Abstract
Soil and groundwater samples were collected from paddy fields in the middle reaches of the Yangtze River Basin to study the occurrence and the risks associated with organochlorine pesticides (OCPs) and organophosphorus pesticides (OPPs) in soil and groundwater. Results showed that OCPs and OPPs were widely distributed throughout the study area. The levels of OCPs and OPPs in the soil were much lower than those specified by soil quality standards. However, the levels of four OCPs (heptachlors, aldrin, dieldrin, and γ-hexachlorocyclohexane) in groundwater were higher than those permitted by drinking water standards. The health risk assessment method suggested by the US Environment Protection Agency was used to evaluate the regional risks from selected pesticides. Results showed that there were low health risks from OCPs and OPPs in soil at the regional scale, but high risks from heptachlor, aldrin, and endrin in groundwater, suggesting an urgent need for groundwater protection. There are widespread concerns on dichlorodiphenyltrichloroethane and hexachlorocyclohexane, but little focus on other pesticides in China. However, our results suggest that the presence of, and risks from, other pesticides in groundwater should be a focus from the region aspect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Historically, chemical pesticides have been heavily applied to agricultural land (Bidleman and Leone 2004). While most of these pesticides have degraded over time, their effects still persist and can be found in the global hydrological cycle and atmospheric circulation (Su et al. 2008; Jamieson et al. 2017). In fact, it is now indicated that the pesticide pollution status is worse than previously believed. For example, some pesticides have been continuously used in some areas in spite of being banned (Zhang et al. 2013). In the past decades, many studies have examined pesticide contaminants in some soils, sediments, and surface water, because of their enrichment processes and effects on the ecological systems (Manz et al. 2001; Hu et al. 2009; Zhou et al. 2006). However, studies related to contaminants in groundwater are still in their early stages worldwide, with the systematic regional survey of groundwater pollution in China as an example (Qiu 2010). Till date, the Chinese groundwater survey has focused on a few types of pollutants, such as inorganic compounds, heavy metals, and individual organic compounds (Hu et al. 2011), with the results indicating that groundwater in China is very seriously polluted (Zhang et al. 2013). Being a country with extensive agriculture, non-point source pollution has a considerable influence on the quality of groundwater in rural China. In the past few decades, large quantities of chemical pesticides have been applied to farmlands (Zhu et al. 2005). The level of chemical pesticide contamination in soil has varied in different regions in China (Cai et al. 2008). Compared to the soil temperature, groundwater temperature is relatively low and stable, indicating that groundwater provides the optimal conditions for the long-term retention of chemical pesticides (Cavalier et al. 1991). As an important drinking water source in China, enough concern should be given to the protection of groundwater resources (Qiu 2010; Zhang et al. 2013). Till date, however, groundwater pollution from pesticides has not been paid sufficient attention.
As a special class of organic pesticides, many organochlorine pesticides (OCPs) persist in the environment (Su et al. 2008; Harner et al. 1999; Wang et al. 2007a, b). Therefore, the persistence and pollution risk of OCPs have been the focus of research for environmental and geological scientists. Since organophosphorus pesticides (OPPs) degrade more easily than OCPs, it is generally believed that OPPs have fewer negative effects on the environment. However, because of the intensive annual applications of OPPs and the enrichment process in the soil environment, some OPPs continue to persist for a long time (Edwards 1966; Vollner and Klotz 1997). Moreover, since they are more easily water-soluble than OCPs, OPPs can migrate into groundwater more easily under flooded conditions (Ragnarsdottir 2000). In addition, OPPs have adverse effects on human health when they accumulate to certain concentrations (Lee et al. 2013). Therefore, information about the occurrence and concentrations of OCPs and OPPs in groundwater is very important to protect groundwater resources (Rousis et al. 2016).
The Yangtze Basin is a core area for rice planting in China. The high humidity and temperature makes it a breeding ground for agricultural pests and diseases, because of which chemical pesticides are used frequently. The unique conditions of high precipitation and a shallow groundwater table in the Yangtze River Basin suggest that the area is susceptible to groundwater pollution (Wang et al. 2007a, b). However, it is difficult to implement counter measures to protect groundwater resources because of insufficient information about the composition, levels, and sources of pesticides in the soil-groundwater systems in the Yangtze River Basin. Therefore, further studies should be carried out to support groundwater protection and pesticide management in the Yangtze River Basin.
We can achieve an understanding of the levels of pollution by investigating the concentrations of the pollutants in the environment (Zhang et al. 2013). However, as the thresholds for human health vary for different pesticide components, the pollutant concentrations cannot be used directly to reflect their impact on human health. Health risk assessments (HRAs) use the ratio of the exposure dose to the tolerable dose of pollutants to assess the effect of pollutants on human health (Bhanti and Taneja 2007). In general, HRAs also use the average pollutant concentrations in environmental media to evaluate the risks. However, when the average health risk status is used, it is difficult to accurately reflect the distribution of the regional risk (Covello and Merkhoher 2013). Regional risk assessments use concentrations from spatial monitoring to evaluate the distribution of the degree of hazard from pollutants (Lawson et al. 1999). It may be more useful to assess the environmental effects of pollutants (Wen et al. 2006). Health risk assessment has been widely used worldwide, and the evaluation methods and perspectives varied. Li et al. (2014) assessed the health risks of pollutants in the groundwater of a park in Northwest China by comparing with the maximum acceptable level recommended by the International Commission on Radiologic Protection. George et al. (2015) analyzed the health risks of pollutants in the groundwater of a city in India using a quantitative microbial risk assessment (QMRA) method. Wu and Sun (2016) carried out a study on the shallow groundwater health risks in the agricultural and industry area referencing the health risks assessment model recommended by the US Environmental Protection Agency (EPA). Li et al. (2016) studied the non-carcinogenic and carcinogenic health risks of groundwater in the semi-arid area of Northwest China using the models recommended by the Ministry of Environmental Protection of China. Khan et al. (2016) evaluated the toxicological risks of trace metals in edible vegetables of an area in Pakistan by comparing with the standard values set by the World Health Organization (WHO) and the US EPA. In this study, the HRA of the US EPA was used in combination with a mosaic chart to evaluate the risks from pesticides in the soil-groundwater system in an agricultural area in the middle reaches of the Yangtze River Basin. By comparing the risks from OCPs and OPPs in soil and groundwater, priority pollutants can be identified in soil as well as groundwater.
The main objectives of this study were (1) to investigate the concentrations and sources of OPPs and OCPs in soil and groundwater in a paddy field, (2) to compare the differences in the composition of pesticides in soil and groundwater, and (3) to reveal the regional pollution risks from OPPs, OCPs, and other priority pollutants in the soil and groundwater in the middle reaches of the Yangtze River Basin.
Materials and methods
Study area and sampling method
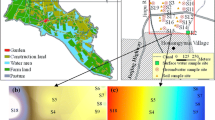
The study area was located in Hubei Province, Central China (30°40′N–31°5′N, 112°35′E–120°60′E). It lies 76.54 km north of the Yangtze River, covering an area of 229.59 km2. This area experiences a northern subtropical monsoon climate, with an annual mean precipitation of 1112.4 mm and annual mean temperature of 16.3 °C. Sixty-five percent of the annual rainfall occurs between April and August. The principal crop is rice, while the soil texture is loam with a soil organic matter content of 16.2 ± 0.8 g kg−1 and a soil pH of 5.5 to 7.5. The groundwater level depth is 1.0 to 1.5 m. And the fraction of organic carbon in the aquifer is 3.7 ± 0.2 mg L−1. The shallow groundwater aquifer lithology in the study area is loose sediment. The groundwater is easily recharged by precipitation and irrigation and discharged by evaporation. The recharge and discharge of groundwater are balance. The groundwater table is relatively stable. Flood irrigation mode is dominated in the study area. Surface runoff and deep percolation easily occur when rainfall or irrigation happens. DDT and other OCPs were widely used in the study area before 1980s, while in the recent years, OPPs are common in use. The irrigation water mostly comes from the Tianmen River, which is located to the south of the study area, flowing from north to south and going into the Yangtze River.
Sixteen groundwater samples were collected in 1-L clean, dry, amber glass bottles using a Baylor tube. Thirty cores of topsoil (0–20 cm) and subsoil (20–40 cm) were collected using a pre-cleaned stainless steel scoop. Once collected, all the samples were incubated on ice and then preserved at a low temperature (4 °C) before being transported to the laboratory. Soil and groundwater designations are presented in Fig. 1.
Extraction and Analysis methods
Soil samples were thawed and then freeze-dried for 24 h on arrival at the laboratory. They were subsequently ground and sieved through a 100-mesh stainless steel sieve, after which they were extracted using the methods mentioned in previous studies (Li et al. 2010; Chen et al. 2011). Briefly, a mixture of surrogate standards (2,4,5,6-tetrachloro-m-xylene for OCPs and nitrobenzene-d5, 2-fluorobiphenyl, and 4-terphenyl-d14 for OPPs, 30 ng) was added to 15 g samples, prior to extraction. Then, the samples were Soxhlet-extracted with dichloromethane (DCM) for 24 h. The extraction solvent was then collected and concentrated to 0.2 mL under a gentle stream of nitrogen. Groundwater samples were liquid–liquid extracted using DCM after adding recovery surrogates. Activated copper granules were added to the collection flasks to remove sulfur. All the extracted solvents were subjected to column chromatography through a silica gel/alumina column (Erger et al. 2012) and were eluted with 70 mL of DCM-hexane (3:7, v/v). The effluent thus obtained was concentrated to 0.2 mL under a gentle stream of nitrogen, and an internal standard (penta-chloronitrobenzene) was added before the detection of OCPs (Zhang et al. 2013).
OCPs were determined by gas chromatography with electron capture detection (GC-ECD), following the method of Zhang et al. (2011). The initial temperature of the quartz capillary chromatographic column (Rtx-CLP II, Restek, US; 30 m × 0.32 mm × 0.25 µm) was 50 °C (equilibrium time = 1 min). The temperature was increased to 180 °C at a rate of 20 °C min−1, held for 1 min, after which it was further increased to 240 °C at 8 °C min−1 and held for 10 min. Injections (1 µL) were carried out splitless initially, with the split being opened after 1 min. The temperatures of the injector and the ECD were 250 and 280 °C, respectively. The column flow rate was 1.2 mL min−1.
OPPs were determined by the gas chromatography flame photometric detector (GC-FPD) method, as followed by Wang and Du (2010). The initial temperature of the quartz capillary chromatographic column (Rtx-1701, Restek, US; 30 m × 0.25 mm × 0.25 µm) was 50 °C (equilibrium time = 1 min). The temperature was increased to 250 °C at 20 °C min−1 and held for 1 min. Injections (1 µL) were carried out splitless initially, with the split being opened after 1 min. The injector and FPD temperatures were 250 and 280 °C, respectively. The column, air, and hydrogen flow rates were 1.2, 80, and 70 mL min−1, respectively.
Qc/qa
The internal calibration method based on a six-point calibration curve was used to quantify individual OCPs/OPPs. Procedural blanks and spiked samples that contained all the reagents were tested after every 10 samples to check the procedural performance and matrix effects. A standard solution (0.3 µg mL−1) was analyzed to calibrate the instrument. The mean recoveries of the surrogates ranged from 81 to 112%. The relative standard deviation (RSD) ranged from 2 to 8%. The detection limit was 0.001 ng g−1 for organochlorines and 0.01 ng g−1 for organophosphate pesticides in the soil samples, and 10 ng L−1 for organochlorines and 50 ng L−1 for organophosphate pesticides in the groundwater samples.
Health risk assessment (HRA)
Generally, the risks from pollutants can be evaluated using environmental quality standards (EQS). However, EQS in various countries include only a limited number of pollutants. In fact, the majority of chemical pesticides are not included in EQS. Therefore, the pesticide pollution risks should be assessed by the selection of other methods. The HRA of the US EPA is often recommended for the evaluation of pollutants on human health through various exposure pathways (Reis et al. 2014) and has been frequently used by scientists worldwide. The greatest merit of this method is that it can be applied when the reference dose of the pollutant is unknown. For the ingestion pathway, the potential non-carcinogenic risk for OCPs and OPPs in soil and groundwater is expressed in the form of the hazard quotient (HQ) as follows:
where C1 represents the concentration of the contaminant in soil (mg kg−1), IR1 is the intake rate of soil (mg day−1), ED1 is the exposure duration (years), EF1 is the exposure frequency (days year−1), AT is the average time (days), and the RfD is the oral reference dose in mg kg−1 day−1.
where C2 represents the concentration of the contaminant in groundwater (mg L−1), IR2 is the intake rate of groundwater (L day−1), ED2 is the exposure duration (years), EF2 is the exposure frequency (days year−1), AT is the average time (days), and RfD is the oral reference dose (mg kg−1 day−1).
Results and discussion
Composition and levels of OCPs and OPPs
Information from the soil and water samples is presented in Table 1. Fourteen OCPs and one OPP were detected in soil samples, while fifteen OCPs and four OPPs were detected in groundwater samples. The detection rates for both ∑OCPs and ∑OPPs were 100%, indicating that OCPs and OPPs were widely distributed throughout the study area. The concentration of ∑OCPs in the topsoil, subsoil, and groundwater ranged from 1.08 to 8.75 µg kg−1 dry weight (dw), from 2.83 to 10.29 µg kg−1 dw, and from 1835.83 to 11,599.40 µg L−1, with mean concentrations of 3.55 ± 1.64 µg kg−1 dw, 4.51 ± 1.69 µg kg−1 dw, and 8783.80 ± 2075.67 µg L−1, respectively. The concentrations of ∑OPPs in the topsoil, subsoil, and groundwater located between 0.40 and 5.58 µg kg−1 dw, 0.54 and 5.33 µg kg−1 dw, and between 1738.80 and 2194.30 µg L−1, with mean concentrations of 1.49 ± 0.86 µg kg−1 dw, 1.04 ± 0.83 µg kg−1 dw, and 2105.10 ± 111.35 µg L−1, respectively.
The levels of OCPs and OPPs are shown in Fig. 2a. The main OCP compounds in soil were ∑DDT and ∑heptachlor, the values of which accounted for 38.6–46.3% and 25.4–34.2% of ∑OCPs, respectively. The ∑DDT concentration accounted for the bulk of the OCPs in soil, which was consistent with results from other studies undertaken in northern China, southern China, and northwestern China (Wang et al. 2009; Yang et al. 2013; Zhang et al. 2013). Chlorpyrifos, as the most commonly used insect pesticide in paddy fields, dominated the OPPs in soil in the study area. As shown in Fig. 2b, the concentration of OCPs in groundwater was much higher than that of OPPs. The mean concentration of heptachlors was 4705.03 ± 1177.33 ng L−1, which was higher than the concentrations of the other OCPs. The concentration of OCPs in groundwater was higher than the concentration of OPPs, indicating that the pesticides in groundwater were related not only to their water solubility, but also to their persistence (OCPs have low water solubility, but high persistence, while it is vice versa for OPPs). The water solubility and half-life of OCPs and OPPs are presented in Table S1 (Supplementary Material).
The value of ∑DDT (1.73 ng g−1) in paddy soils was lower than those found in other areas in the Yangtze River Basin, such as Wuhan City (151.6 ng g−1; Zhou et al. 2013), Shanghai City (21.41 ng g−1; Jiang et al. 2009), Taihu Lake Region (60.2 ng g−1; Gao et al. 2005), Yangtze River Delta (73.0 ng g−1; Sun et al. 2016) and other parts of China (Wang et al. 2007a, b; Gao et al. 2013). It was also much lower than that in India (Mishra et al. 2012), while being similar to that in Chulla North Province of South Korea (Kim and Smith 2001). The comparison of the OCP residues to those found in other agricultural areas showed that the level of DDT residue in paddy soils in the study area was much lower than those in wheat, corn, vegetable, and orchard fields (Gaw et al. 2006; Tao et al. 2008; Wang et al. 2009), which suggested a lower OCP pollution than that in the other areas.
The concentrations of Σheptachlor, ΣDDT, ΣHCH, and endrin in groundwater in the study area were between 1 and 3 times higher than those in other areas of China (Hu et al. 2011; Zhang et al. 2013) and 1 to 2 times higher than the concentrations in other areas abroad (Sankararamakrishnan et al. 2005; Shukla et al. 2006; Gonçalves et al. 2007; Ghose et al. 2009). The concentration of OPPs in groundwater was also very high. The heavy pesticide pollution in groundwater in the study area could be related to heavy rainfall, flood irrigation, paddy environment, and shallow groundwater table. The concentration of heptachlor in groundwater was also high, despite its low migration ability (log Koc 4.38; water solubility 0.18 mg L−1) (US EPA 1996). This indicated that the groundwater could also be polluted by pesticides with low migration ability.
Health risk assessment for OCPs and OPPs in the soil and groundwater environment
The levels of pesticides in soils were much lower than the Class 1 level, which is the baseline concentration according to the Chinese Soil Environmental Quality Standards (50 µg kg−1 dw; PRC EPA 1995). This indicated that there was minimal risk from the soil environment.
Although groundwater is not used for drinking in the study area, it should still be protected. The mean concentration of heptachlor (4478.39 ng L−1) in the groundwater was about 10 times higher than the American drinking water standard (400 ng L−1; US EPA 2008) and 100 times higher than the drinking water standard of the World Health Organization (WHO 2011; 30 ng L−1). The mean concentrations of aldrin (683.31 ng L−1) and dieldrin (196.01 ng L−1) were much higher than the WHO drinking water standard (30 ng L−1). The mean concentration of γ-HCH (645.62 ng L−1) was higher than that of the American drinking water standard (200 ng L−1; US EPA 2008). However, these four OCPs were not included in the current Chinese groundwater quality standards (PRC EPA 1993). The highest concentrations of ΣDDT and ΣHCH in groundwater were lower than the Chinese drinking water standard (1000 ng L−1; PRC EPA 2006). The mean concentrations of OPPs in groundwater were all higher than 300 ng L−1. These results showed that groundwater in the middle reaches of the Yangtze River Basin was highly polluted by pesticides. Moreover, the pollution due to OCPs is more serious than that due to OPPs. Therefore, there was an urgent need to take measures against the OCP pollution in groundwater.
A comprehensive evaluation of the health risk to humans from OCPs and OPPs was carried out in soil and groundwater. Seven pesticides were detected in soil, while 12 pesticides detected in groundwater, using formulas (1) and (2). The RfD of selected pesticides is shown in Table 2. Since the acceptable daily intake (ADI) was available for all the pesticides in Table 2, while RfD was not available for some pesticides. Therefore, ADI was used to evaluate the health risks for the pesticides in this study. The assessment for pesticides in soil is illustrated in Fig. 3a, b. As shown in Table 1, even though the concentration of ΣDDT in soil was high, the health risk associated with soil was low. There were higher risks to health due to Σheptachlor in both, topsoil and subsoil, compared to the other pesticides. There was a higher risk to health due to chlorpyrifos in subsoil, although the risk was lower in topsoil. Therefore, heptachlor and chlorpyrifos were the priority pollutants. As shown in Fig. 3c, the risks to health due to pesticides were much higher in groundwater than in soil. Heptachlor, aldrin, and endrin, with HQ values of more than 0.1, were the priority pollutants in groundwater. This indicated that the pesticides in groundwater posed higher risks to human health than those present in soil.
Source analysis
The composition of isomers and metabolites of pesticides in the soil environment was often used to identify the source and the residue time (Kim and Smith 2001; Wang et al. 2007a, b; Jiang et al. 2009; Mishra et al. 2012). DDT in the environment was mainly derived from technical DDT and technical dicofol. These compounds were mainly comprised of p,p′-DDT (65–80%) and o,p′-DDT (≥80%), respectively (Qiu and Zhu 2010). o,p′-DDT was not detected in the study area, which indicated that technical DDT was the main source of DDT residue in the paddy environment. DDT could be broken down into DDD and DDE in the environment (Mishra et al. 2012). It would take more than 20 years for the DDT in the soil environment to degrade completely (Harner et al. 1999). In the study area, DDT in the soil was mainly in the form of p,p′-DDD and p,p′-DDE, which indicated that there were no new inputs of technical DDT in the recent past. As shown in Fig. 4a, the ratio of DDE/DDTs in the topsoil at most of the sampling sites was greater than 0.8. However, it was lower than 0.7 in the subsoil. DDT was often degraded to DDE under aerobic conditions by dehydrodechlorination, while it was transformed to DDD in an anoxic environment with reductive dechlorination, either microbially or chemically (Zoro et al. 1974; Baxter 1990). Therefore, the DDE/DDT ratio was higher in topsoil than in subsoil. Moreover, as shown in Fig. 4a, the DDE/DDT ratio in groundwater was mainly between 0.7 and 0.8, which indicates that the DDT in groundwater degraded more slowly than in topsoil, but more quickly than in subsoil.
Technical HCH, including several isomers, comprises 60–70, 10–12, and 5–12% of α-HCH, β-HCH, and γ-HCH, respectively. Another commercial HCH, lindane, comprises almost 100% of γ-HCH (Wang et al. 2009). The vapor pressure of γ-HCH was much higher than that of β-HCH, while its half-life is much shorter (Table S1, Supplementary Material), which indicated that γ-HCH will degrade more easily in the soil environment. As shown in Fig. 4b, the ratio of γ-HCH/HCHs in soil and groundwater was mainly located between 0.3 and 0.5, which was significantly higher than that in technical HCH, suggesting that the HCH residue in the environment mainly originated from the use of lindane. The ratio of γ-HCH/HCHs was greater than 0.8 in the topsoil and subsoil at some sampling sites, which suggested recent inputs of lindane. However, our investigations indicated that there had been no recent applications of lindane. Therefore, the high concentrations of γ-HCH in soil may be attributed to long-range atmospheric transport (LRAT). The log Koc and water solubility values of β-HCH were much higher than those of their isomers (Manz et al. 2001). In addition, α-/γ-HCH can be transformed to β-HCH (Walker et al. 1999). Furthermore, the symmetrical arrangement of chlorine atoms in the molecular structure of β-HCH was supposed to be more resistant to microbial degradation in soil than the other isomers of HCH (Kalbitz et al. 1997). These properties resulted in the accumulation of β-HCH in the soil environment. However, the ratio of β-HCH/(β-HCH + α-HCH) in soil was mainly between 0 and 0.1, indicating that high levels of α-HCH may be transported by LRAT. The ratio of β-HCH/(β-HCH + α-HCH) was much higher in groundwater than in soil (Fig. 4b). This can be mainly attributed to the limited influence of LRAT on the presence of α-HCH in groundwater.
Heptachlor can be transformed to heptachlor epoxide in the soil environment with a DT50 of 250 days (FAO 2000). The average heptachlor/Σheptachlor ratios in the topsoil and subsoil were 0.83 ± 0.38 and 0.93 ± 0.03, respectively. These values indicated that heptachlor was slightly degraded in the soil environment. Moreover, the heptachlor residue in the study area mainly came from recent inputs. The heptachlor/Σheptachlor ratio (0.95 ± 0.01) was also high in groundwater, suggesting that heptachlor inputs came from recent applications in paddy fields, rather than from LRAT.
The fate of pesticides in the environment is influenced by a series of factors, such as biodegradation, volatilization, and leaching (Li et al. 2015; Regnery et al. 2011). On the one hand, as seen from Table S1, which shows the physical and chemical properties of OCPs and OPPs, the OPPs, such as parathion and chlorfenvinphos, have greater solubility than OCPs. Therefore, it could be easier for these OPPs to migrate into groundwater. However, the level of OCPs was higher than that of OPPs in the groundwater. The reason is more complex. Due to the greater Henry constant for chlorpyrifos, parathion, and chlorfenvinphos than that for OCPs, the OPPs were more easily volatile. However, as the local groundwater depth is shallow (1.0–1.5 m), even with the lower solubility of OCPs, a large amount of them could infiltrate into groundwater, since their migration path into the groundwater is shorter. In addition, as most of the OCPs were persistent organic matter, they were also more likely to remain in the groundwater. Comprehensively considering the above-mentioned factors, the level of OCPs in groundwater could be higher than OPPs. Accordingly, they would pose a higher health risk. In this study, low levels of OCPs were detected in the soil because the OCP residues in soil undergo long-term biochemical degradation and volatilization. It also suggested that OCPs had not been used for a long time in the study area, which was consistent with the fact that OCPs have been forbidden for many years in the area. Table S1 also shows that the half-life of parathion was only about one week. A high concentration of parathion in the groundwater illustrated that it was still in use at present in the study area. Other researchers, such as Li et al. (2015), Regnery et al. (2011), did not find OPPs in the groundwater, suggesting that both, the rapid degradation and volatility of OPPs were the most important factors that prevent it from entering groundwater. However, this study also suggests that the groundwater depth may be another key factor influencing the presence of OPPs in the groundwater.
Conclusions
The OCP concentration in groundwater was higher than that recommended by the drinking water quality standard, while that in soil was much lower than that in the soil quality standard, suggesting a seriously polluted groundwater in the paddy fields in the middle reaches of the Yangtze River Basin. A regional HRA showed that the health risks due to OCPs and OPPs were much higher in groundwater than in soil. The priority pollutants in groundwater—heptachlor, aldrin, and endrin—were at dangerous levels. More attention should be given to groundwater pollution through pesticides in the middle reaches of the Yangtze Basin. Source analysis indicated that there were no recent inputs of technical DDT. High levels of α-HCH may be transported by LRAT. Heptachlor inputs came from recent applications, rather than from LRAT. High levels of OCPs detected in the groundwater were mainly due to the shallow groundwater depth and the persistent of OCPs.
References
Baxter, R. M. (1990). Reductive dechlorination of certain chlorinated organic compounds by reduced hematin compared with their behavior in the environment. Chemosphere, 21, 451–458.
Bhanti, M., & Taneja, A. (2007). Contamination of vegetables of different seasons with organophosphorous pesticides and related health risk assessment in northern India. Chemosphere, 69, 63–68.
Bidleman, T. F., & Leone, A. D. (2004). Soil–air exchange of organochlorine pesticides in the Southern United States. Environmental Pollution, 128, 49–57.
Cai, Q. Y., Mo, C. H., Wu, Q. T., Katsoyiannisc, A., & Zeng, Q. Y. (2008). The status of soil contamination by semivolatile organic chemicals (SVOCs) in China: A review. Science of the Total Environment, 389, 209–224.
Cavalier, T. C., Lavy, T. L., & Mattice, J. D. (1991). Persistence of Selected Pesticides in Ground-Water Samples. Ground Water, 29(2), 225–231.
Chen, F., Ying, G. G., Kong, L. X., Wang, L., Zhao, J. L., Zhou, L. J., et al. (2011). Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei. China Environmental Pollution, 159, 1490–1498.
Covello, V.T., &Merkhoher, M.W., (2013). Risk assessment methods: approaches for assessing health and environmental risks. Springer Science & Business Media.
Edwards, C.A., (1966). Insecticide residues in soils. In Residue Reviews (pp. 83-132). Springer US.
Erger, C., Balsaa, P., Werres, F., & Schmidt, T. C. (2012). Multi-component trace analysis of organic xenobiotics in surface water containing suspended particular matter by solid phase extraction/gas chromatography–mass spectrometry. J. Chroma. A, 1249, 181–189.
FAO, (2000). Assessing soil contamination A reference manual. http://www.fao.org/docrep/003/x2570e/x2570e07.htm.
Gao, H. J., Jiang, X., Wang, F., Bian, Y. R., Wang, D. Z., Dend, J. C., et al. (2005). Residual levels and new inputs of chlorinated POPs in agricultural soils from Taihu Lake region. Pedosphere, 15(3), 301–309.
Gao, J., Zhou, H., Pan, G., Wang, J., & Chen, B. (2013). Factors influencing the persistence of organochlorine pesticides in surface soil from the region around the Hongze Lake. China. Sci. Total Environ., 443, 7–13.
Gaw, S. K., Wilkins, A. L., Kim, N. D., Palmer, G. T., & Robinson, P. (2006). Trace element and ΣDDT concentrations in horticultural soils from the Tasman, Waikato and Auckland regions of New Zealand. Science of the Total Environment, 355, 31–47.
George, J., An, W., Joshi, D., Zhang, D., Yang, M., & Suriyanarayanan, S. (2015). Quantitative Microbial Risk Assessment to Estimate the Health Risk in Urban Drinking Water Systems of Mysore, Karnataka. India. Water Qual. Expos. Hea., 7, 331–338.
Ghose, N. C., Saha, D., & Gupta, A. (2009). Synthetic Detergents (Surfactants) and Organochlorine Pesticide Signatures in Surface Water and Groundwater of Greater Kolkata. India. J. Water Resource Protect., 1, 290–298.
Gonçalves, C. M., Esteves da Silva, J. C., & Alpendurada, M. F. (2007). Evaluation of the pesticide contamination of groundwater sampled over two years from a vulnerable zone in Portugal. J. Agr. Food Chem., 55, 6227–6235.
Harner, T., Wideman, J. L., Jantunen, L. M. M., Bidleman, T. F., & Parkhurst, W. J. (1999). Residues of organochlorine pesticides in Alabama soils. Environmental Pollution, 106, 323–332.
Hu, Y., Qi, S., Zhang, J., Tan, L., Zhang, J., Wang, Y., et al. (2011). Assessment of organochlorine pesticides contamination in underground rivers in Chongqing, Southwest China. Journal of Geochemical Exploration, 111, 47–55.
Hu, L., Zhang, G., Zheng, B., Qin, Y., Lin, T., & Guo, Z. (2009). Occurrence and distribution of organochlorine pesticides (OCPs) in surface sediments of the Bohai Sea, China. Chemosphere, 77, 663–672.
Jamieson, A. J., Malkocs, T., Piertney, S. B., Fujii, T., & Zhang, Z. (2017). Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nature Ecology and Evolution, 1, 51.
Jiang, Y. F., Wang, X. T., Jia, Y., Wang, F., Wu, M. H., Sheng, G. Y., et al. (2009). Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. Journal of Hazardous Materials, 170, 989–997.
Kalbitz, K., Popp, P., Geyer, W., & Hanschmann, G. (1997). β-HCH mobilization in polluted wetland soils as influenced by dissolved organic matter. Science of the Total Environment, 204, 37–48.
Khan, Z. I., Ahmad, K., Ashraf, M., Shoaib, N., Parveen, R., Bibi, Z., et al. (2016). Assessment of toxicological health risk of trace metals in vegetables mostly consumed in Punjab, Assessment of toxicological health risk of trace metals in vegetables mostly consumed in Punjab. Environmental Earth Sciences, 75(5), 1–5.
Kim, J. H., & Smith, A. (2001). Distribution of organochlorine pesticides in soils from South Korea. Chemosphere, 43, 137–140.
Lawson, A., Biggeri, A., Böhning, D., Lesaffre, E., Viel, J. F., & Bertollini, R. (1999). Disease mapping and risk assessment for public health. Hoboken: Wiley.
Lee, P. C., Rhodes, S. L., Sinsheimer, J. S., Bronstein, J., & Ritz, B. (2013). Functional paraoxonase 1 variants modify the risk of Parkinson’s disease due to organophosphate exposure. Environment International, 56, 42–47.
Li, P., Li, X., Meng, X., Li, M., & Zhang, Y. (2016). Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Exposure Health, 8(3), 361–379.
Li, J., Liu, X., Zhang, G., & Li, X. D. (2010). Particle deposition fluxes of BDE-209, PAHs, DDTs and chlordane in the Pearl River Delta, south China. Science of the Total Environment, 408, 3664–3670.
Li, P., Qian, H., & Wu, J. (2014). Origin and assessment of groundwater pollution and associated health risk: A case study in an industrial park, northwest China. Environmental Geochemistry and Health, 36(4), 693–712.
Li, X., Rao, Z., Yang, Z., Guo, X., Huang, Y., Zhang, J., et al. (2015). A survey of 42 semi-volatile organic contaminants in groundwater along the grand canal from Hangzhou to Beijing, East China. International Journal of Environmental Research and Public Health, 12(12), 16070–16081.
Manz, M., Wenzel, K. D., Dietze, U., & Schüürmann, G. (2001). Persistent organic pollutants in agricultural soils of central Germany. Science of the Total Environment, 277, 187–198.
Mishra, K., Sharma, R. C., & Kumar, S. (2012). Contamination levels and spatial distribution of organochlorine pesticides in soils from India. Ecotoxicology and Environmental Safety, 76, 215–225.
PRC EPA (1993). Chinese groundwater quality standards. http://kjs.mep.gov.cn/hjbhbz/bzwb/shjbh/shjzlbz/199410/t19941001_66500.htm. (in Chinese).
PRC EPA (1995). Chinese Soil Environmental Quality Standards. http://kjs.mep.gov.cn/hjbhbz/bzwb/trhj/trhjzlbz/199603/W020070313485587994018.pdf. (in Chinese).
PRC EPA (2006). Chinese drinking water standard. http://www.nhfpc.gov.cn/cmsresources/zwgkzt/wsbz/new/20070628143525.pdf. (in Chinese).
Qiu, J. (2010). China faces up to groundwater crisis. Nature, 466, 308.
Qiu, X., & Zhu, T. (2010). Using the o, p′-DDT/p, p′-DDT ratio to identify DDT sources in China. Chemosphere, 81, 1033–1038.
Ragnarsdottir, K. V. (2000). Environmental fate and toxicology of organophosphate pesticides. Journal of the Geological Society, 157(4), 859–876.
Regnery, J., Püttmann, W., Merz, C., & Berthold, G. (2011). Occurrence and distribution of organophosphorus flame retardants and plasticizers in anthropogenically affected groundwater. Journal of Environmental Monitoring, 13(2), 347–354.
Reis, A. P., Patinha, C., Noack, Y., Robert, S., Dias, A. C., & Ferreira da Silva, E. (2014). Assessing the human health risk for aluminium, zinc and lead in outdoor dusts collected in recreational sites used by children at an industrial area in the western part of the BassinMinier de Provence, France. Journal of African Earth Sciences, 99, 724–734.
Rousis, N. I., Zuccato, E., & Castiglioni, S. (2016). Monitoring population exposure to pesticides based on liquid chromatography-tandem mass spectrometry measurement of their urinary metabolites in urban wastewater: A novel biomonitoring approach. Science of the Total Environment, 571, 1349–1357.
Sankararamakrishnan, N., Kumar Sharma, A., & Sanghi, R. (2005). Organochlorine and organophosphorous pesticide residues in ground water and surface waters of Kanpur, Uttar Pradesh, India. Environment International, 31, 113–120.
Shukla, G., Kumar, A., Bhanti, M., Joseph, P. E., & Taneja, A. (2006). Organochlorine pesticide contamination of ground water in the city of Hyderabad. Environment International, 32, 244–247.
Su, Y., Hung, H., Blanchard, P., Patton, G. W., Kallenborn, R., Konoplev, A., et al. (2008). A circumpolar perspective of atmospheric organochlorine pesticides (OCPs): Results from six Arctic monitoring stations in 2000–2003. Atmospheric Environment, 42, 4682–4698.
Sun, J., Pan, L., Zhan, Y., Lu, H., Tsang, D. C., Liu, W., et al. (2016). Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Science of the Total Environment, 544, 670–676.
Tao, S., Liu, W. X., Li, Y., Yang, Y., Zuo, Q., Li, B. G., et al. (2008). Organochlorine pesticides contaminated surface soil as reemission source in the Haihe Plain, China. Environmental Science and Technology, 42, 395–400.
US EPA (1996). Soil screening guidance. http://www.epa.gov/superfund/health/conmedia/soil/pdfs/part_5.pdf.
US EPA (2008). NSF International standard/American national standard for drinking water treatment units. https://openlibrary.org/works/OL16588363W/NSF_International_standard_American_national_standard_for_drinking_water_treatment_units.
Vollner, L., & Klotz, D., (1997). Leaching and degradation of pesticides in groundwater layers. In I. A. E. Agency (Ed), Environmental behaviour of crop protection chemicals (pp. 187–203). Vienna, Austria.
Walker, K., Vallero, D. A., & Lewis, R. G. (1999). Factors influencing the distribution of lindan and other hexachlorocyclohexanes in the environment. Environmental Science and Technology, 33, 4373–4378.
Wang, Y., & Du, R. (2010). Simultaneous extraction of trace organophosphorous pesticides from plasma sample by automated solid phase extraction and determination by gas chromatography coupled with pulsed flame photometric detector. Forensic Science International, 198, 70–73.
Wang, F., Jiang, X., Bian, Y. R., Yao, F. X., Gao, H. J., Yu, G. F., et al. (2007a). Organochlorine pesticides in soils under different land usage in the Taihu Lake region, China. Journal of Environmental Sciences-China, 19, 584–590.
Wang, Y., Merkel, B. J., Li, Y., Ye, H., Fu, S., & Ihm, D. (2007b). Vulnerability of groundwater in Quaternary aquifers to organic contaminants: a case study in Wuhan City. China Environmental Geology, 53(3), 479–484.
Wang, X., Ren, N., Qi, H., Ma, W., & Li, Y. (2009). Levels, distributions, and source identification of organochlorine pesticides in the topsoils in Northeastern China. Journal of Environmental Sciences-China, 21, 1386–1392.
Wen, T. H., Lin, N. H., Lin, C. H., King, C. C., & Su, M. D. (2006). Spatial mapping of temporal risk characteristics to improve environmental health risk identification: a case study of a dengue epidemic in Taiwan. Science of the Total Environment, 367(2), 631–640.
Wu, J., & Sun, Z. (2016). Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Exposure Health, 8(3), 311–329.
Yang, D., Qi, S., Zhang, J., Wu, C., & Xing, X. (2013). Organochlorine pesticides in soil, water and sediment along the Jinjiang River mainstream to Quanzhou Bay, southeast China. Ecotoxicology and Environmental Safety, 89, 59–65.
Zhang, C., Liao, X., Li, J., Xu, L., Liu, M., Du, B., et al. (2013). Influence of long-term sewage irrigation on the distribution of organochlorine pesticides in soil–groundwater systems. Chemosphere, 92, 337–343.
Zhang, Y., Yang, J., Shi, R., Su, Q., Gao, Y., & Zhu, X. (2011). Development of an analytical method based on accelerated solvent extraction, solid-phase extraction clean-up, then GC–ECD for analysis of fourteen organochlorine pesticides in Cereal Crops. Chromatographia, 73, 385–391.
Zhou, Q., Wang, J., Meng, B., Cheng, J., Lin, G., Chen, J., et al. (2013). Distribution and sources of organochlorine pesticides in agricultural soils from central China. Ecotoxicology and Environmental Safety, 93, 163–170.
Zhou, R., Zhu, L., Yang, K., & Chen, Y. (2006). Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. Journal of Hazardous Materials, 137, 68–75.
Zhu, Y., Liu, H., Xi, Z., Cheng, H., & Xu, X. (2005). Organochlorine pesticides (DDTs and HCHs) in soils from the outskirts of Beijing, China. Chemosphere, 60, 770–778.
Zoro, J. A., Hunter, J. M., & Eglinton, G. (1974). Degradation of p,p′-DDT in reducing environments. Nature, 247, 235–237.
Acknowledgements
This research was financially supported by the Scientific and Technological Project in Henan Province (Grant No. 172102110101), Excellent Youth Foundation of He’nan Scientific Committee (Grant No. 174100510021), Scientific Research Foundation of the Higher Education Institutions of Henan Province (Grant No. 16A570001), and the National Public Benefit (Environmental) Research Foundation of China (Grant No. 201109009). Here we also thank professor Liu Xin and Han Yuping from North China University of Water Conservancy and Electric Power for their contributions on the preparation of the original manuscript.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, H., Lei, H., He, X. et al. Spatial distribution of organochlorine and organophosphorus pesticides in soil-groundwater systems and their associated risks in the middle reaches of the Yangtze River Basin. Environ Geochem Health 41, 1833–1845 (2019). https://doi.org/10.1007/s10653-017-9970-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-9970-1