Abstract
To study the influence of long-term pesticide application on the distribution of organochlorine pesticides (OCPs) in the soil–groundwater system, 19 soil samples and 19 groundwater samples were collected from agricultural area with long-term pesticide application history in Northern China. Results showed that the composition of OCPs changed significantly from soil to groundwater. For example, ∑DDT, ∑HCH, and ∑heptachlor had high levels in the soil and low levels in the groundwater; in contrast, endrin had low level in the soil and high level in the groundwater. Further study showed that OCP distribution in the soil was significantly influenced by its residue time, soil organic carbon level, and small soil particle contents (i.d. <0.0002 mm). Correlation analysis also indicates that the distribution of OCPs in the groundwater was closely related to the levels of OCPs in the soil layer, which may act as a pollution source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 65% of domestic water, 50% of industrial water, and 33% of agricultural water in Northern China originate from groundwater supplies. Residents from more than 400 in 655 cities in China consume groundwater (The People’s Republic of China Ministry of Environmental Protection 2011). Thus, groundwater protection is an important factor in water safety of China. However, many areas face groundwater pollution (Qiu 2010). As a result, the Chinese government is now committed to controlling chemical pollution and potential future pollution of groundwater resources (The People’s Republic of China Ministry of Environmental Protection, 2011). The protection of agricultural environments from pesticide pollution is also included in this program. However, recent studies have mainly focused on pesticide residues in soil environments (Covaci et al. 2001; Guan et al. 2009; Kuranchie-Mensah et al. 2012), and only a few studies have concentrated on pesticide residues in soil–groundwater systems (Zhang et al. 2013). The composition of pesticide residues in groundwater may be inconsistent with that in soil, because the cycling and composition of pesticides in the environment are influenced by the co-action of multiple factors, such as degradation, adsorption, and migration (Shukla et al. 2006; Arias-Estévez et al. 2008; Mishra et al. 2012). The lack of valuable information on the composition and level of pesticide residues makes it difficult to plan for groundwater protection.
The composition and level of pesticide residues vary in different land-use types (An et al. 2004; Hao et al. 2008; Jiang et al. 2009). In China, pesticides were widely used in vegetable gardens before 2000 (Wang et al. 2006). Some studies indicate that organochlorine pesticide (OCP) residues in vegetable soil ranked first among various land-use types (An et al. 2005). Thus, the monitoring and control of pesticides in the soil–groundwater systems of vegetable gardens are indispensable.
In China, a vegetable planting base is a large and centralized vegetable planting area with high investment and high returns. To ensure a good harvest, large amounts of water, fertilizers, and pesticides are often applied. Thus, pesticide contamination in vegetable planting bases merits more attention. Tianjin is an important vegetable planting base in the Beijing–Tianjin region located in the center of Northern China. Semi-closed greenhouse is used for vegetable cultivation in this area, which is a common method in Northern China. In this cultivation mode, tomatoes, cucumbers, eggplants, and other fruits and vegetables can be produced perennially. Given the popularity of pollution-free organic vegetable cultivation, the application method and rate of pesticides in greenhouses have changed over time. Consequently, the categories and levels of pesticide residues in soil–groundwater systems may have also changed. Sufficient irrigation may also have some effect on the distribution of pesticide residues.
The objectives of this study are as follows: (1) to investigate the levels and distributions of 21 types of organochlorine pesticides (OCPs) in the soil–groundwater system of a typical land-use type greenhouse vegetable planting base in Tianjin City, China; (2) to study the factors that influence the distribution of OCPs in soil and groundwater, including topography, groundwater depth, soil physical and chemical properties, and the residue level in the environmental matrix.
Materials and methods
Study area and sampling methods
Study area
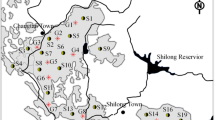
The study site has a total area of 1.01 × 105 ha and is located in the vegetable planting base of Xiqing District. This vegetable planting base accounts for approximately 1/3 of the total cultivation area in Xiqing District (Fig. 1a). More than 50,000 greenhouses operate in this vegetable planting base. Each greenhouse is approximately 100 m in length and 5 m in width. Vegetables have been planted in the study area for more than 15 years. Although the planted crops vary in different greenhouses and seasons, the crop rotation method employed in the greenhouses is the same. Information on the use time and dosage of pesticides is given in Table S1 (see Supporting Information).
Information of the study area and the distribution of the sample sites: a study area location and sampling site distribution; b groundwater depth from the ground surface of the study area plotted by the interpolation of the groundwater depth in the sampling wells; c groundwater flow field in the study area
Sufficient irrigation methods are generally adopted in this area. The texture of the soil was primarily clay loam with an organic matter content of 0.38–0.465%, pH level of 5.54–7.05, and cation exchange capacity (CEC) of 14.20 to 20.40 cmol kg−1. The groundwater level in the study area ranged from 0.7 to 4 m below the soil surface. The groundwater flow field is shown in Fig. 1b. This study focuses on pesticide pollution in shallow groundwater. Groundwater samples were collected from 1 to 3 m below the soil surface. The recharge of the groundwater mainly comprised rainfall, irrigation, groundwater flow outside of the study area, and water exchange with the Nan Canal.
Sampling methods
To avoid the negative effects of continuous pesticide application year-round, the sampling time was set for February 24 and 25, 2011, when the vegetables had been harvested and pesticide usage had ceased. A total of 19 groundwater samples (2 L each) were collected using bailers tubes: 17 samples (G1–G17) were obtained from the greenhouse, and the remaining two samples (G18 and G19) were collected from uncultivated meadow far from the greenhouse. The groundwater depth for each of the monitoring wells was recorded using a groundwater level gauge (HY.SWJ-1 50, Huayu, China). Groundwater depth interpolation and groundwater flow field map were plotted using ArcGIS 10.0 software (ESRI, USA) (Fig. 1). The distribution of the sampling sites, groundwater depth, and the groundwater flow field are shown in Fig. 1. Groundwater depth was shallow at sites G13, G17, and G18, and groundwater flow direction was from west to east. Soil samples (150 g each) were collected near the 19 groundwater sampling sites, among which 17 sites (S1–S17) were located in the greenhouse and the remaining two sites were used as control sites (S18 and S19). The pesticide residue levels inside and outside the greenhouse were compared to characterize the pesticide pollution characteristics in the greenhouse.
The soil profile subsamples were collected from both the topsoil (0–25 cm) and subsoil (25–50 cm) using a soil auger. Each of the final samples consisted of a mixture of five subsamples and was placed in a brown glass bottle. The Nan Canal is an artificial river that is approximately 100 m away from the greenhouse. Two water samples (2 L), upstream (R1) and downstream (R2), the Nan Canal, were collected in brown glass bottles. The samples were placed on ice to avoid degradation and volatilization of the pesticides. Finally, all samples were taken back to the laboratory within 2 days, extracted within 1 week, and preserved in a refrigerator at 4 °C.
Chemicals, extraction, and analysis
Chemicals
A mixture standard solution of 21 types of OCPs was purchased from Chem Service, Inc., USA, containing: α-hexachlorocyclohexane (α-HCH), β-hexachlorocyclohexane (β-HCH), γ-hexachlorocyclohexane (γ-HCH), δ-hexachlorocyclohexane (δ-HCH), o,p′-dichlorodiphenyltrichloroethane (o,p′-DDT), p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT), p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE), p,p′-dichlorodiphenyldichloroethane (p,p′-DDD), heptachlor, heptachlor epoxide, α-endosulfan, β-endosulfan, endosulfan sulfate, cis-chlordane, trans-chlordane, aldrin, dieldrin, endrin, endrin ketone, methoxychlor, and endrin aldehyde at a concentration of 0.3 µg mL−1.
Organic solvents, including n-hexane, acetone, and ethyl acetate, were purchased from Fisher Scientific International, Inc., USA. Copper powder (purity 99.99%) and quartz sand (purity 99.999%) were purchased from Alfa Aesar, USA. A solid-phase extraction column (SPEC, 1000 mg 6 mL−1) was purchased from Agilent, USA. The surrogate (2,4,5,6-tetrachloro-m-xylene and PCB 209) and the internal standard (pentachloronitrobenzene) were purchased from AccuStandard, Inc., USA.
Extraction
Pesticides in the soil samples were extracted with an automated Dionex ASE 300 extractor (Sunnyvale, USA) (Zhang et al. 2009). Prior to extraction, the soil samples were freeze dried, ground in a mortar, and passed through a 0.5-mm sieve. A 10.00 g screened soil sample, along with 3.0 g copper powder and 2.0 g diatomite, was placed in a steel cell. Before the extraction process, 20.0 μL of the 0.3 µg mL−1 surrogate solution was added. The extraction pressure was 1500 psi, the extraction temperature was set to 110 °C, and an n-hexane/acetone solvent (1:1, vol/vol) was used. The preheating and static times were both set to 5 min, and 60% of the cell volume was used. The extracted liquid was merged twice with the extraction, dehydrated by anhydrous sodium sulfate, and then filtered with a 0.4-μm polyvinylidene fluoride filter (organic phase). The organic phase was concentrated to approximately 1 mL and was further cleaned with a Florisil solid-phase extraction (SPE) column (1 g, 6 mL; Supelco, USA), which was preconditioned with 10 mL n-hexane/acetone (1:1, vol/vol) and 2 mL distilled water. After sample loading, the SPE column was eluted with 30 mL of n-hexane/ethylacetate (8:2, vol/vol). The eluted liquid was collected and concentrated to 1 mL under a centrifugal vacuum evaporator system (CVE3100, Eyala, Japan). Approximately 20 μL of 10 µg mL−1 internal standard solution was added prior to analysis.
Water samples were passed through a 0.45-μm glass fiber (i.d., 50 mm) and then concentrated by SPE. Approximately 20 μL of surrogate solution was added prior to the extraction. HyperSep C18 cartridges (500 mg 6 mL−1, Thermo Electron Corporation, USA) were preconditioned thrice (5 min each) with 15 mL ethyl acetate and 20 mL distilled water, sequentially. The filtered water samples were loaded onto the cartridges at a flow rate of approximately 10 mL min−1. After sample loading, the cartridges were washed with 10 mL distilled water and then vacuum dried for approximately 1 h. The cartridges were eluted thrice with 5 mL methanol/acetone (7:3, vol/vol). The extract was concentrated to less than 1 mL with a gentle nitrogen stream, reconstituted with 8 mL n-hexane, and concentrated again to less than 1 mL. Anhydrous sodium sulfate was used to remove the water residue. The extract was finally concentrated to 0.4 mL for analysis. Approximately 20 μL of internal standard solution was added prior to analysis.
Analysis methods
OCPs were identified and quantified using a QP2010 GC/MS spectrometer (Shimadzu, Japan) with a DB-5MS silica-fused capillary column (30 m, 0.32 mm i.d., 0.25 μm film thickness; Agilent Technology). The column temperature was programmed to start from 50 °C (1 min) to 180 °C (1 min) at 20 °C min−1 and then to 240 °C (10 min) at 8 °C min−1. The mass spectrometer was operated in electron impact mode, and the ion source temperature was maintained at 220 °C. Samples (1 μL) were auto-injected in splitless mode for 0.75 min at a temperature of 250 °C.
Quality control and quality assurance
Quantification was performed using the internal calibration method based on the six-point calibration curve for individual OCPs. The OCP concentrations reported in this paper were recovery corrected. For every set of ten samples, a procedural blank and spiked sample comprising all reagents was used to check the procedural performance and matrix effects. The surrogate solution was analyzed for the instrument correction of the calibration.
Physical–chemical properties of the pesticides
The main physical and chemical properties of the pesticides are listed in Table S2 (Supporting Information).
Data and figure process software
The statistical analysis of the data was conducted by using SPSS 16.0 software (SPSS International, USA), and the statistical analysis figures were plotted using Origin 8.0 (OriginLab, USA). The geographic map was plotted using ArcGIS 10.0 (ESRI, USA), and spatial analysis was carried out with Surfer 8.0 (Golden Software, USA).
Results and discussion
Composition and levels of OCPs in the study area
The descriptive statistics of OCP concentrations in soil and water samples are shown in Tables 1 and 2. The concentrations of total OCPs in topsoil, subsoil, groundwater, and surface water were 35.1–1329.2, 6.0–313.8 ng g−1 dw, 44–432, and 160–188 ng L−1, respectively; the mean values were 240.5 ± 308.9, 122.6 ± 82.9 ng g−1 dw, 216 ± 120, and 172 ng L−1, respectively. ∑DDT, ∑HCH, and ∑heptachlor were the main OCPs in the soil, which accounted for 92.3% of ∑OCPs in topsoil and 90.5% of ∑OCPs in subsoil. The fraction of ∑DDT was primarily comprised of p,p′-DDE (Table 1), which is often considered the result of the dehydrodechlorination reaction of DDT in forming DDE under aerobic environments (Pfaender and Alexander 1972). This result was consistent with the aerobic soil environment and the long-term non-application of technical DDT in the study area. The main isomer of HCH in the soil was β-HCH (Table 1). The vapor pressures of α-HCH and γ-HCH were higher than the vapor pressure of β-HCH; however, the half-lives of α-HCH and γ-HCH were lower than the half-life of β-HCH (Table S2, Supporting Information). Therefore, α-HCH and γ-HCH would be easier to degrade and volatilize than β-HCH, and the residue level of β-HCH would be higher than that of α-HCH and γ-HCH in the soil environment. Heptachlor epoxide is the main part of ∑heptachlor (Table 1), thus indicating that technical heptachlor had high degradation in the soil. Unlike the composition of OCPs in the soil, the OCPs were comprised mainly of endrin in the groundwater (91.1% of total OCPs) and surface water (82.6% of total OCPs).
The levels of OCPs in the greenhouse were significantly higher than those in the uncultivated meadow, suggesting that OCP application in the greenhouse had a significant influence on soil OCP residues (Table 1). The mean concentrations of ∑DDT and ∑HCH (160.7 and 39.8 ng g−1 dw in topsoil and 67.7 and 24.8 ng g−1 dw in subsoil) in the study area were higher than those in the kailyard and paddy fields in most areas in China (Table S3, Supporting Information), farmlands in Central Germany (Manz et al. 2001), and cotton fields in the USA (Kannan et al. 2003). However, the mean concentrations of ∑DDT and ∑HCH were lower than those in paddy fields and tea gardens in India (Mishra et al. 2012) and orchards in New Zealand (Gaw et al. 2006). The respective concentration of ∑heptachlor and endrin was 21.5 and 14.3 ng g−1dw in topsoil and 18.5 and 6.9 ng g−1 dw in subsoil, which were higher than the concentrations in the farmlands, kailyards, orchards, and paddy fields in other areas of China (Table S4, Supporting Information), farmlands in the Southern USA (Bidleman and Leone 2004), various land-use types in Sao Paulo, Brazil (Rissato et al. 2006), and urban areas in the Czech Republic (Shegunova et al. 2007). These results indicate that the application amount of technical heptachlor and endrin in the present study was higher than that in other areas because of the persistence of heptachlor epoxide (the main metabolite of technical heptachlor in the study area) and endrin in the soil environment (Table S2, Supporting Information). This finding indicates that pesticide pollution in the vegetable planting base soil of the study area was more serious than in many areas. However, as shown in Table 3, both the concentrations of ∑DDT and ∑HCH in the soil of the study area were still much lower than the Chinese soil quality criteria of class 2 (The People’s Republic of China National Environment Protection Agency and The People’s Republic of China State Bureau of Technical Supervision 1996), which is considered the human health standard limit. Therefore, the pollution levels of both ∑DDT and ∑HCH were low in the soil of the study area.
The mean concentration of endrin (179 ng L−1) in the groundwater in the study area was lower than that in the paddy field of the Taihu Basin in Eastern China (Wang et al. 2007) but was higher than that in the irrigation area of Taiyuan City in Northern China (Zhang et al. 2013) and the US EPA criteria (US Environmental Protection Agency 1986).
The mean concentration of ∑HCH (18 ng L−1) in the groundwater in the study area was higher than that in farmlands in most areas of China (see Table S5, Supporting Information) and was significantly higher than that in farmland in Bangladesh (Matin et al. 1998) and various land-use types in Sao Paulo, Brazil (Rissato et al. 2006). However, the mean concentration of ∑HCH in the groundwater in the study area was considerably lower than that in the urban area of Hyderabad, India (Shukla et al. 2006), and the Chinese groundwater quality criteria of class 2 (The People’s Republic of China State Bureau of Technical Supervision 1994), which is considered the groundwater background value (Table 3). Table 3 also shows the concentration of ∑DDT in the groundwater of the study area at background level. These findings reveal the serious groundwater pollution caused by endrin in the vegetable planting base. Endrin in the groundwater of uncultivated meadows (farmlands in the 1980s) had the same levels as that in the groundwater of the vegetable planting base area (Table 2), suggesting that the pesticide residue in the groundwater may be caused by long-term application.
Distribution of OCPs in the study area
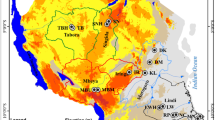
The concentration of the main OCPs in the soil–groundwater system was interpolated using Surfer 8.0 (Golden Software, USA). The level of OCPs was the same in most greenhouses (Fig. 2). However, high concentration peaks were observed, e.g., the level of ∑DDT in the topsoil of S11 was higher than that of other greenhouse (Fig. 2a). The ratio of o,p′-DDT/p,p′-DDT in the S11 greenhouse was 10.25, suggesting that large amounts of dicofol were used in addition to DDT (Qiu and Zhu 2010). The level of o,p′-DDT in other greenhouses was significantly lower than the level of p,p′-DDT, suggesting that the DDT residues in these greenhouses mainly originated from technical DDT (Kim and Smith 2001). The concentration peaks of ∑HCH in the topsoil were found in the greenhouses of S10 and S14 (Fig. 2b), which mainly originated from technical HCH because of the high ratio of β-HCH/γ-HCH (Li et al. 2000). Concentration peaks of ∑heptachlor were found in greenhouse S9 both in the topsoil and in the subsoil (Fig. 2c, g), where heptachlor had been significantly degraded into heptachlor epoxide. Thus, the heptachlor residue in the soil was mainly from past heptachlor applications. The concentration peaks of ∑DDT in the subsoil were located in the greenhouses of S2 and S3 (Fig. 2e), where the DDT mainly originated from technical DDT and partly from dicofol. The ∑HCH in the subsoil (Fig. 2f) had a concentration peak in greenhouse S11, where the ratio of γ-HCH/β-HCH was 2.52, suggesting that the HCH residue in this greenhouse mainly originated from technical lindane. High concentrations of endrin were observed in the topsoil of greenhouse S11 (Fig. 2d) and in the subsoil of S6, S7, S8, S14, S1, S18, and S19. This result indicates that the endrin residue in S11 originated from new applications, whereas the endrin in other greenhouses originated from previous applications and leached into the subsoil under long-term irrigation. The distribution trend of endrin in the subsoil was consistent with that in groundwater (Fig. 2i), suggesting that endrin leached into groundwater from the soil.
Distribution of OCPs in space: a ∑DDT in topsoil; b ∑HCH in topsoil; c ∑heptachlor in topsoil; d endrin in topsoil; e ∑DDT in subsoil; f ∑HCH in subsoil; g ∑heptachlor in subsoil; h endrin in subsoil; i endrin in groundwater. The red and green colors denote the high- and low-concentration regions, respectively
Factors to influence the distributions of OCPs
Factors, including pesticide application (application time, type, and amount), soil pesticide residue level, soil physicochemical properties, groundwater depth, groundwater flow field, and the residue level in the environmental matrix, were analyzed to study their influences on OCP distribution in the soil–groundwater system.
The metabolites or isomers of some OCPs could reflect the residue time of OCPs in the environment and further reveal the application time (i.e., new input or unused for a long time) and type. The fitting curves of the three main pesticides (DDT, HCH, and heptachlor) in the soil and their main metabolites or isomers were plotted to study the relationship between application time and OCP distribution (Fig. 3). No significant correlation was found between ∑DDT and p,p′-DDE in the topsoil and subsoil. However, a significant positive correlation was found between ∑DDT and p,p′-DDE in the topsoil (R 2 = 0.972, p < 0.01) and subsoil (R 2 = 0.909, p < 0.01) when the surface soil data of sample S11 were excluded (Fig. 3a). The above analysis shows that the DDT residue in greenhouse S11 may be mainly from dicofol, but that the residue in other greenhouses may be mainly comprised of p,p′-DDE, a degradation product of DDT. This result indicates that the distribution of DDT in the soil was significantly influenced by application time and application type. As shown in Fig. 3b, a significant positive correlation exists between ∑HCH and β-HCH in the topsoil (R 2 = 0.999, p < 0.01) and subsoil (R 2 = 0.929, p < 0.01). However, the correlation was not significant when the data of S18 in the top soil (S11 and S19 in subsoil) were considered. This result reveals that the HCH distribution in the soil was significantly influenced by its application time and regionally influenced by its application type.
Both β-HCH and aldrin were significantly correlated with k4 (i.e., the proportion of soil particles with a diameter <0.002 mm) in the topsoil (p < 0.05), indicating that β-HCH and aldrin might be easily absorbed in the clay soil. By contrast, both p,p′-DDD and p,p′-DDE were significantly correlated with TOC in the topsoil and subsoil (p < 0.05).This result could be attributed to the high adsorption capacity of p,p′-DDD and p,p′-DDE by soil organic matter (Wang et al. 2006). However, most of other OCPs in the soil layer (i.e., topsoil and subsoil) did not correlate with the soil physical and chemical indicators (i.e., pH, CEC, TOC, and soil mechanical composition). This result could be attributed to the effect of other factors on pesticide distribution in soil, such as soil texture, pesticides properties, soil moisture, soil temperature, microorganisms, light degradation, and hydrolysis.
Zhang et al. (2013) identified that groundwater flow direction was one of the main driving factors of OCP distribution in the soil–groundwater system in the Xiaodian irrigation area. However, in this study, the OCP distribution in the groundwater (Fig. 2i) was not consistent with the groundwater flow field (Fig. 1c). Thus, the hydrodynamic dispersion effect had no significant influence on the migration of pesticides in groundwater. This result could be attributed to the differences of groundwater depths in the study area which were not significant.
The level of endrin in the groundwater had a significant correlation with the levels in the soil (Fig. 3d). This indicates that the soil layer may paly a role as a pollution source, which could affect the distribution of OCPs in the groundwater.
Conclusions
∑DDT, ∑HCH, and ∑heptachlor were the main components of OCPs in the soil in the vegetable planting base in Northern China, while endrin was the main component of OCPs in the groundwater. Long-term pesticide application had a significant influence on the distribution of pesticides in the soil–groundwater system. The driving factors that influenced OCP distribution in soil were pesticide application time, pesticide type, and soil properties, whereas the major factor influencing OCP distribution in groundwater was OCP residue levels in the soil.
References
An, Q., Dong, Y., Wang, H., & Ge, C. J. (2005). Residues and distribution character of organochlorine pesticides in soils in Nanjing area. Acta Scientiae Circumstantiae, 25, 470–474. (In Chinese).
An, Q., Dong, Y., Wang, H., Wang, X., Wang, M., & Guo, Z. (2004). Organochlorine pesticide residues in cultivated soils, in the south of Jiangsu, China. Acta Pedologica Sinica, 41, 414–419. (In Chinese).
Arias-Estévez, M., López-Periago, E., Martínez-Carballo, E., Smimal-Gándara, J., Mejuto, J. C., & García-RíO, L. (2008). The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agriculture, Ecosystems & Environment, 123, 247–260.
Bidleman, T. F., & Leone, A. D. (2004). Soil–air exchange of organochlorine pesticides in the Southern United States. Environmental Pollution, 128, 49–57.
Covaci, A., Hura, C., & Schepens, P. (2001). Selected persistent organochlorine pollutants in Romania. Science of the Total Environment, 280, 143–152.
Gaw, S. K., Wilkins, A. L., Kim, N. D., Palmer, G. T., & Robinson, P. (2006). Trace element and ΣDDT concentrations in horticultural soils from the Tasman, Waikato and Auckland regions of New Zealand. Science of the Total Environment, 355, 31–47.
Guan, Y. F., Wang, J. Z., Ni, H. G., & Zeng, E. Y. (2009). Organochlorine pesticides and polychlorinated biphenyls in riverine runoff of the Pearl River Delta, China: assessment of mass loading, input source and environmental fate. Environmental Pollution, 157, 618–624.
Hao, H., Sun, B., & Zhao, Z. (2008). Effect of land use change from paddy to vegetable field on the residues of organochlorine pesticides in soils. Environmental Pollution, 156, 1046–1052.
Jiang, Y. F., Wang, X. T., Jia, Y., Wang, F., Wu, M. H., Sheng, G. Y., et al. (2009). Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. Journal of Hazardous Materials, 170, 989–997.
Kannan, K., Battula, S., Loganathan, B. G., Hong, C. S., Lam, W. H., Villeneuve, D. L., et al. (2003). Trace organic contaminants, including toxaphene and trifluralin, in cotton field soils from Georgia and South Carolina, USA. Archives of Environmental Contamination and Toxicology, 45, 30–36.
Kim, J. H., & Smith, A. (2001). Distribution of organochlorine pesticides in soils from South Korea. Chemosphere, 43, 137–140.
Kuranchie-Mensah, H., Atiemo, S. M., Palm, L. M. N. D., Blankson-Arthur, S., Tutu, A. O., & Fosu, P. (2012). Determination of organochlorine pesticide residue in sediment and water from the Densu river basin, Ghana. Chemosphere, 86, 286–292.
Li, Y. F., Scholtz, M. T., & van Heyst, B. J. (2000). Global gridded emission inventories of α-hexachlorocyclohexane. Journal of Geophysical Research, 105, 6621–6632.
Manz, M., Wenzelm, K. D., Dietze, U., & Schüürmann, G. (2001). Persistent organic pollutants in agricultural soils of central Germany. Science of the Total Environment, 277, 187–198.
Matin, M. A., Malek, M. A., Amin, M. R., Rahman, S., Khatoon, J., Rahman, M., et al. (1998). Organochlorine insecticide residues in surface and underground water from different regions of Bangladesh. Agriculture, Ecosystems & Environment, 69, 11–15.
Mishra, K., Sharma, R. C., & Kumar, S. (2012). Contamination levels and spatial distribution of organochlorine pesticides in soils from India. Ecotoxicology and Environmental Safety, 76, 215–225.
Pfaender, F. K., & Alexander, M. (1972). Extensive microbial degradation of DDT in vitro and DDT metabolism by natural communities. Journal of Agricultural and Food Chemistry, 20, 842–846.
Qiu, J. (2010). China faces up to groundwater crisis. Nature, 466, 308.
Qiu, X., & Zhu, T. (2010). Using the o, p′-DDT/p,p′-DDT ratio to identify DDT sources in China. Chemosphere, 81, 1033–1038.
Rissato, S. R., Galhiane, M. S., Ximenes, V. F., de Andrade, R. M., Talamoni, J. L. B., Libânio, M., et al. (2006). Organochlorine pesticides and polychlorinated biphenyls in soil and water samples in the Northeastern part of São Paulo State, Brazil. Chemosphere, 65, 1949–1958.
Shegunova, P., Klánová, J., & Holoubek, I. (2007). Residues of organochlorinated pesticides in soils from the Czech Republic. Environmental Pollution, 146, 257–261.
Shukla, G., Kumar, A., Bhanti, M., Joseph, P. E., & Taneja, A. (2006). Organochlorine pesticide contamination of ground water in the city of Hyderabad. Environment International, 32, 244–247.
The People’s Republic of China Ministry of Environmental Protection. (2011). The groundwater pollution prevention and control planning (2011–2020). http://www.zhb.gov.cn/gkml/hbb/bwj/201111/t20111109_219754.htm, October 28, 2011.
The People’s Republic of China National Environment Protection Agency, & The People’s Republic of China State Bureau of Technical Supervision. (1996). Soil quality criteria of china. http://kjs.mep.gov.cn/hjbhbz/bzwb/trhj/trhjzlbz/199603/W020070313485587994018.pdf, March 1, 1996.
The People’s Republic of China State Bureau of Technical Supervision. (1994). Quality standard for ground water. http://kjs.mep.gov.cn/hjbhbz/bzwb/shjbh/shjzlbz/199410/t19941001_66500.htm, October 1, 1994.
US Environmental Protection Agencey. (1986). National recommended aquatic life criteria table. http://water.epa.gov/scitech/swguidance/standards/criteria/current/index.cfm, May 1, 1986.
Wang, F., Jiang, X., Bian, Y. R., Yao, F. X., Gao, H. J., Yu, G. F., et al. (2007). Organochlorine pesticides in soils under different land usage in the Taihu Lake region, China. Journal of Environmental Sciences-China, 19, 584–590.
Wang, X., Piao, X., Chen, J., Hu, J., Xu, F., & Tao, S. (2006). Organochlorine pesticides in soil profiles from Tianjin China. Chemosphere, 64(9), 1514–1520.
Zhang, L., Dong, L., Shi, S., Zhou, L., Zhang, T., & Huang, Y. (2009). Organochlorine pesticides contamination in surface soils from two pesticide factories in Southeast China. Chemosphere, 77, 628–633.
Zhang, C., Liao, X., Li, J., Xu, L., Liu, M., Du, B., et al. (2013). Influence of long-term sewage irrigation on the distribution of organochlorine pesticides in soil-groundwater systems. Chemosphere, 92, 337–343.
Acknowledgements
This work was financially supported by the Research Foundation for Talented Scholars of North China University of Water Conservancy and Electric Power, the Scientific Research Foundation of the Higher Education Institutions of Henan Province (Grant No. 16A570001), the National Public Benefit (Environmental) Research Foundation of China (Grant No. 201109009), and the Program for New Century Excellent Talents in University (Grant No. NCET-13-0794).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no any actual or potential conflict of interest to this work, including any financial, personal, or other relationships with other people or organizations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, HW., Lei, HJ., He, XS. et al. Levels and distributions of organochlorine pesticides in the soil–groundwater system of vegetable planting area in Tianjin City, Northern China. Environ Geochem Health 39, 417–429 (2017). https://doi.org/10.1007/s10653-016-9899-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9899-9