benzo[a]pyrene and benz[a]anthracene were the principal responsible for carcinogenic power of samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution has been aggravated all over the world by industrialization, urbanization and technological development. In fact, all of these processes entail the dumping of toxicants into receiving water bodies, which induces undesirable effects onto aquatic environment (Moses et al. 2015). Wastewater released from different industrial and domestic sources contain different types of contaminants ranging from inorganic pollutants (e.g., nitrates, phosphates, heavy metals) to organic compounds (e.g., persistent organic pollutants POPs, chlorinated hydrocarbons, pesticides) (Hamid et al. 2016; Haddaoui et al. 2016; Wang et al. 2014). The presence of toxicants deteriorates the water quality and also poses risk to consumption by humans (Mudhaf et al. 2009). As belonging to toxic and quite persistent organics, polycyclic aromatic hydrocarbons (PAHs) are a numerous class of compounds whose molecular structure is formed by ≥2 fused aromatic rings, which ubiquitously threaten the environment (Zhang et al. 2014) due to their carcinogenic and mutagenic properties (Aziz et al. 2014; Qin et al. 2014; Deng et al. 2006). The PAH contamination of aquatic systems is more and more gaining attention by environmental protection agencies and scientific institutions (Bai et al. 2014). At this regard, 16 PAH species have been designated as priority pollutants by the US Environmental Protection Agency (US EPA) (Sereshk and Bakhtiari 2014) and 7 of them have been classified as certain, probable or possible carcinogens to humans by International Agency for Research on Cancer (Qin et al. 2013a, b). PAHs affecting natural environments are mainly derived from man’s activities, e.g., combustion of biomass and fossil fuels, petroleum processing and spill, industrial manufacturing and vehicular traffic. Additionally, PAHs can be originated by natural sources, such as forest fires, volcanic eruptions and biosynthesis activities (Yanker et al. 2002; Lima et al. 2005; Parinos et al. 2013). PAHs can be transferred into water through several ways, such as anthropogenic and natural oil spills, surface runoff, industrial and municipal sewage, and water surface/air exchange (Tsapakis et al. 2006; Zhiguo et al. 2010). Therefore, the PAH behavior, transport, fate, sources and risk posed to ecological systems require extensive studies focused on the aquatic compartment (Guo et al. 2007). Recent researches have confirmed the adverse impact of PAHs on water bodies spread over different regions of the world (Zhang et al. 2012a, b, c; Lu et al. 2014; Sun et al. 2016; Luo et al. 2008a). The identification of PAHs sources found in the environment represents the first step to initiate every appropriate management and remediation plan. The concentration ratio approach between pairs of compounds is among the widely used techniques to investigate PAHs origin (Yuan et al. 2001; Yanker et al. 2002). To characterize the pollution sources of waters, the PAH molecular signature was examined, using PAH concentration ratios as diagnostic. Due to large differences in stability, solubility and absorption, PAH concentrations change during the migration process from sources to waters; thus, PAHs with relatively high stabilities and similar molecular weights were chosen for this investigation (Yanker et al. 2002; Luo et al. 2008a, b).
Hydrocarbon pollution in Tunisia has been well documented in recent years (Louati et al. 2001; Trabelsi and Driss 2005; Zaghden et al. 2005a, b; 2017; Zrafi-Nouira et al. 2008, 2009, 2010; Zrafi et al. 2013); however, most studies have been focused on marine environment, while only a few of them have been aimed at assessing hydrocarbon contamination in wastewaters and their receiving bodies (wadies, lakes, rivers, etc.). Watersheds receive a variety of wastewaters from agriculture runoff, industrial and municipal effluents; in particular, pollution caused by xenobiotic contaminants associated with industrial and agricultural effluents dumped into Wadi El Bey watershed has attracted great concern. This study was aimed at producing the first database concerning PAH loads in the effluents dumped there.
In light of the collected data, our study aimed at: (1) investigating the spatial–temporal distribution of PAHs at a relatively large scale through the analysis of industrial and agricultural dumping in different seasons; (2) discriminating the possible sources of PAHs by means of diagnostic concentration ratios approach; and (3) assessing the environmental risks associated to these contaminants affecting surface wastewater of Wadi El Bey watershed.
Materials and method
The study area
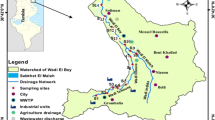
Wadi El Bey watershed is located in the northeast of Tunisia, southeast of the Cap Bon Peninsula (Fig. 1). It covers a total area of approximately 664 km2 that includes several urban agglomerations. Among them, Soliman, Bou Argoub, Grombalia and Menzel Bouzelfa host several industrial districts (food-processing industry, paper mills, tannery, etc.) and an important agricultural activity that employs huge amounts of organic and chemical fertilizers. The hydrographic network overall consists of Wadi El Bey, the most important river in the governorate, and its major tributaries Wadi Masri, Tahouna and el Malah, whose waters are controlled by hillside dams. Wadi El Bey has become heavily polluted not only due to natural outlet of runoff, but also due to domestic effluents, industrial discharges and wastes of agricultural drainage produced in the region, which contain metal and organic pollutants such as polycyclic aromatic hydrocarbons. The river pours all of its waters into the sebkha of Soliman, wetlands of relevant ecological value and wintering place for migratory birds. The area has been declared as sensitive for its biological diversity by the Convention signed in 1971 by the Delegates at International Conference of Ramsar, Iran, for the conservation and sustainable use of wetlands (Mili 2016); the sebkha also acts as a flood zone absorbing the floods of Wadi El Bey during the pluvial periods and eventually draining into the Gulf of Tunis through a dune cordon. The direct and permanent dumping of treated and untreated waters in Wadi El Bey exerts an important pressure on quality of the whole river system comprising the wadi, sebkha and coastal zone. Sewage treatment plants and industrial units located on its banks represent sources of significant punctual pollution affecting water quality in all components of hydro-system. Diffuse pollution of agricultural and natural areas located in the watershed and runoff from urban areas also contribute to deterioration of the quality of these waters, especially during the winter season. For this study, the selection of the sampling sites privileged the Wadi El Bey watershed sites that received industrial effluents and agricultural drainage, posing special attention to these sources of pollution.
Sampling location in the Wadi El Bey watershed. W-IGr represents the discharge of industrial area—Grombalia, W-IB represents the discharge of the Beer industry, W-TI represents the Tannery discharges (Oued Tahouna), W-Ag-WM represents the agricultural drainage—Wadi Maleh, and W-Ag-BK represents the agricultural drainage—Béni Khaled
Sample collection
Surface waters were collected from 2013 to 2015 at five sites during the four year seasons (summer, winter, spring and autumn). Sampling was carried out by means of 1-L amber glass screw-cap bottles. At least 2 samples for season were collected in each site. The distribution of the sampling points is shown in Table 1 using the Global Positioning System. After collection, the samples were stored at 4 °C until analysis.
Hydrocarbon extraction and cleanup (liquid chromatography)
Before analysis, water samples were filtered, the analytical procedure used to extract the hydrocarbon content from waters was a modification of the method described by Saliot (1989). To summarize, total hydrocarbons (TH) in water samples (50 mL) were liquid/liquid-extracted three times for 1 h with 100 mL of pure chloroform, using a separatory funnel. Standard mixtures of deuterated PAHs in toluene (1000 μg/mL) were used to calculate the recovery efficiency; the average percentage recovery of total PAHs achieved 96%. The extracts were concentrated to 2 mL using a rotary evaporator and reduced to dryness under gentle nitrogen flow. Afterward, the extracts were weighed and separated into non-aromatic hydrocarbon (NAH) and aromatic hydrocarbon (AH) fractions through adsorption liquid chromatography. A column of silica gel was used for this purpose, while pure n-hexane and a mixture of n-hexane/chloroform (2:1 in volume) were chosen to elute NAH and AH fractions, respectively. The mini-column (5 mm internal diameter, 150 mm length) was filled with 1.0 g of silica gel, type 60 for chromatography (63–200 mm). Prior to use, the silica was conditioned overnight at 110 °C. Following solvent evaporation, NAHs were dissolved in cyclohexane, fortified with a mixture of perdeuterated PAHs acting as internal standards for analysis and processed through gas chromatography coupled to mass spectrometry. TH was thus expressed by the sum of non-aromatic and aromatic hydrocarbons, while highly polar compounds were not accounted for. (TH = NAH + AH).
Gas chromatography–mass spectrometry (GC–MS) analysis
The analytes were detected and quantified using a Trace GC Ultra gas chromatograph equipped with an AS-8000 auto-sampler and Trace DSQ-II quadrupole mass spectrometer (both from Thermo, Rodano, Italy). The separation of analytes was obtained through a Restek 30-m-long Rtx®-5MS-type capillary column (i.d. = 250 μm, film = 0.25 μm, provided by Superchrom, Milan, Italy). The column temperature was held at 90 °C for 2 min, then raised to 170 °C at + 20 °C/min, and kept constant for 2 min; a second ramp (+ 5 °C/min rate) held the column up to 290 °C, where elution was completed within 8 min. The carrier gas was helium (1 mL/min). Sample injection was operated in the split-less mode (T = 280 °C, valve closure = 1 min). The analyte detection was performed in the positive selective ion monitoring (SIM) mode, and signals were acquired by a dedicated software (Excalibur) purchased from Thermo. Target compounds were identified by comparing retention times and mass spectrums of GC peaks with those of authentic standard solution and using a certified standard reference National Institute of Standards and Technology (NIST, USA) and homemade spectrum libraries. To ensure quality analyses, standards were injected and analyzed under the same conditions as those used for the samples. Each analyte was referred to its perdeuterated homolog when present, or to the closest internal reference compound in the chromatogram. Compounds were quantified by comparing the peak areas with those of the co-injected standards. A total of 26 PAHs from naphthalene to dibenz[a,h]anthracene were investigated together with methylated homologs.
Quality assurance and quality control
Before analysis, standard solutions (mixtures of PAHs) were run to check column performance, peak height and resolution. A PAH mixture in toluene (purchased from Sigma, Milan, Italy) was used to prepare the standard and calibration stock solutions. After checking the linearity of the instrumental response, the efficiency of the whole analytical procedure and the absence of matrices effects were valued by spiking equivalent portions of a sample bulk with increasing amounts of standard. The amounts of analytes measured were plotted versus the concentration added. The intercept point of the regression line on the concentration axis was equal to the amount of analyte in the original sample. The standard addition graph for all PAHs in sediments extracted was linear with coefficients of determination (R2) better than 0.98.
Statistical analyses
The concentration data of the individual and total polycyclic aromatic hydrocarbon were statistically analyzed using Statistica software. A total of 40 samples were used for the statistical analysis. Results were expressed as mean ± SE (standard error). Comparisons among multiple groups of samples, for each site and each season, were achieved by one-way ANOVA followed by Tukey’s multiple comparison Test. Statistical significance was defined as P < 0.05.
Principle component analysis (PCA) is a multivariate analytical tool used to reduce unrelated original index variables and to extract a small number of factors, which explain most of the variance of the original index (Jiacheng et al. 2015). PCA was performed on individual PAH concentrations of wastewater with the varimax rotation method (Hu et al. 2014). Two factors were obtained, and the profiles of the factor loadings were used to infer the possible sources of PAHs.
Results and discussion
Spatial and seasonal distribution of PAH
Figure 2 illustrates the spatial–temporal distribution of PAHs in wastewater resulting by the analysis of samples collected in different sampling sites and in the four seasons. The total concentrations of 26 PAHs (ΣPAHs) in surface water showed a wide variability, ranging from 1.22 ± 0.01 to 91.7 ± 0.09 µg/L.
Seasonal distribution of polycyclic aromatic hydrocarbon in industrial and agricultural effluents. The blue histogram shows the concentration of PAHs (ng/L) in autumn. The red histogram shows the concentration of PAHs (ng/L) in winter. The green histogram shows the concentration of PAHs (ng/L) in spring. The orange histogram shows the concentration of PAHs (ng/L) in summer
In industrial effluents, ΣPAH ranged from 1.30 to 91.7 µg/L with the mean reaching 15.0 ± 0.01 µg/L. The W-IGr site (influents from Grombalia industrial area) experienced the maximum concentration of PAHs; this finding probably depended on the high number of industries (> 30) wasting their effluents into the Wadi El Bey watershed.
In agricultural effluents, ΣPAH ranged from 1.22 to 18.7 µg/L (mean = 4.86 ± 0.68 µg/L), the maximum occurring in W-Ag-Bk (agricultural drainage, Beni Khaled site); therefore, industrial districts resulted more polluted than agricultural areas.
As put in evidence in Fig. 2, in general in most of the sampling sites, ∑PAHs concentrations raised during the cold seasons. This could be caused by the high effluent volumes dumped into the Wadi El Bey watershed during the warm period (flow rates ~ 2 m3/s in summer and ~ 0.10 m3/s in winter). Indeed, hydrological conditions and the strength of pollutant sources are the major factors modulating the PAHs presence in the water bodies. In our case, the contaminants were probably diluted by big water volumes recorded in the hot season; resulting in low PAH concentrations in that year time (Lu et al. 2014). Furthermore, the low concentration of PAH in W-Ag-WM in winter can be attributed to the high inputs of agricultural effluents discharged during winter. The contaminants could be diluted by large volume of effluents (W-Ag-WM) in cold season.
Apart from PAH inputs, the PAH concentration in surface water can be affected by losses and outputs, due to evaporation, absorption by suspended particulates, integration into the food chains, biodegradation and photodegradation (Tsapakis et al. 2006; Guigue et al. 2014). An important role in the surface water output of low molecular weight PAHs (LPAHs) can be played by evaporation. Evaporation to the atmosphere is the most important process that hydrocarbons undergo with regard to their vapor pressure, after introduction/deposition to surface waters (Yamada et al. 2003). Degradation by bacteria and photodegradation lead to synergetic reactions that enhance the degradation (Watkinson and Griffiths 1987; Literathy et al. 1989). Seasonal variations of removing processes have also been widely described. In this study, in summer when temperature is near 35 °C, evaporation was maximal and the flow was very low. In these conditions, concentrations of PAH in the reservoir water will be decreased. Lower molecular weight PAHs such as naphthalene and anthracene are more soluble and degradable; however, higher molecular weight compounds such as pyrene and benz[a]anthracene are more recalcitrant (Obayori and Salam 2010). In our study area, naphthalene ranged from 32.1 to 228.2 ng/L during the cold season and 19.2 to 95.3 ng/L in warm season, which highlighted the increase in weathering effects during the warmer months.
Table 2 presents a worldwide comparison of PAH levels recorded determination in water bodies. Based on that, total PAH concentrations were relatively high in surface waters of the Wadi El Bey watershed. However, it should be taken in account that most studies were limited to the 16 US EPA priority PAHs, hence, the concentration there reported was not fully representative of total PAH contents in the corresponding study areas (Yuhu et al. 2015). The PAH concentrations in the industrial and agricultural discharges affecting Wadi El Bey exceeded those valued in previous studies in Tunisia (Gulf of Tunis, Gulf of Gabes) (Mzoughi and Chouba 2011; Fourati et al. 2017) and also of most areas all over the world listed Northwestern Mediterranean Sea (France), Anacostia River (USA), Marseille coastal area (France), Venice Lagoon (Italy) (Lu et al. 2014; Hwang and Foster 2016; Guigue et al. 2011; Manadori et al. 2006). Furthermore, PAHs contamination in surface water could be categorized into four grades, namely micro-polluted, 10–50 ng/L; light-polluted, 50–250 ng/L; moderately polluted, 250–1000 ng/L and heavily polluted, > 1000 ng/L (Chen 2008). ∑PAHs in wastewater discharged into Wadi El Bey watershed ranged from 1.218 to 91.7 µg/L, so that all sampling sites could be classified as heavily PAH polluted.
PAH composition according to ring number
PAHs under study were divided into five groups according to number of aromatic rings (2–6). The concentrations of the five PAH groups in effluents from the Wadi El Bey watershed are shown in Table 3, which reports also the respective seasonal trends. The percent distribution was quite similar in most sites within the same season. Indeed, 4-ring PAHs were the most abundant in the majority of sampling sites (87% in winter at W-TI), followed by 3-ring congeners (81% in spring at W-Ag-WM). Six-ring PAHs were scarce and were detected only in the autumn. As total, high molecular weight homologs HMW PAHs (4 to 6 rings) were predominant in most sampling sites and seasons, the concentrations ranging from 0.084 to 63.2 µg/L with the maximum detected in W-IGr during the winter; instead, low molecular weight (LMW) PAHs (2 and 3 rings) predominated only in W-Ag-WM site (agricultural drainage, Wadi Maleh) during the winter and spring and in W-Ag-Bk in the autumn. LMW PAHs, such as Nap, Ant and Phe, are more labile and are expected to degrade faster than HMW compounds; moreover, their relatively high vapor pressure compared to HMW PAHs favors volatilization (Fernandes et al. 1997; Macias-Zamora et al. 2002). In fact in the summer, when temperatures increase, the net volatilization of LMW hydrocarbons occurs (Fingas 1995; Dachs et al. 2002; Palm et al. 2004). Besides, low concentrations of LMW hydrocarbons in summer have been often ascribed to higher rates of photo- and biodegradations (Yamada et al. 2003; Palm et al. 2004). For example, in site W-IGr the concentration of LMW decreases from 25.26 µg/L in winter to 6.94 µg/L in summer and this is due to evaporation of compounds because of the high temperature registered in this site in warm season. The PAH fingerprint in the samples provides important information about the sources affecting the sites from which they come (Cao et al. 2005). Many studies reported that the predominance of alkylate, 2- and 3-ring PAHs indicated the petrogenic nature of pollution sources, e.g., crude oil, unburned petroleum and its products, while that of 4- to 6-ring PAHs is related to pyrogenic sources, i.e., to incomplete combustion of organic matters such as coal and petroleum (Xu et al. 2007; Cheng et al. 2009). This is of concern, because 4- and 5-ring PAHs display toxicity and environmental persistence properties (Cerniglia 1992).
Source identification of PAHs
Diagnostic concentration ratios of PAHs
PAHs are introduced into the environment mainly via industrial wastes, fossil fuel combustion, petroleum spills, vehicle exhausts, as well as through nonpoint sources such as urban runoff and atmospheric fallout (Countway et al. 2003).
According to formation mechanisms, PAHs can be classified as pyrogenic and petrogenic. Pyrogenic PAHs are formed during incomplete combustion of organic matter, while petrogenic PAHs are derived from petroleum (crude oil and its refined products). PAH molecular indices based on different thermodynamic stability have been widely used to identify potential PAH sources in environmental samples (Budzinski et al. 1997; Yunker et al. 2002; Tuncel and Topal 2015).
Since the molecular fingerprints of PAHs change with the nature of emission, indices based on concentration ratios between PAH species have been derived to identify sources affecting environmental compartments, despite this approach can be regarded only as qualitative, because of variability of calculated ratio rates due to distinct volatility and persistence of compounds. To reduce possible artifacts, congeners of similar volatility (i.e., isomeric species) and good stability are usually chosen. The sole important exception is the BaP/BeP ratio that, owing to high persistence of the latter compared to the former, looks indicative of the aging of samples (Yanker et al. 2002; Liu et al. 2008; Cecinato et al. 2014). In this study, several PAH ratios were examined. They include the (Phe/Ant), Flu/(Flu + Pyr), InP/(InP + BgP), (BaA/(BaA + Chr), (MPhe/Phe) pairs, all of them proposed by the scientific literature for interpreting the petrogenic and pyrogenic nature of PAHs (Table 4) (Yanker et al. 2002; Keshavarzifard et al. 2014; Zakaria et al. 2002). As for Phe/An and Fluo/Pyr ratios, values < 10 and > 1, respectively, indicate that contamination results from combustion processes (Soclo et al. 2000). In fact, anthracene and fluoranthene are less thermodynamically stable than phenanthrene and pyrene, respectively. Though these substances are often associated with each other in natural matrices, the predominance of Flu over Pyr looks as typical of pyrolytic processes. In petroleum-derived emissions, pyrene is more abundant than fluoranthene. Moreover, LMW/HMW PAHs ratios < 1 suggest pyrolytic contamination, while ratios > 1 are typical of petrogenic sources (Budzinski et al. 1997).
According to our data (Table 4), Phe/Ant was < 10 and Flu/Pyr > 1 in W-IGr (spring), W-BI (winter, spring, summer), W-TI (autumn, summer) and W-Ag-WM (spring, summer). Thus, at the four sites pollution was of pyrolytic nature; on the other hand, the others sites seemed to present both pyrolytic and petrogenic contamination. The source assignment was confirmed by the LMW/HMW PAH ratio rates (e.g., see data for W-Ag-WM both in the winter and spring).
Furthermore, BaA/(BaA + Chr) ratios of < 0.2, 0.2–0.35 and > 0.35 are associated with petroleum, mixed origin and combustion sources, respectively (Gao et al. 2007; Bouloubassi et al. 2012). In this study, most sites were characterized by BaA/(BaA + Chr) rates exceeding 0.35, which implied wood and coal combustion. On the other hand, the mixed origin for PAHs was supposed in W-IGr (autumn, spring), W-BI (spring), W-TI (summer), W-Ag-WM (winter).
BaA/(BaA + Chr) and Ant/(Ant + Phe) ratios were plotted versus Flu/(Flu + Pyr) for each data point and season to analyze how PAHs were distributed with respect to possible origins (Fig. 3). According to graphs, the sites were likely affected by a blend of petrogenic and pyrolytic inputs. Pyrogenic emissions show MPhe/Phe rates < 1, while fresh petroleum-related contamination is characterized by rates ranging 2–6 (Garrigues et al. 1995), and values falling between 1 and 2 are indicative of mix of pyrogenic and petrogenic emissions (coal, used lubricants, automobile exhausts) (Luo et al. 2008a, b). In our study, MPhe/Phe rate was > 2 at most sites indicating fresh petroleum inputs, the only exceptions occurring at W-IGr in the winter and at W-Ag-Bk in the spring (1 < MPhe/Phe < 2).
Plots of isomeric ratios BaA/(BaA + Chr) and Ant/(Ant + Phe) versus Flu/(Flu + Pyr) in different seasons. The red color points represent the ratio in winter. The blue color points represent the ratio in summer. The orange points represent the ratio in autumn. The green points represent the ratio in spring
Khalili et al. (1995) and Guo et al. (2003) found BaP/(BaP + Chr) ratios equal to 0.49 and 0.73, respectively, in gasoline and diesel engine exhausts, while 0.07–0.24 values (Chen et al. 2005) were indicative of grass, wood and coal combustion. According to that (Table 3), the Wadi El Bey area was impacted by vehicular emissions.
The ratio of benzo[b]fluoranthene to benzo[k]fluoranthene (BbF/BkF) has been used to distinguish among pyrogenic sources (Morillo et al. 2008; Walker and Dickhut 2001). The BbF/BkF ratio has shown distinct rates in the cases of aluminum smelters (2.5–2.9), coke ovens (3.7), motor vehicles (1.3), creosote (1.2) and wood soot (0.9) (Dickhut et al. 2000; Stout et al. 2003; Tobiszewski and Namiesnik 2012). In our study, this ratio seemed to suggest the occurrence of wood soot in W-IGr and W-TI as well as of coke oven dust in W-BI in the spring and of creosote in W-BI during the summer.
In conclusion, according to PAH diagnostic ratios the pyrolytic sources were predominant, though somewhere petrogenic emission could contribute.
PCA analysis
PCA is a statistical tool that resets large amounts of data and data sets (Simpson et al. 1996). PCA was applied to data set to facilitate the visualization of similarities and differences. For this purpose, the varimax rotation was adopted with principal components (factors) present in eigen values > 1 to identify possible sources.
PCA loading plots for wastewater from industrial and agricultural effluents dumped in the watershed of Wadi El Bey are shown in Fig. 4. Component 1 (PC1) could explain 41.69% of total variance, with high loadings on Nap, Acy, Phe, Ant, Pyr and Fl (overall 2- and 3-ring PAHs). InP is an indicator of diesel combustion (Li and Kamens 1993). This seems to indicate that petrochemical wastewater and exhausts coming from industrial area were the main pollution source in the study area. Factor 2 was highly weighted by 4- and 5-ring PAHs (Chry, BaA, BaP, BaF and Fluo). Vehicles are known to release considerable percentages of Chry (Harrison et al. 1996), while BaA is commonly produced by both gasoline and diesel combustion (Lee et al. 2004). Thus, PCA confirmed that wastewater contamination of Wadi El Bey was determined by both petrogenic (3-ring) and pyrolytic sources, whose relative importance depended on the site.
Environmental risk assessment
Potential toxicity of carcinogenic PAHs
Although many PAHs and by-products display long-term toxicity, attention is overall paid to seven compounds (BaA, BaP, BbF, BkF, Chry, DBA and Ind), classified by IARC as possible, probable or certain carcinogens for humans (CPAHs) (Keshavarzifard et al. 2015). The three major routes of exposure of PAHs to humans are inhalation, ingestion and dermal contact (Neff 1979; Weast 1968). In this study, low-income people living close to the watershed of Wadi El Bey are currently exposed to polluted water. Ingestion pathway seems to pose low-risk exposure pathway even when untreated water is used. Attention to dermal absorption should be useful for protecting children, who play in the rivers as a recreational activity. Moreover, people in poverty situation use directly river water to carry out domestic activities; those living close to the study area could be exposed to PAHs via dermal contact. In this study, the concentration of PAH in industrial effluents is much higher than that in agricultural effluents; that’s why workers in the wastewater treatment facilities (specially industrial activities) must be protected from direct contact with the wastewater in order to reduce the likelihood of skin cancer.
The concentrations of CPAHs in wastewaters from the watershed of Wadi El Bey ranged from 484 to 1415 µg/L (Table 5); the maximum was recorded at W-IB, and the minimum at W-Ag-WM. CPAHs in the watershed of Wadi El Bey was quite high (2–26%) compared to other rivers in the world (e.g., Gomti River 6.93%) (Malik et al. 2011).
Though benzo[a]pyrene is the only PAH for which adequate toxicological data are available (WHO 2005; Chen and Chen 2011), toxic equivalency factors (TEFs) have been established to index the carcinogenicity of other PAHs relative to BaP and have been used to estimate benzo[a]pyrene-equivalent doses (Nadal et al. 2004). Consequently, TEFs were used to evaluate the overall toxicity and assess the subsequent risks induced by PAHs affecting environmental samples. TEFs used in our study were as follows: 1.0 for BaP; 0.1 for BaA, BbF, BkF and InP; and 0.01 for Chr (US EPA 2010; Nekhavhambe et al. 2014) (DBA was not taken in account, since it was never detected in examined samples). According to IARC (1987), the benzo[a]pyrene-equivalent toxicities (TEQ) of each i-PAH and of their sum are:
After conversion of neat concentrations of the seven carcinogenic PAHs into BaP (eq.dose) values, total toxicity (TEQ) was calculated. Total TEQ values calculated for samples from industrial and agricultural effluents varied from 75.2 to 237.3 ng/L (Table 5). Though the highest CPAH was measured at W-BI, the maximum value of total TEQ was found at W-TI, due to high content of BaP there.TEQ values were found to exceed 1 for all PAH congeners except Ind; therefore, harmful biological effects could be expected from carcinogenic PAHs affecting all wastewaters in the region.
Relative BaP (eq.doses) comprised in total TEQ resulted to be in the following order: BaP (33–87%) > BaA > BKF > BbF > Chry > Ind (Table 5). Similar contributions of BaP (43.6–70%) were calculated in other studies in different environments (Nadal et al. 2004; Savinov et al. 2003) confirming the importance of BaP as indicator of the overall PAH carcinogenity.
Ecological risk assessment
Ecological risk assessment (ERA) using risk quotients (RQ) is used to assess the impact caused by pollutants (i.e., human activities) onto living organisms belonging to ecosystems (Qiao et al. 2006). ERA includes chemical, physical and biological monitoring of waters and sediments (Ünlü and Alpar 2009). In the early stages of risk assessment RQ, namely the quotient of the measured or estimated environmental concentration divided by the toxicant reference value, was proposed for the individual value estimate (US EPA 2001). In recent decades, some indicators and methods of different complexities have been proposed for ERA of toxic chemicals in waters (Sany et al. 2012). The pollution ability to cause undesired aftermaths onto the environment can be estimated by means of ERA cumulative indexes (Solomon et al. 2000). PAHs always occur in water as a complex mixture of compounds, leading to cumulative effects on the ecological environment (Guo et al. 2011). Thus, PAHs in sediments pose a potential risk for aquatic ecosystems, and the ecological risk assessment has been shown to be a useful tool in characterizing the PAHs-associated risk posed to surrounding organisms and ecosystems (Wu et al. 2011). Kalf et al. (1997) proposed to assess the ecological risk of organic substances by using risk quotient (RQ), and Cao et al. (2010) improved the accuracy method by introducing the toxic equivalency factors. This new method was applied in this study to assess the ecological risk of PAHs in industrial and agricultural effluents discharges in Wadi El Bey watershed. The risk quotients (RQ) for each of PAHs were calculated as follows:
where CPAH is the concentration of each PAH accounted for and CQV is the corresponding quality value in the medium (Kalf et al. 1997). In the present study, the negligible concentrations (NCs) and the maximum permissible concentrations (MPCs) of PAHs in water, sediment and bank soil as reported by Kalf et al. were chosen as the quality values. MPCs refer to the concentration of pollutants above which the risk of adverse effects is considered unacceptable to ecosystems, and NCs refer to the concentration in the environment below which the occurrence of adverse effects is considered to be negligible (Crommentuijn et al. 2000).
Therefore, RQNCs and RQMPCs are defined as follows:
where CQV (NCs) is the quality value of the NCs of PAHs in the medium, and CQV (MPCs) is the quality value of the MPCs of PAHs in the medium. And the RQΣPAHs, RQΣPAHs (NCs) and RQΣPAHs (MPCs) are defined as follows:
Based on the ecosystem risk assessment of 16 individual PAHs, the sum of RQ (NCs) and RQ (MPCs) of individual PAHs exceeding 1.0 was adopted to estimate the cumulative risk assessment through RQ∑PAHs (NCs) and RQ∑PAHs (MPCs) (Table 6).
This approach allowed to classify the PAH-associated risk taking in account all toxic compounds independently of the medium nature, as well as to compare the risk for ecosystems induced by PAHs in distinct mediums (Sany et al. 2012). Table 5 reports NCs and MPC in waters for each PAH, and the corresponding RQ (NCs) and RQ (MPCs) values are calculated for Wadi El Bey watershed sites. In principle, RQ (NCs) < 1.0 indicated that the single PAHs might be of negligible concern, while RQ (MPCs) > 1.0 would indicate that contamination of single PAHs was severe and some control measures and remedial actions had to be undertaken. In case of RQ (NCs) > 1.0 and RQ (MPCs) < 1.0, contamination of single PAHs was at middle levels and control measures or remedial actions seemed to be useful.
As shown in Table 7, RQ(MPCs) corresponding to Nap (W-TI), Acy (W-IGr), Ace (W-IGr, W-Ag-WM), Flu (all sites), Phe (W-IGr), Ant (W-IGr), Flua (W-IGr, W-BI), Pyr (W-IGr, W-BI), BaA (all sites), Chry (W-IGr, W-BI, W-TI, W-Ag-Bk), BbF (W-IGr, W-BI, W-TI, W-Ag-Bk), BkF (W-IGr, W-BI, W-TI, W-Ag-Bk), BaP (all sites) and Ind (W-Ag-WM, W-Ag-Bk) exceeded 1, which indicated that severe toxicity might be caused to the aquatic organisms by PAHs. The mean values of RQ (NCs) in all sampling sites and for all individual PAHs exceeding 1 showed a middle-level risk for ecosystem.
RQ (MPCs) of ∑PAHs were all > 1 and all RQ (NCs) of ∑PAHs were > 800 (Table 6), so the cumulative risk posed by PAHs to ecosystem in industrial and agricultural effluents wasted in Wadi El Bey watershed was very high.
It could be observed from Fig. 5 that high molecular weight PAHs were the principal responsible for risk to ecosystem. In fact, HMW are highly mutagenic and carcinogenic, while low molecular PAHs are less mutagenic. Risk assessment indicates Nap, Acy, Ace, Phe, Ant, Flt, Pyr, BaA, BbF, Bkf, Bap and Chry had moderate or high ecotoxicological risks in the Wadi El Bey watershed, and the total concentrations of the 16 PAHs reached relatively higher levels. Therefore, more extensive investigations of sources of these PAHs, control measures and remedial actions should be conducted to decrease the contamination of 3-4-5 rings in the study region.
In particular, the main pollution sources (petroleum and combustion) should be controlled to decrease the level of PAHs.
Conclusion
The comprehensive survey of 26 PAHs in industrial and agricultural effluents discharges has been conducted in Wadi El Bey. PAH concentrations in industrial effluents were higher than in agricultural discharges with maximum in the W-IGr site. According to PAH levels, contamination in Wadi El Bey was relatively high. The PAH profiles indicated that the 4- and 5-ring PAHs were the main compounds at most sites. Diagnostic concentration ratios suggest that PAHs could originate from petrogenic, mixed and pyrolytic sources, depending on the site. According to water quality guidelines and calculated risk quotients, Wadi El Bey wastewaters were very polluted and posed moderate to high risk to the ecosystem. The results of this study would contribute to understanding the levels, sources and ecosystem risk of PAHs in the agricultural and industrial effluents wasted into Wadi El Bey watershed, directly connected to the Mediterranean Sea. This can provide useful information for human health and water resources protection in this dynamic region of the world. Finally, data on the compositional patterns and the identification of pollution source could be used as the baseline for further local or regional studies.
Abbreviations
- Ant:
-
Anthracene
- BaA:
-
Benz[a]anthracene
- BbF:
-
Benzo[b]fluoranthene
- BkF:
-
Benzo[k]fluoranthene
- BgP:
-
Benzo[ghi]perylene
- BeP:
-
Benzo[e]pyrene
- BaP:
-
Benzo[a]pyrene
- Chry:
-
Chrysene
- DBA:
-
Dibenz[a,h]anthracene
- Fluo:
-
Fluoranthene
- Indeno:
-
İndeno[1,2,3-cd]pyrene
- Mphe:
-
Methylphenanthrene
- Naph:
-
Naphthalene
- Pery:
-
Perylene
- Phe:
-
Phenanthrene
- Pyr:
-
Pyrene
- B(c)phe:
-
Benzo(c)phenanthrene
References
Aziz, F., Syed, J. H., Malik, R. N., Katsoyiannis, A., Mahmood, A., & Li, J. (2014). Occurrence of polycyclic aromatic hydrocarbons in the Soan River, Pakistan: Insights into distribution, composition, sources and ecological risk assessment. Ecotoxicology and Environmental Safety, 109, 77–80.
Bai, Y., Meng, W., Xu, J., Zhang, J., Guo, C., Lv, J., et al. (2014). Occurrence, distribution, environmental risk assessment and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in water and sediments of the Liaohe River Basin, China. Bulletin of Environment Contamination and Toxicology, 93, 744–747.
Bouloubassi, I., Roussiez, V., & Lorre, A. (2012). Sources, dispersal pathways and mass budget of sedimentary polycyclic aromatic hydrocarbons (PAH) in the NW Mediterranean margin, Gulf of Lions. Marine Chemistry, 142–144, 18–28.
Budzinski, H., Jones, I., Bellocq, J., Piérrad, C., & Garrigues, P. (1997). Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Marine Chemistry, 58, 85–97.
Cao, Z., Liu, J., Luan, Y., Li, Y., Ma, M., Xu, J., et al. (2010). Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbon in the Luan River, China. Ecotoxicology, 19, 827–837.
Cao, Z. H., Wang, Y. Q., Ma, Y. M., Xu, Z., Shi, J. L., Zhuang, Y. Y., et al. (2005). Occurrence and distribution of polycyclic aromatic hydrocarbons in reclaimed water and surface water of Tianjin, China. Journal of Hazardous Materials, A122, 51–59.
Cecinato, A., Guerriero, E., Balducci, C., & Mutio, V. (2014). Use of the PAH fingerprints for identifying pollution sources. Urban Climate, 10, 630–643.
Cerniglia, C. E. (1992). Biodegradation of polycyclic aromatic hydrocarbons. Bio-degradation, 3, 351–368.
Chen, Y. (2008). The spatial and temporal distribution, source and bioavailability of PAHs in Qiantang River (pp. 15–16). Hangzhou: College of Environmental and Resource Science, Zhejiang University.
Chen, C. W., & Chen, C. F. (2011). Distribution, origin, and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in sediments of Kaohsiung Harbor, Taiwan. Marine Pollution Bulletin, 63, 417–423.
Chen, Y. G., Sheng, G. Y., & Bi, X. H. (2005). Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environmental Science and Technology, 39, 1861–1867.
Cheng, W. C., Liu, C. H., & Leung, D. Y. C. (2009). On the correlation of air and pollutant exchange for street Canyons in combined wind-buoyancy driven flow. Atmospheric Environment, 43, 3682–3690. https://doi.org/10.1016/j.atmosenv.2009.04.054.
Countway, R. E., Dickhut, R. M., & Canuel, E. A. (2003). Polycyclic aromatic hydrocarbon (PAH) distributions and associations with organic matter in surface waters of the York River, VA Estuary. Organic Geochemistry, 34, 209–224.
Crommentuijn, T., Sijm, D., Bruijn, J. D., Vanleeuwen, K., & Vandeplassche, E. (2000). Maximum permissible and negligible concentrations for some organic substances and pesticides. Journal of Environmental Management, 58, 297–312.
Dachs, J., Lohmann, R., Ockenden, W. A., Méjanelle, L., Eisenreich, S. J., & Jones, K. C. (2002). Oceanic biogeochemical controls on global dynamics of persistent organic pollutants. Environmental Science Technology, 36(20), 4229–4237.
Deng, H., Peng, P., Huang, W., & Song, J. (2006). Distribution and loadings of polycyclic aromatic hydrocarbons in the Xijiang River in Guangdong, South China. Chemosphere, 64, 1402–1408.
Dickhut, R. M., Canuel, E. A., Gustafson, K. E., Liu, K., Arzayus, K. M., Walker, S. E., et al. (2000). Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environmental Science and Technology, 34, 4635–4640.
Fernandes, M. B., Sicre, M. A., Boireau, A., & Tronszynski, J. (1997). Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Marine Pollution Bulletin, 34, 857–867.
Fingas, M. F. (1995). A literature review of the physics and predictive modelling of oil spill evaporation. Journal of Hazardous Materials, 42, 157–175.
Fourati, R., Tedetti, M., Guigue, C., Goutx, M., Garcia, N., Zaghden, H., et al. (2017). Sources and spatial distribution of dissolved aliphatic and polycyclic aromatic hydrocarbons in surface coastal waters of the Gulf of Gabès (Tunisia, Southern Mediterranean Sea). Progress in Oceanography. https://doi.org/10.1016/j.pocean.2017.02.001.
Gao, S., Sun, C., & Zhang, A. (2007). Pollution of polycyclic aromatic hydrocarbons in China. Developments in Environmental Science, 7, 237–287.
Garrigues, P., Budzinski, H., Manitz, M. P., & Wise, W. A. (1995). Pyrolytic and petrogenic inputs in recent sediments: a definitive signature through phenanthrene and chrysene compound distribution. Polycyclic Aromatic Compounds, 7, 275–284.
Götz, R., Bauer, O. H., Frissel, P., & Rock, K. (1998). Organic trace compounds in water of the river Elbe near Hamburg. Chemosphere, 36, 2103–2118.
Guigue, C., Tedetti, M., Ferretto, N., Garcia, N., Méjanelle, L., & Goutx, M. (2014). Spatial and seasonal variabilities of dissolved hydrocarbons in surface waters from the Northwestern Mediterranean Sea: Results from 1 year intensive sampling. Science of the Total Environment, 466–467, 650–662.
Guigue, C., Tedetti, M., Giorgi, S., & Goutx, M. (2011). Occurrence and distribution of hydrocarbons in the surface microlayer and subsurface water from the urban coastal marine area off Marseilles, northwestern Mediterranean Sea. Marine Pollution Bulletin, 62, 2741–2752.
Guo, W., He, H., Yang, Z., Lin, C., Quan, X., & Wang, H. (2007). Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere, 68(1), 93–104.
Guo, W., He, M. C., Yang, Z. F., Lin, C. H., & Quan, X. C. (2011). Aliphatic and polycyclic aromatic hydrocarbons in the Xihe River, an urban river in China’s Shengyang City: Distribution and risk assessment. Journal of Hazardous Materials, 186, 1193–1199.
Guo, H., Lee, S. C., Ho, K. F., Wang, X. M., & Zou, S. C. (2003). Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmospheric Environment, 37, 5307–5317.
Haddaoui, I., Mahjoub, O., Mahjoub, B., Boujelben, A., & Di Bella, B. (2016). Occurrence and distribution of PAHs, PCBs, and chlorinated pesticides in Tunisian soil irrigated with treated wastewater. Chemosphere, 146, 195–205.
Hamid, A., Yaqub, G., Riaz, A., & Sadiq, Z. (2016). Physico-chemical characterization and quantification of selected persistent trace organic pollutants containing wastewater samples. Asian Journal of Chemistry, 28(3), 683–6860.
Harrison, R. M., Smith, D. J. T., & Luhana, L. (1996). Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environmental Science and Technology, 30, 825–832.
Hu, N. J., Huang, P., Liu, J. H., Ma, D. A., Shi, X. F., Mao, J., et al. (2014). Characterization and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in sediments in the Yellow River Estuary, China. Environmental Earth Sciences, 71, 873–883.
Hwang, H. M., & Foster, G. D. (2016). Characterization of polycyclic aromatic hydrocarbons in urban storm water runoff flowing into the tidal Anacostia River, Washington, DC, USA. Environmental Pollution, 140, 416–426.
IARC. (1987). Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon France, 92. http://monographs.iarc.fr/ENG/Monographs/vol92/index.php
Jiacheng, L., Yuchuan, S., Xiao, S., & Daoxian, Y. (2015). Polycyclic aromatic hydrocarbon contamination in a highly vulnerable underground river system in Chongqing, Southwest China. Journal of Geochemical Exploration. https://doi.org/10.1016/j.gexplo.2016.05.013.
Kalf, D. F., Crommentuijn, T., & van de Plassche, E. J. (1997). Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs). Ecotoxicology and Environmental Safety, 36(1), 89–97.
Keshavarzifard, M., Zakaria, M. P., Hwai, Yusuff F. M., & Mustafa, S. (2015). Distributions and source apportionment of sediment-associated polycyclic aromatic hydrocarbons (PAHs) and hopanes in rivers and estuaries of Peninsular Malaysia. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-015-4093-7.
Keshavarzifard, M., Zakaria, M. P., Hwai, T. S., Mustafa, S., Vaezzadeh, V., Magam, S. M., et al. (2014). Baseline distributions and sources of polycyclic aromatic hydrocarbons (PAHs) in the surface sediments from the Prai and Malacca Rivers, Peninsular Malaysia. Marine Pollution Bulletin, 88, 366–372.
Khalili, N. R., Scheff, P. A., & Holsen, T. M. (1995). PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric Environment, 29, 533–542.
Lee, J., Gigliotti, C. L., Offenberg, J. H, Eisenreich, J. S., & Turpin, B. J. (2004). Sources of polycyclic aromatic hydrocarbons to the Hudson River Airshed. Atmospheric Environment, 38, 5971–5981.
Li, C. K., & Kamens, R. M. (1993). The use of polycyclic aromatic hydrocarbons as source signatures in receptor modeling. Atmospheric Environment. Part A. General Topics, 27, 523–532.
Lima, A. L. C., Farrington, J. W., & Reddy, C. M. (2005). Combustion-derived polycyclic aromatic hydrocarbons in the environment. Environmental Forensics, 6(2), 109–131.
Literathy, P., Haider, S., Samban, O., & Morel, G. (1989). Experimental studies on biological and chemical oxidation of dispersed oil in seawater. Water Science Technology, 24, 845–856.
Liu, Y., Beckingham, B., Ruegner, H., Li, Z., Ma, L., Schwientek, M., et al. (2013). Comparison of sedimentary PAHs in the rivers of Ammer (Germany) and Liangtan (China): differences between early- and newly industrialized countries. Environmental Science and Technology, 47, 701–709.
Liu, Y., Ling, C. N., Zhao, J. F., Huang, Q. H., Zhu, Z. I., & Gao, H. W. (2008). Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of rivers and an estuary in Shanghai, China. Environmental Pollution, 154, 298–305.
Louati, A., Elleuch, B., Kallel, M., Oudot, J., Saliot, A., & Dagaut, J. (2001). Hydrocarbon contamination of coastal sediments from the Sfax area (Tunisia), Mediterranean Sea. Marine Pollution Bulletin, 42, 445–452.
Lu, Y., Song, S., Wang, R., Liu, Z., Meng, J., Sweetman, A. J., et al. (2014). Impacts of soil and water pollution on food safety and health risks in China. Environment International, 77, 5–15.
Luo, X. J., Chen, S. J., Mai, B. X., Sheng, G. Y., Fu, J. M., & Zeng, E. Y. (2008a). Distribution, source apportionment, and transport of PAHs in sediments from the Pearl River Delta and the northern South China Sea. Archives of Environmental Contamination and Toxicology, 55(1), 11–20.
Luo, X., Chen, S., Yu, M., Mai, B., Sheng, G., & Fu, J. (2008b). Distribution and partition of polycyclic aromatic hydrocarbons in surface water from the Pearl River Estuary. Environmental Science, 29(9), 2385–2391.
Macias-Zamora, J. V., Mendoza-Vega, E., & Villaescusa-Celaya, J. A. (2002). PAHs composition of surface marine sediments: A comparison to potential local sources in Todos Santos Bay, B.C., Mexico. Chemosphere, 46, 459–468.
Malik, A., Verma, P., Singh, P., & Singh, A. K. (2011). Distribution of polycyclic aromatic hydrocarbons in water and bed sediments. Environmental Monitoring and Assessment, 172, 529–545.
Manadori, L., Gambaro, A., Piazza, R., Ferrari, S., Stortini, A. M., & Capotaglio, G. (2006). PCBs and PAHs in a sea-surface microlayer and sub-surface water samples of the Venice Lagoon (Italy). Marine Pollution Bulletin, 52, 184–192.
Mili, S. (2016). Instant Cities on the Wet coastal zones- Tunisia. Procedia Environmental Sciences, 34, 525–538.
Morillo, E., Romero, A. S., Maqueda, C., Madrid, L., Ajmone-Marsan, F., Grcman, H., et al. (2008). Soil pollution by PAHs in urban soils: A comparison of three European cities. Journal of Environmental Monitoring, 9, 1001–1008.
Moses, E. A., Etuk, B. A., & Udosen, E. D. (2015). Levels, distribution and sources of polycyclic aromatic hydrocarbons in surface water in the lower reach of Qua Iboe River Estuary, Nigeria. American Journal of Environmental Protection, 4(6), 334–343. https://doi.org/10.11648/j.ajep.20150406.20.
Mudhaf, H. F., Alsharifi, F. A., & Abu-Shady, A. S. I. (2009). A survey of organic contaminants in household and bottled drinking waters in Kuwait. Science of the Total Environment, 407, 1658–1668.
Mzoughi, N., & Chouba, L. (2011). Distribution and partitioning of aliphatic hydrocarbons and polycyclic aromatic hydrocarbons between water, suspended particulate matter, and sediment in harbours of the West coastal of the Gulf of Tunis (Tunisia). Journal of Environmental Monitoring, 13, 689–698.
Nadal, N., Schuhmacher, M., & Domingo, J. (2004). Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environmental Pollution, 132, 1–11.
Neff, M. (1979). Polycyclic aromatic hydrocarbons in the aquatic environment: Sources, fates and biological effects (p. 1979). London: Applied Science Publishers.
Nekhavhambe, T. J., van Ree, T., & Fatoki, O. S. (2014). Determination and distribution of polycyclic aromatic hydrocarbons in rivers, surface runoff, and sediments in and around Thohoyandou, Limpopo Province, South Africa. Water SA, 40, 415–424.
Obayori, O. S., & Salam, L. B. (2010). Degradation of polycyclic aromatic hydrocarbons: Role of plasmids. Scientific Research and Essay, 5(25), 4093–4106.
Palm, A., Cousins, I., Gustafsson, O., Axelman, J., Grunder, K., Broman, D., et al. (2004). Evaluation of sequentially-coupled POP fluxes estimated from simultaneous measurements in multiple compartments of an air-water-sediment system. Environmental Pollution, 128(1–2), 85–97.
Parinos, C., Gogou, A., Bouloubassi, I., Stavrakakis, S., Plakidi, E., & Hatzianestis, I. (2013). Sources and downward fluxes of polycyclic aromatic hydrocarbons in the open southwestern Black Sea. Organic Geochemistry, 57, 65–75.
Qiao, M., Wang, C. X., Huang, S. B., Wang, D. H., & Wang, Z. J. (2006). Composition, sources, and potential toxicological significance of PAHs in the surface sediments of Meiliang Bay, Taihu Lake, China. Environment International, 32, 28–33.
Qin, N., He, W., Kong, X. Z., Liu, W. X., He, Q. S., Yang, B., et al. (2013a). Ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the water from a large Chinese lake based on multiple indicators. Ecological Indicators, 24, 599–608.
Qin, N., He, W., Kong, X. Z., Liu, W. X., He, Q. S., Yang, B., et al. (2014). Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in the water–SPM–sediment system of Lake Chaohu, China. Science of the Total Environment, 496, 414–419.
Qin, N., He, W., & Wang, Y. (2013b). Residues and health risk of polycyclic aromatic hydrocarbons in the water and aquatic products from Lake Chaohu. Acta Scientiae Circumstantiae, 33(1), 230–239.
Saliot, A. (1989). Natural hydrocarbons in sea water. In E. K. Duursma & R. Dawson (Eds.), Marine organic chemistry (pp. 327–374). Amsterdam: Elsevier.
Sany, B. T., Salleh, A., Sulaiman, A. H., & Tehrani, G. M. (2012). Ecological risk assessment of poly aromatic hydrocarbons in the North Port, Malaysia. World Academy of Science, Engineering and Technology, 69, 43–46.
Savinov, V. M., Savinova, G. G., Matishova, S., Dahle, K., & Næs, K. (2003). Polycyclic aromatic hydrocarbons (PAHs) and organochlorines (OCs) in bottom sediments of the Guba Pechenga, Barents Sea, Russia. Science of the Total Environment, 306(9–56), 69.
Sereshk, Z. H., & Bakhtiari, A. R. (2014). Distribution patterns of PAHs in different tissues of annulated sea snake (Hydrophis cyanocinctus) and short sea snake (Lapemiscurtus) from the Hara Protected Areaon the North Coast of the Persian Gulf, Iran. Ecotoxicology and Environmental Safety, 109, 117–120.
Simpson, C. D., Mosi, A. A., Cullen, W. R., & Reimer, K. J. (1996). Composition and distribution of polycyclic aromatic hydrocarbons in surficial marine sediments from Kitimat Harbour, Canada. Science of the Total Environment, 181, 265–278.
Soclo, H. H., Garrigues, P. H., & Ewald, M. (2000). Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: Case studies in Cotonou (Benin) and Aquitaine (France) areas. Marine Pollution Bulletin, 40, 387–396.
Solomon, K., Giesy, J., & Jones, P. (2000). Probabilistic risk assessment of agrochemicals in the environment. Crop Protection, 19, 649–655.
Stout, S. A., Leather, J. M., & Corl, W. (2003). A user’s guide for determining the sources of contaminants in sediments. Technical report 1907. San Diego, CA: Spawar Systems Center, pp. 1–85.
Sun, C., Zhang, J., Ma, Q., Chen, Y., & Ju, H. (2016). Polycyclic aromatic hydrocarbons (PAHs) in water and sediment from a river basin: Sediment–water partitioning, source identification and environmental health risk assessment. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-016-9807.
Tobiszewski, M., & Namiesnik, J. (2012). PAH diagnostic ratios for the identification of pollution emission sources. Environmental Pollution, 162, 110–119.
Trabelsi, S., & Driss, M. R. (2005). Polycyclic aromatic hydrocarbons in superficial coastal sediments from Bizerte Lagoon, Tunisia. Marine Pollution Bulletin, 50, 344–359.
Tsapakis, M., Apostolaki, M., Eisenreich, S., & Stephanou, E. G. (2006). Atmospheric deposition and marine sedimentation fluxes of polycyclic aromatic hydrocarbons in the eastern Mediterranean basin. Environmental Science and Technology, 40(16), 4922–4927.
Tuncel, S. G., & Topal, T. (2015). Polycyclic aromatic hydrocarbons (PAHs) in sea sediments of the Turkish Mediterranean coast, composition and sources. Environmental Science and Pollution Research International, 22(6), 4213–4221.
Ünlü, S., & Alpar, B. (2009). Evolution of potential ecological impacts of the bottom sediment from the gulf of Gemlik; Marmara Sea, Turkey. Bulletin of Environment Contamination and Toxicology, 83, 903–906.
US EPA. (2001). Risk assessment guidance for superfund, human health evaluation manual (Part E, supplemental guidance for dermal risk assessment). EPA/540/R/99/005. US Environmental Protection Agency. USA.
US EPA. (2010). Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2010). EPA/600/R-08/139F, 2009. US Environmental Protection Agency, Washington.
Walker, S. E., & Dickhut, R. M. (2001). Sources of PAHs to sediments of the Elizabeth River, VA. Soil and Sediment Contamination, 10, 611–632.
Wang, J., Li, Q., Qi, R., et al. (2014). Sludge bulking impact on relevant bacterial populations in a full-scale municipal wastewater treatment plant. Process Biochemistry, 49, 2258–2265. https://doi.org/10.1016/j.procbio.2014.08.005. [Cross Ref].
Wang, X. C., Sun, S., Ma, H. Q., & Liu, Y. (2006). Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Jiaozhou Bay, Qingdao (China). Marine Pollution Bulletin, 52, 129–138.
Watkinson, R. J., & Griffiths, D. (1987). Biodegradation and photooxidation of crude oils in a tropical marine environment. In J. Kuiper & W. J. Van Den Brink (Eds.), Marine ecosystems (pp. 67–77). Dordrecht: Ž. Nartinus Nifjoff Publisher.
Weast, R. C. (1968). Handbook of chemistry and physics, 1968–1969 (49th ed., p. 1968). Cleveland, OH: The Chemical Rubber Company.
WHO. (2005). Draft Guidelines for Safe Recreation-water Environments: Coastal and Fresh Waters. WHO (EOS/Draft/98.14), Genebra.
Wu, B., Zhang, R., Chen, S. P., Timothy, F., Aimin, L., & Xuxiang, Z. (2011). Risk assessment of polycyclic aromatic hydrocarbons in aquatic ecosystems. Ecotoxicology. https://doi.org/10.1007/s10646-011-0653-x.
Xu, J., Yu, Y., Wang, P., Gu, W. F., Dai, S. G., & Sun, H. G. (2007). Polycyclic aromatic hydrocarbons in the surface sediments from the Yellow River China. Chemosphere, 67, 1408–1414.
Yamada, M., Takada, H., Toyoda, K., Yoshida, A., Shibata, A., Nomura, A., et al. (2003). Study on the fate of petroleum derived polycyclic aromatic hydrocarbons (PAHs) and the effect of chemical dispersant using an enclosed ecosystem, mesocosm. Marine Pollution Bulletin, 47(1), 105–113.
Yanker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, D. (2002). PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33(4), 489–515.
Yuan, D. X., Yang, D. N., Yang, L. W., & Qian, Y. R. (2001). Status of persistent organic pollutants in the sediment from several estuaries in China. Environmental Pollution, 114, 101–111. Google Scholar.
Yuhu, L., Ping, L., Wandong, M., Song, Q., Zhou, H., Han, H., et al. (2015). Spatial and temporal distribution and risk assessment of polycyclic aromatic hydrocarbons in surface seawater from the Haikou Bay, China. Marine Pollution Bulletin, 92, 244–251.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchel, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33, 489–515.
Zaghden, H., Kallel, M., Elleuch, B., Oudot, J., & Saliot, A. (2005a). Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Sfax, Tunisia, Mediterranean Sea. Marine Chemistry, 105, 70–89.
Zaghden, H., Kallel, M., Louati, A., Elleuch, B., Oudot, J., & Saliot, A. (2005b). Hydrocarbons in surface sediments from the Sfax coastal zone, (Tunisia) Mediterranean Sea. Marine Pollution Bulletin, 50, 1287–1294.
Zakaria, M. P., Takada, H., Tsutsumi, S., Ohno, K., Yamada, J., Kouno, E., et al. (2002). Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: A widespread input of petrogenic PAHs. Environmental Science and Technology, 36, 1907–1918.
Zhang, L., Dong, L., Ren, L., Shi, S., Zhou, L., Zhang, T., et al. (2012a). Concentration and source identification of polycyclic aromatic hydrocarbons and phthalic acid esters in the surface water of the Yangtze River Delta, China. Journal of Environmental Sciences, 24(2), 335–342.
Zhang, Y., Guo, C. S., Xu, J., Tian, J. Z., Shi, G. L., & Feng, Y. C. (2012b). Potential source contributions and risk assessment of PAHs in sediments from Taihu Lake, China: Comparison of three receptor models. Water Research, 46, 3065–3073.
Zhang, L., Qiu, L., Wu, X., Liu, X., You, L., Pei, D., et al. (2012c). Expression profiles of seven glutathione S-transferase (GST) genes from Venerupis philippinarum exposed to heavy metals and benzo [a] pyrene. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 155(3), 517–527.
Zhang, G. J., Zang, X. H., Li, Z., Wang, C., & Wang, Z. (2014). Polydimethylsiloxane/metal-organic frameworks coated fiber for solid-phase microextraction of polycyclic aromatic hydrocarbons in river and lake water samples. Talanta, 129, 600–605.
Zhiguo, C., Jingling, L., Luan, Y., Yongli, L., Muyuan, M., Xu, J., et al. (2010). Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology, 19, 827–837.
Zrafi, I., Bakhrouf, A., Rouabhia, M., & Saidane-Mosbahi, D. (2013). Aliphatic and aromatic biomarkers for petroleum hydrocarbon monitoring in Khniss Tunisian-Coast, (Mediterranean Sea). Procedia Environmental Sciences, 18, 211–220.
Zrafi-Nouira, I., Khedir-Ghenim, Z., Bahri, R., Cheraeif, I., Rouabhia, M., & Saidane-Mosbahi, D. (2009). Hydrocarbon in seawater and interstitial water of Jarzouna-Bizerte Coastal of Tunisia (Mediterranean Sea): Petroleum origin investigation around refinery rejection place. Water, Air, and Soil pollution, 202(1), 19–31.
Zrafi‐Nouira, I., Khedir‐Ghenim, Z., Zrafi, F., Bahri, R., Cheraief. I., Rouabhia, M., Saidane Mosbahi, D. (2008). Hydrocarbon pollution in the sediment from the Jarzouna‐Bizerte coastal area of Tunisia (Mediterranean Sea). Bulletin of Environmental Contamination and Toxicology, 80: 566–572.
Zrafi-Nouira, I., Nimer, M. D., Bahri, R., Mzoughi, N., Aissi, A., Ben Abdenebii, H., et al. (2010). Distribution and sources of polycyclic aromatic hydrocarbon around a petroleum refinery rejection area in Jarzouna-Bizerte (Coastal Tunisia). Soil and Sediment Contamination, 19(3), 292–306.
Acknowledgements
This work was supported by the Ministry of Higher Education and Scientific Research of Tunisia.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gdara, I., Zrafi, I., Balducci, C. et al. Seasonal occurrence, source evaluation and ecological risk assessment of polycyclic aromatic hydrocarbons in industrial and agricultural effluents discharged in Wadi El Bey (Tunisia). Environ Geochem Health 40, 1609–1627 (2018). https://doi.org/10.1007/s10653-018-0075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0075-2