Abstract
Many studies have conducted to determine the best management practice to reduce the mobility and phytoavailability of the trace metals in contaminated soils. In this study, geochemical speciation and phytoavailability of Zn for sunflower were studied after application of nanoparticles (SiO2 and zeolite, with an application rate of 200 mg kg−1) and bacteria [Bacillus safensis FO-036b(T) and Pseudomonas fluorescens p.f.169] to a calcareous heavily contaminated soil. Results showed that the biotic and abiotic treatments significantly reduced the Zn concentration in the aboveground to non-toxicity levels compared to the control treatment, and the nanoparticle treatments were more effective than the bacteria and control treatments. The concentration of CaCl2-extractable Zn in the treated soils was significantly lower than those of the control treatment. The results of sequential extraction showed that the maximum portion of total Zn belonged to the fraction associated with iron and manganese oxides. On the contrary, the minimum percent belonged to the exchangeable and water-soluble Zn (F1). From the environmental point of view, the fraction associated with iron and manganese oxides is less bioavailable than the F1 and carbonated fractions. On the basis of plant growth promotion, simultaneous application of the biotic and abiotic treatments significantly increased the aboveground dry biomass yield and also significantly reduced the CaCl2-extractable form, uptake by aboveground and translocation factor of Zn compared to the control treatment. Therefore, it might be suggested as an efficient strategy to promote the plant growth and reduce the mobile and available forms of toxic metals in calcareous heavily contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is a trace metal of anthropogenic or geogenic origin, which is an essential nutrient for plants, animals, and human. Excessive trace metals accumulation in the soil, particularly in the soil solution, is due to human activities such as industrialization, mining, and intensive agricultural activities (Marchiol et al. 2007; Mousavi et al. 2010a, b, 2013, 2017; Xiu-Zhen et al. 2012). The normal and maximum permissive concentration of Zn in the soil is 3–50 and 300 mg kg−1, respectively (Kloke 1980). Nevertheless, Zn concentrations exceeded 300 mg kg−1 may encounter a threat to public health. According to Khurana and Chatterjee (2011); in sunflower, tissue Zn concentrations associated with the threshold of toxicity and toxicity are 190, and 240 mg kg−1 on a dry matter basis, respectively. Therefore, the remediation of Zn contaminated soils has attracted raising attention and is considered to be a noteworthy environmental issue. Selecting a suitable remediation method in accordance with the types and concentrations of contaminants, site properties, and use of the polluted soil is vital to reduce the environmental risks (Bolan et al. 2014). The fixation of trace metals in the soil is an undertaking remediation method because of being simple, effective, inexpensive, and in situ (Ok et al. 2011). In-situ remediation methods are based on the addition of amendments into a polluted soil. It may cause some changes in the geochemical fractions of the contaminants (Sanderson et al. 2014). Prevalent procedures for the remediation of contaminated soils are land application of organic matter residues, natural, and artificial additives such as zeolites, hydrous oxides of Al, Fe, and Mn, and phytoremediation techniques (Bolan et al. 2014). However, these immobilizing agents, though economical and environmentally friendly, have not been extensively evaluated as nanoparticles (NPs). Moreover, their effects on the chemical speciation of trace metals in high levels of soil contamination in calcareous condition have remained unknown.
The availability of trace metals in the soils depends on their properties and chemical form, binding state, environmental parameters, and soil properties. The geochemical speciation of trace metals must be taken into account in environmental contamination experiments because total concentrations in the soil present limited data on the mobilization and bioavailability of them, which can be misguiding when judging environmental effects due to the possible overestimation of exposure risk (Shaheen and Rinklebe 2014). Silicon application significantly reduced Zn concentration and accumulation in various plant tissues (Anwaar et al. 2014; Bokor et al. 2014a, b) which is not inert but acts as a physical barrier in plants (Liang et al. 2006). This element not only deposits in the cell walls but is also involved in vital activities, especially under environmental stresses (Liang et al. 2006). da Cunha et al. (2008) reported that the application of Si in the form of calcium silicate changed the Zn distribution in the soil, and it was found in more stable fractions: complexed with organic matter and Fe–Mn oxides. Based on the reports of Adrees et al. (2015) and Yao et al. (2017), Si-rich treatments deposit metals principally as their silicates, phosphates, and hydroxides in treated soils. Zeolite is an aluminosilicate mineral with widespread industrial applications, and it is commonly used as a metal-immobilizing agent (Bolan et al. 2014; Navel and Martins 2014), and it can significantly induce the redistribution of trace metals in the soil between the available (exchangeable and soluble) and non-available (oxide, carbonate and bonded to organic matter and residual) forms (Janos et al. 2010). However, little information is available about the potential of SiO2 and zeolite in nano-sized on the geochemical speciation and phytoavailability of trace metals in calcareous heavily contaminated soils.
The rhizosphere bacteria that can colonize plant roots and promote plant growth are usually called plant growth-promoting bacteria (PGPBs) (Kloepper and Schroth 1978). The potential of PGPBs to alleviate plant stress in metal-contaminated soils is well documented (Mani et al. 2015; Kamran et al. 2016). These bacteria are known for their effects on trace metal mobility and bioavailability because they release chelating agents and siderophores, acidic materials, and phosphate solubilizers. They also cause redox changes (Ma et al. 2011), which directly and/or indirectly change the chemical speciation of these toxic metals in the soil. However, in the recent studies (Wu et al. 2006; Li and Wong 2010; Xu et al. 2012), no attention has been paid to study the potential of these bacteria on speciation and bioavailability of metals in calcareous heavily contaminated soils at the presence of nanoparticles and in greenhouse conditions.
Until now, numerous methods have been suggested for determining the available concentrations of trace metals in soil; however, the study of highly contaminated calcareous soils is still limited. For example, the DTPA–TEA (diethylentriaminepentaacetic acid–triethanolamine) soil test was developed by Lindsay and Norvell (1978) to identify near-neutral and calcareous soils with insufficient levels of available micronutrients. In some recent studies on the contaminated calcareous soils which have some similarities to this work, a limited range of extractants was studied in lower levels of contamination (Calvarro et al. 2014; Cornu et al. 2014). Hence, a comprehensive research on chemical behavior of trace metals (e.g., their availability and geochemical fractions) is required and can be used as an important tool for interpreting the chemical interactions between these elements and the components of calcareous soils under high levels of soil contamination. The main objective of this work was to study the geochemical speciation and phytoavailability of Zn for sunflower (Helianthus annuus L.) in a natural contaminated calcareous soil treated with different nanoparticles and PGPBs. The potential of different extractants was studied in respect to extract the available form of Zn in the mentioned soil as one of the focal points of the experiment, as well.
Materials and methods
Soil/amendments preparation and characterization
The soil, from calcisols in WRB system, was collected (1–30 cm) from the surrounding of the NILZ Company in Zanjan, Iran (36°36′40″ and 36°38′40″N; 48°37′33″ and 48°38′48″E). There are many industrial and mining activities in Zanjan province, Zanjan, Iran, which may cause trace metals entry, especially Zn, into the food chains. For this reason, this area was selected. Also, some soil samples were collected from surrounding area to measure and determine the total Zn concentration. For example, total Zn concentration in some industrial enterprises nearby the studied area was: Calcimine Company 4733 mg kg−1, Zanjan Zinc Khales Sazan Company 5024 mg kg−1; Dandi road 8132 mg kg−1; 10th Km of Zanjan–Dizaj Abad road 7667 mg kg−1, Pars Rouy Company 3478 mg kg−1. Obtained results confirm that not only the studied area but also it’s around have high levels of Zn contamination. After air-drying, the soil was sieved (2 mm) to determinate some physical [soil texture (Gee and Bauder 1986)], chemical [pH, EC, CEC, OC%, calcite%, Si, N, P, K, Pb, Cd and Zn concentration (Sparks 1996)] and biological properties [microbial population and respiration (Weaver et al. 1994)]. These properties were found as follows: soil texture loamy; pH 7.19; EC 4.64 dS m−1; CEC 17.94 meq 100 g−1; OC 0.39%; calcite 15.25%; Si 158.98 mg kg−1; N 0.07%; P 8.6 mg kg−1; K 344 mg kg−1; total Zn 7027.54 mg kg−1; total Pb 52.48 mg kg−1; total Cd 26.7 mg kg−1; microbial population 150,000 MPN g−1 and microbial respiration 0.25 mg g−1. Because of many adverse effects induced by sterilization in the soil (Perkins et al. 2013) a non-sterilized soil was used in this study.
The zeolite used in this study was a natural clinoptilolite-zeolite powder (< 60 µm in diameter and with an 85–95% purity) prepared by Afrand Tusca company (http://zeolite-afrand.com/about_e.html), in Tehran, Iran. Initial compositions of the zeolite were found as follows (unit%): SiO2 67.5, Al2O3 12.8, Li2O 7.3 CaO 3.1, K2O 2.1, Na2O 1.03, Fe2O3 1.3, MgO 0.8, TiO 0.3, MnO 0.04, P2O5 0.03, and its CEC was 160–180 meq 100 g−1. Then, the zeolite was changed to NPs (< 100 nm) by using a Planetary Ball Mill (PM 600) in Material and Energy Research Center (https://en.merc.ac.ir/), in Karaj, Iran. In addition, the SiO2-NPs were obtained from Sigma-Aldrich (purity > 95%). The morphology and characteristics of the zeolite/SiO2-NPs were studied by field emission scanning electron microscopy (FE-SEM; Hitachi S-4700, Tokyo, Japan), and energy-dispersive X-ray spectra (EDS) were achieved using FE-SEM.
In this research, two native bacterial species, namely Bacillussafensis FO-036b (T) and P. fluorescens p.f.169, were used as biotic treatment. They were separated and purified from the soils in the vicinity of the zinc and lead mines of Haft Emarat in Arak, Iran (longitude of 35°48′35″E and latitude of 50°58′18″N) (Motesharezadeh and Savaghebi-Firoozabadi 2010). Some characteristics of the studied strains including their ability in production of siderophore (Schwyn and Neilands 1987), ACC-deaminase (Penrose and Glick 2001) and indole-3-acetic acid (IAA) (Patten and Glick 2002) which confirm they are plant growth-promoting rhizobacteria (PGPR) were determined, and their resistance to high levels of Pb, Zn, and Cd was also tested.
Administration of the amendments to the soil and pot experiment
This factorial greenhouse pot experiment was arranged in a completely randomized design. The treatments were biotic in 3 levels (inoculation with Bacillus safensis, inoculation with P. fluorescens, and control or non-inoculation) and abiotic treatment consisted of 3 levels, SiO2-NPs and zeolite-NPs (with an application rate of 200 mg kg−1), and control. Because in recent studies on silicon application in contaminated soils, the applied rate most was around 200 mg kg−1 (da Cunha and do Nascimento 2009; Li et al. 2009; Inal et al. 2009; Syu et al. 2016); therefore, this level was preferred for both SiO2 and zeolite.
This experiment was conducted in the plastic pots with 14.5 cm height and 8.5 cm opening mouth diameter. Each pot was filled with 3 kg air-dried soil passed through a 4 mm sieve and for preventing the waterlogging at the bottom of the pots, they are leaky for drainage, and they were uniformly sprayed with Zeolite/SiO2-NPs in February 16, 2016. The pots were then incubated for 60 days (Wu et al. 2015) at room temperature (23–27 °C), and soil moisture was held near field capacity (FC) by weighting everyday, and the lost water was added again (Huang et al. 2009). The soil of each pot was mixed well once every week to maintain intimate contact with the nanoparticles. After incubation step in April 15, 2016, all the incubated soil samples were air-dried and passed through a 2-mm sieve. The soil of each pot was sampled to determine the geochemical forms of Zn by method of Tessier et al. (1979).
After that, sunflower seeds (Helianthus annuus L. ‘Azargol cultivar’—Seed and Plant Improvement Institute in Karaj, Iran) were surface sterilized by soaking for 12 min in 1.5% sodium hypochlorite and thoroughly rinsed with sterile deionized water. They were then germinated, and six germinated seeds (later thinned to three) were transplanted into the pots. For inoculation, 1 ml of mixed inoculum (B. safensis and P. fluorescens with the same bacterial populations of 5 × 108 cfu ml−1) was applied per germinated seed which was injected under the germinated seed in the pot in April, 15, 2016. During the plant growth, irrigation was done up to field capacity with deionized water, and the pots moisture content was controlled by weighting every day, and the lost water was added again. During the study, environmental parameters were as follows: temperature 28 °C/20 °C (day/night), average temperature 26 °C, relative humidity 48%, light intensity 14,000 lx, and photoperiod 14/10 h (Huang et al. 2009).
Chemical and statistical analyses
After 2 months from the transplanting date, on June 13, 2016, the plants were harvested and the aboveground biomasses were separated from the roots (Jones et al. 1991), and Zn concentration was measured by atomic absorption (Shimadzu, Japan A-670). Soil samples were collected after harvesting from the pots and prepared for analyses as well.
As one of the objectives of this experiment, six extractants for the available form of Zn were compared to find a suitable one indicating the highest correlation with plant response. To do this, the ability of Zn extraction was studied in 12 replications. The extractants were H2O (Abreu et al. 2006); 1 M MgCl2, pH 7 (Tessier et al. 1979); 0.01 M CaCl2 (Houba et al. 2000); Ca (NO3)2 (Mench et al. 1994); DTPA + TEA (Lindsay and Norvell 1978) and HNO3 (Chang et al. 1984). After that, the superior extractant was selected based on having the highest determination coefficient with plant response (dry biomass yield and Zn uptake in plant tissues). The superior extractant was also compared to the extractant proposed by Tessier et al. (1979) and Elliott et al. (1990) for the extraction of exchangeable and water-soluble fractions. For studying the Zn geochemical fractions, the sequential extraction method suggested by Tessier et al. (1979) and Elliott et al. (1990) was used. Based on these procedures, Zn was partitioned into five fractions: exchangeable and water soluble (F1:1 M MgCl2, pH 7), bound to carbonates (F2: 1 MNaOAc adjusted to pH 5 with acetic acid HOAc), bound to Fe–Mn oxides [F3: 0.04 M NH2OH–HCl in 25% (v/v) HOAc], bound to organic matter (F4:0.02 M HNO3 and 5 ml of 30% H2O2 adjusted to pH 2 with HNO3, 5 ml of 3.2 M NH4OAc in 20% [v/v] HNO3), and residual (F5: HF–HC104 mixture). The separation of the steps was done by decantation of the supernatant after centrifugation at a rate of 20,000 rpm for 30 min. Finally, the concentrations of Zn in different fractions were measured by atomic absorption (Shimadzu, Japan A-670).
At each extraction step and in each measurement, a blank sample was analyzed in 3 replications for evaluating the quality of the reagents and to detect possible trace metal contaminations. Translocation factor (TF) from roots to aboveground (the ratio of Zn accumulation in aboveground to root was measured and phytoavailability of Zn was calculated, according to the Eq. (1) (Cao et al. 2009);
where CP is the metal concentration (mg kg−1) in plant; YA is the aboveground biomass (kg pot−1); CS is the metal concentration (mg kg−1) in soil; and M is the soil mass (kg pot−1).
Statistical analysis of data was performed by MSTATC (MSTATC, East Lansing, Mich.) and SPSS 16.0 (SPSS Inc., Chicago, IL) software, and the means were compared according to the Duncan’s multiple-range test (P < 0.05).
Results and discussion
Characterizing the bacteria and zeolite/SiO2 NPs
Figure 1 presents the scanning electron microscopy (SEM) images of SiO2 NPs and zeolite NPs at 100 and 200 nm magnifications, respectively (Fig. 1a, b). In addition, energy-dispersive X-ray spectra (EDS) of SiO2 NPs and zeolite NPs are presented in Fig. 2(a, b, respectively). Figure 1a, b shows that zeolite with the average particle size of < 60 nm can be clearly recognized as the coarser powders than the SiO2 which has the average particle size of < 15 nm; therefore, SiO2 it is considered as the NPs with the higher specific surface area than the zeolite NPs that might be had a more effect on trace metals stabilization. Energy-dispersive X-ray spectra (EDS) of the studied NPs further confirm the presence of Si, O, Fe, Al, Na, and K in the zeolite-NPs (Fig. 2).
The results of some laboratory studies showed that the studied strains are restraint to toxic metals and have the PGPR characteristics found as follows: siderophore positive, ACC-deaminase positive, indole-3-acetic acid positive, resistance to Pb (2500 mg kg−1), resistance to Zn (3000 mg kg−1), and resistance to Cd (2500 mg kg−1); these results are consistent with the Khare et al. (2010) and Issazadeh et al. (2014). Also, in order to test if the bacteria were successfully inoculated or not, both during the plant growth period and after harvesting step, the microbial population and respiration were measured in the inoculated soils. The results showed that the inoculated soils significantly had more microbial activity than the non-inoculated soil. For example, the maximum microbial respiration/population belonged to the P. fluorescens + SiO2-NPs treatment (data not shown).
Comparing extractants for available and geochemical fractions of Zn
The results showed that Zn extracted by MgCl2 has the highest coefficient of determination (R 2 > 0.8) with the plant response, Zn uptake in the plant tissue and dry biomass yield, while the lowest coefficient of determination belonged to Ca (NO3)2 (R 2 < 0.1) and H2O (R 2 < 0.3). The results for Zn uptake by plant are presented in Fig. 3. Generally, the order of different extractants in terms of determination coefficient of plant responses, both dry biomass yield and Zn uptake by plant, with the extractable Zn by the extractants was as follows: MgCl2 > CaCl2 > DTPA–TEA > HNO3 > H2O ≫ Ca (NO3)2.
Calcium chloride, magnesium chloride, calcium nitrate, and 0.1 M nitric acid are used to extract the exchangeable and water-soluble form of different metals such as Zn (Jones et al. 1991). Distilled water (H2O) is able to extract the water-soluble fraction. However, this form is not a suitable index of metals bioavailability (Jones et al. 1991). CaCl2 and MgCl2 are currently used as the universal extractants. Magnesium chloride is the most frequently used extractant for the measurement and quantification of ion-exchangeable of different metals such as Zn. The rationalization of using this extractant considers two important characteristics: the high ion-exchange capacity of Mg(II) and the almost insignificant complex-formation potential of chloride ions. Moreover, according to Houba et al. (2000), 0.01 M CaCl2 has ionic strength, which is similar to that of for the soil solution and Ca is the main cation in the soil solution and on the adsorption complex of soils. The Ca2+ and Mg2+ ions from the extractants also present additional positive effects of promoting the aggregate stability of the soil, as the soil colloidal material in the suspension is coagulated. Calcium chloride is also un-buffered. Therefore, the necessary reactions and interactions may occur at the initial pH of the soil. Accordingly, these extractants are better able to extract other adsorbed cations than solutions containing other cations (Houba et al. 2000). Amoakwah et al. (2013) reported that CaCl2 extraction method generally extracted appreciable amounts of Zn and Cd after the application of the amendments. Soil analysis by DTPA–TEA (diethylentriaminepentaacetic acid–triethanolamine) method was developed by Lindsay and Norvell (1978) to identify near-neutral and calcareous soils with insufficient levels of available micronutrients. Because the availability level of Zn in the studied soil was significant, the weak response of this extractant in Zn extraction is justifiable.
The results of Zn speciation after incubation showed that the highest portion of the total Zn belonged to the Zn associated with Fe–Mn oxides fraction (F3), from 31.27 to 43.88%, and followed by the residual fraction (F5). The soluble + exchangeable fraction (F1) had the lowest portion, ranging from 0.11 to 0.23% of the total content, and the carbonated fraction was significant which refer to the calcareous nature of the studied soil (Saffari et al. 2016). Generally, the percentage of different fractions in the soil after incubation period was as follows: associated with iron and manganese oxides > residual > associated with carbonates > associated with organic matter > soluble + exchangeable Zn (data not shown).
After harvesting the sunflower, Zn geochemical fractions in the soil were studied, as well. The results showed that zeolite-NPs (Z) and SiO2-NPs (S) as abiotic treatment were superior to the biotic treatment and significantly affected the Zn speciation (P < 0.05). The interactive effect of biotic and abiotic amendments was significant only on F2 and F4 fractions (data not shown). The nanoparticles significantly reduced the exchangeable and water-soluble Zn (F1) compared to the control treatment (P < 0.05) up to about 10.28 and 12.62% by zeolite-NPs and SiO2-NPs, respectively (Table 1). The decrease of F1 fraction in the soil treated with SiO2 and zeolite-NPs compared to the control treatment reduces the Zn uptake by plants (Table 3). In nanoparticles, most of the atoms are on the surface which they are unsaturated and can easily cohere with other ions, possessing considerable chemical reactivity. Therefore, such materials can selectively adsorb metal ions and have a significant adsorption capacity (Anjum et al. 2016). The superiority of the CS compared to CZ with regard to decrease in F1 fraction can be related to its smaller particle size and therefore greater specific surface area than the zeolite (Fig. 1). Therefore, these amendments can be applied to alter Zn contamination, aiming to decrease its solubility and availability (da Cunha et al. 2008; Abbaspour and Golchin 2011; Bolan et al. 2014; Bokor et al. 2014a, b; Navel and Martins 2014; Adrees et al. 2015). The high potential of Si to reduce the availability of Zn might be caused by its effect on decreasing the soil acidity, as well which makes the metals be principally deposited as their silicates, phosphates, and hydroxides (Adrees et al. 2015). The porous and lattice structure of zeolites increases ion-exchange sites in soils in addition to offering absorption sites for small molecules, thereby being able to fix heavy metals in the soil (Abbaspour and Golchin 2011).
The biotic treatment has no significant effect on exchangeable Zn (F1) (Table 1). This might be due to the considerable contamination level of the soil. B. safensis (B) and P. fluorescens (P) are known as plant growth-promoting bacteria (PGPB) that reduce the plant stress in metal-contaminated soils (Khare et al. 2010 and Issazadeh et al. 2014) but high contamination levels decrease their potential. Plant growth-promoting bacteria are known to affect the trace metal mobility and bioavailability due to release of chelating, acidifying, phosphate solubilizing, and redox changing agents (Ma et al. 2011), which prompt the changes in geochemical speciation of these toxic metals in the soil. Except for F1 and F3 fractions, their effects on other fractions were considerable. The F2 fraction (carbonate-Zn) was significantly (P < 0.05) affected by the interaction effect of biotic and abiotic materials. The highest decrease in the concentration of F2 fraction (about 17% less than the control treatment) was happened by the P + S treatment which followed by SiO2-NPs without any biotic treatment (i.e., CS treatment), being about 16% less than the control treatment (Table 2). The high effectiveness of the SiO2-NPs in decreasing the carbonated Zn, both separately and in combination with the bacteria, is likely due to its larger specific surface area and therefore its higher chemical reactivity than the zeolite. Regarding to the importance of F2 fraction with respect to its availability compared to the F3, F4, and F5 fractions (Tessier et al. 1979), more research is needed for more précises and comprehensive investigations on the mechanism of the ensued reactions at presence of these amendments for their simultaneous recommendation in the contaminated soils.
The F3 fraction was positively affected by abiotic amendments. The maximum increase of this fraction was induced by zeolite-NPs (13.3% compared to the control treatment), which had a significant difference from that by SiO2-NPs (Table 1). The mobility of Zn is limited by the existence of Fe–Mn oxides (Kumpiene et al. 2008). The predominant fraction of Zn belonged to this fraction that increased with the application of abiotic amendments, which is in agreement with the findings of Shim et al. (2014). With respect to the EDS of applied mineral particles (Fig. 1), the existence of different oxides in the zeolite structure (such as Fe, Al, and Si oxides) helps to promote the formation of this fraction. In a research, it was reported that the application of Si in the form of Ca2SiO4 changed the Zn distribution in the soil and was found in more stable fractions such as complexes with Fe–Mn oxides (da Cunha et al. 2008). Zeolite, as an aluminosilicate mineral, has a high cation exchange capacity, which is due to the substitution of Si4+ with Al3+ in tetrahedral sites, causing these materials act as natural cation exchangers (Inglezakis et al. 2002). Janos et al. (2010) reported that low levels of zeolite can significantly redistribute the trace metals in the soil between available (exchangeable and water soluble) and non-available (carbonates, oxides, bonded to organic matters, and residual) forms.
The bacteria and NPs significantly increased the organic fraction (F4). The organic fraction increased from 12.02% by BC treatment (B. safensis without any abiotic amendments) to 58.63% by B + S treatment compared to the control treatment (Table 2). Soil bacteria, as the most active organic colloids in the soil system, excrete different organic compounds such as siderophores, which have high association constants compounds, and so they can form stable complexes with other metals (Schalk et al. 2011). They can also form stable complexes with low molecular weight acids which play important roles in the complexation of trace metal ions and enhance their bioavailability for plants (Huang and Chen 2002). Some studies reported that depending on the nature of the metals, metal mobilization may be affected by organic acids produced by plant-associated microorganisms (Braud et al. 2006; Park et al. 2011). Combined biotic and abiotic treatments application was superior to the separate application of biotic or abiotic treatments in respect to promote the F4 fraction and had significant difference with the other treatments (Table 2) that was most likely due to the promotion of microbial activity affected by zeolite/SiO2-NPs. Huang and Chen (2002) showed that soil microorganisms are the most active organic colloids in the soil which by their surface charged and secretion of different organic compounds have a significant role in determination the fate of metals. Therefore, at the presence of the nanoparticles, potential of these bacteria for changing the chemical behavior of metals increases as well. Wu et al. (2006) by studying the effect of mine tailings addition at the presence of bacteria showed that F4-Pb rose dramatically. They reported that increasing the organic compounds resulting from the bacteria activities are the main reasons.
The residual fraction of Zn (F5), which it is not expected to be readily released under natural condition of calcareous soils, was significantly affected by biotic and abiotic amendments. Based on the presented data in Table 1, the residual fraction decreased in the presence of abiotic amendments compared to the control treatment that it was maximum in the pots treated with zeolite-NPs (67.62% less than the control). B. safensis and the zeolite significantly reduced this fraction (50.4% decrease compared to the control) and had no significant difference with each other (Table 1). Even though it was not possible to conclude which fraction received the Zn from the residual fraction, based on Table 2, it might be assumed that under zeolite treatment residual Zn shifted to the carbonated fraction and in B. safensis treatment shifted to organic fraction. From the environmental point of view, under the conditions normally found in calcareous soils, the residual fraction is less bioavailable than the other fractions (Hamidpour et al. 2016). In this work, the potential of B. safensis and zeolite was more than the other treatments to shift Zn to more available fractions. Also, the zeolite-NPs treatment makes the more powerful complexes with the metal ions than the bacteria (Table 1), because the sedimentation is their main mechanism as oxides pool while in the bacteria treatment, adsorption is the dominant mechanism (Wu et al. 2006).
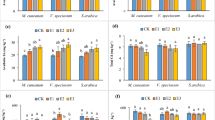
Dry biomass yield, CaCl2-extractable Zn, and status of Zn in the plant tissues
The amendments studied in this work significantly affected dry biomass yields, CaCl2-extractable Zn, and status of Zn in sunflower tissues. On the other hand, the interactive effects of biotic and abiotic treatments were significant on all of them (P < 0.05). The maximum dry biomass yields of the root and shoot were recorded in B + Z and P + S treatments (about 20.34 and 55.05% increase compared to the control treatment, respectively). By contrast, the minimum dry biomass yields of the root and shoot were recorded in the soil treated with SiO2-NPs, without any biotic treatments (CS treatment) and control treatment, respectively (Table 3). Studies showed that PGPBs are able to promote the production of IAA (indole acetic acid), dissolve the phosphate, and demonstrate antagonism toward pathogens and redox changes which affect trace metal mobility and bioavailability (Ma et al. 2011) and finally may promote the plants growth (Raval and Desai 2012; Akbari et al. 2011; Herman et al. 2008). Bansiwal et al. (2006) emphasized that zeolites were prevalently used as soil conditioners, which was a good strategy to make the soil conditions better in arid and semiarid regions (Yasuda et al. 1998). Land application of zeolite promotes the conditions of available nitrogen, phosphorus, calcium, and magnesium in the soil (Abdi et al. 2006). Chander and Joergensen (2002) reported that the influences of zeolites on microbial populations and their activities in soils were entirely unknown; however, they observed an increase in microbial biomass and incorporation of added 14C into microbial biomass after zeolite amendment. Silicon application in polluted soils can control toxic metals bioavailability by affecting on soil properties which finally changes the speciation of the metals in the soil solution due to the formation of different complexes such as silicate complexes (Putwattana et al. 2010). For example, silicon reduces the acidity of growth media which finally decreases the mobility of trace metals. In addition, it was reported that silicate-mediated exudates of root can complex trace metal ions in vitro (Wen et al. 2013). Modulating the metal translocation, restraining trace metal ions translocate from root to shoot, chelating trace metal ions with ligands, compartmenting of trace metals into vacuoles, stimulating of different antioxidants, and structural altering in plants are the important hypothetical mechanisms which alleviate the toxic metals stress by Si (Wen et al. 2013) and promote plant growth in treated contaminated soils. As presented in Table 3, in most cases, the simultaneous application of biotic and abiotic amendments, such as P + Z, B + S, P + S, and B + Z treatments, may lead to the highest dry yields of the root and shoot, being significantly higher than when they were used separately (i.e., CS, CZ, PC, and BC treatments). These findings might be used to adopt an important management strategy in contaminated calcareous soils to achieve an acceptable economic production.
The concentration of trace metals in the aboveground biomass was always less than that in the root (Table 3) (Mousavi et al. 2010a; Mousavi et al. 2010b; Mousavi et al. 2013, 2017). The amendments significantly decreased the concentration of Zn in the plant tissues (P < 0.05), which was variable from 38.99 to 56.4% reduction in the shoot biomass and from 26.24 to 48.89% reduction in the root than the control treatment. The lowest concentration of Zn in the root occurred in the pots treated with B + Z and P + S (about 48.5 and 48.89% less than the control treatment, respectively). To confirm these results, the lowest and maximum microbial respiration/population were measured in the control and P + S treatments, respectively (data not shown). The bacteria in the soil induce surfaces to react strongly with trace metal ions in the soil solution, and they can adsorb a higher amount of them than can inorganic soil materials such as montmorillonite, kaolinite, or vermiculite (Ledin et al. 1996). Xu et al. (2012) and Braud et al. (2009) reported that Cd and Pb uptakes by Pseudomonas inoculated plants reduced due to changing of soluble/exchangeable Cd and Pb to non-available fractions. Because of an extremely high ratio of surface area to volume (about 1–1.5 µm3), bacterial cells have a strong capacity for adsorbing and immobilizing trace metal ions from the soil solution (Wu et al. 2006). By contrast, the highest accumulation of Zn in the root happened in the control treatment, which had a significant difference from the values of the other treatments (Table 3). The associated rhizosphere bacteria, depending on their nature, properties and species as well as on the chemical behavior of the metal, might pose both positive and negative effects on the bioavailability of trace metals in the soil. The different behaviors of the bacteria studied in this research refer to their inherent differences. Meanwhile, their different abilities to change the soil properties, such as dissolved organic carbon (DOC) concentration, and soil acidity, cause many changes on the mobility and bioavailability of trace metals.
Investigation of Zn status in the aboveground plant materials showed that the lowest accumulation of Zn was recorded in the CS and P + S treatments (74.333 and 74 mg kg−1 respectively) (Table 3). They were less than the defined threshold of toxicity and toxicity confines in sunflower (190 and 240 mg kg−1, respectively) by Khurana and Chatterjee (2011). However, the highest accumulation was measured in the control treatment which was up to 76.49 and 39.72%, more than the threshold of toxicity and toxicity confines (Table 3). The positive effects of zeolite and SiO2 in larger particle sizes on trace metal immobilization were reported in different articles (Inglezakis et al. 2002; da Cunha et al. 2008; Janos et al. 2010; Yao et al. 2017). Based on these results, the biotic and abiotic amendments significantly decreased Zn accumulation in the aboveground tissues, and in most cases, the abiotic amendments were better than the biotic amendments in the immobilization of Zn and reduction of its accumulation in the plant tissues, especially in CS and CZ treatments. For example, a lower Zn uptake by the aboveground and root tissues and a lower translocation factor and Zn phytoavailability compared to the most treatments were measured in the soil treated with CS or CZ (Table 3). By contrast, the biotic amendments whether single or combined with the abiotic amendments were better than the others for increasing the Zn concentration, uptake, and translocation factor from the root to the aboveground. There are several possible strategies underlying the decreased trace metals uptake and translocation by Si application. Co-precipitation of the trace metals with Si in metabolically less active tissues, especially in the endodermis cell wall, pericycle, xylem, and phloem, is probably to block the absorption and translocation of the trace metals (da Cunha and do Nascimento 2009; Shi et al. 2005). Furthermore, Si can promote the binding of trace metal to cell walls and compartmentation of more toxic metal into vacuoles (Rogalla and Romheld 2002; Shi et al. 2005), which also limits the trace metal activity and mobility in plant tissues.
All of the amendments studied in this research reduced the phytoavailability of Zn for sunflower, which was variable from 4% less in the B + Z treatment to 40.64% less in the BC treatment than in the control treatment (Table 3). The greatest decrease in Zn phytoavailability was measured in BC (40.64%), CS (37.14%), and B + S (34.68%) treatments. The considerable decrease in Zn phytoavailability under B + S treatment may be due to the formation of insoluble Zn complexes because of the low acidity of SiO2 and the formation of organic matter complexes as a result of activities by B. safensis (Adrees et al. 2015). Among the treatments, the highest dry biomass yield was recorded in the soil treated with the biotic + abiotic amendments. Regarding insignificant differences among most of the treatments, combined treatments of the biotic and abiotic materials might be considered to be a valuable strategy to manage agricultural productions in contaminated calcareous soils. Land application of the bacteria and the NPs in this research significantly decreased CaCl2-extractable Zn, and the maximum decrease was recorded in SiO2-NPs treatment (without any bacteria), being about 38.91% less than the control treatment (Table 3). The application of Si changes the Zn distribution to more stable fractions such as complexed with Fe–Mn oxides (da Cunha et al. 2008). Based on Adrees et al. (2015), Si has a high potential to reduce the availability of Zn by increasing the soil pH, which makes metals be primarily deposited as their silicates, phosphates, and hydroxides.
The final comparison between the extractant which it is used in the Tessier et al. (1979) method and CaCl2, as the superior extractant of this work for the extraction of available Zn, showed that CaCl2 was better than MgCl2 because its determination coefficient with the plant response (Zn uptake by sunflower and dry biomass yield) was more than that of MgCl2 which its results for Zn uptake by plant tissues are presented in Fig. 4. Based on the obtained results, it is concluded that CaCl2 and MgCl2 are good extractants for the available Zn in calcareous heavily contaminated soils, but more studies are needed. Both CaCl2 and MgCl2 are used as the universal extractants in soil solution studies. The similarities of Mg2+ and Ca2+ in their chemical behavior, their predominance in the soil solution and on the adsorption complex of soils (especially Ca2+), high ion-exchange capacity, and the almost insignificant complex-formation potential of chloride ions are the main reasons for their abilities in simulation of the root behavior for the trace metals absorption (Houba et al. 2000; Amoakwah et al. 2013).
Conclusion
The results showed that the studied amendments significantly increased the dry biomass yield of sunflower, confirming a potential role of these amendments in alleviating the Zn toxicity on sunflower which combined biotic and abiotic amendments application was more effective than the individually applied. Zeolite and SiO2 nanoparticles considerably reduced the exchangeable and water-soluble Zn and CaCl2-extractable Zn in comparison with the control and bacteria treatments that had no significant difference with each other. In some cases, the SiO2 treatment was superior to the zeolite that likely refers to its smaller size and subsequently its higher specific surface area than the zeolite. The highest percentage of the total Zn both before and after planting belonged to the oxide fraction. By contrast, the minimum percent belonged to the exchangeable and water-soluble fraction. High percentage of the oxide fraction in the treated soil may be considered as safe from the environmental risk point of view, because this fraction is less bioavailable than the exchangeable and carbonated fractions. However, future researches should focus on higher rates of nanoparticles application (> 200 mg kg−1) and the other trace metals under heavily contamination. In the control treatment, Zn concentration in the aboveground was more than the defined threshold of toxicity and toxicity confines for sunflower which in the nanoparticles and the bacteria treatments it was significantly decreased. Generally, on the basis of toxic metals stress alleviation, combined biotic and abiotic treatments application significantly improved the aboveground dry biomass yield and also reduced the CaCl2-extractable form, uptake by aboveground and translocation factor of Zn compared to the control treatment. Therefore, it might be considered as an efficient method to promote the plant growth and reduce the mobile and available forms of toxic metals in calcareous heavily contaminated soils. Although these results need to be verified on the field scale, they emphasize the potential of studied treatments for land application as a remediation tool for heavily Zn contaminated calcareous soils. Also, the superior extractant need to be approved by conducting more researches on the field scale for suggesting in the contaminated calcareous soils.
References
Abbaspour, A., & Golchin, A. (2011). Immobilization of heavy metals in a contaminated soil in Iran using di-ammonium phosphate, vermicompost and zeolite. Environmental Earth Science, 63, 935–943.
Abdi, G. H., Khui, M. K., & Eshghi, S. (2006). Effects on natural zeolite on growth and flowering on strawberry. International Journal of Agricultural Research, 1, 384–389.
Abreu, C. A., Angela, A. M., Furlani, C., et al. (2006). Quest of water extract analysis of micronutrients in soilless organic substrates. Communications in Soil Science and Plant Analysis, 37, 2327–2338.
Adrees, M., Ali, Sh, Rizwan, M., et al. (2015). Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicology and Environmental Safety, 119, 186–197.
Akbari, P., Ghalavand, A., ModarresSanavy, A. M., et al. (2011). The effect of biofertilizers, nitrogen fertilizer and farmyard manure on grain yield and seed quality of sunflower (Helianthus annuus L.). Journal of Agricultural Science and Technology, 7, 173–184.
Amoakwah, E., Van Slycken, S., Tack, F. M. G., et al. (2013). Assessing the extraction efficiency of CaCl2 and Rhizon extraction methods after the application of organic matter and CaCl2 as soil amendments to enhance the mobility of Cd and Zn. Environmental & Analytical Toxicology, 3, 167. doi:10.4172/2161-0525.1000167.
Anjum, M., Miandad, R., Waqas, M., et al. (2016). Remediation of wastewater using various nano-materials. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2016.10.004.
Anwaar, S. A., Ali, S., Ali, S., et al. (2014). Silicon (Si) alleviates cotton (Gossypiumhirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environmental Science and Pollution Research, 22(5), 3441–3450. doi:10.1007/s11356-014-3938-9.
Bansiwal, A. K., Rayalu, S. S., Labhasetwar, N. K., et al. (2006). Surfactant-modified zeolite as a slow release fertilizer for phosphorus. Journal of Agricultural and Food Chemistry, 54, 4773–4779.
Bokor, B., Bokorová, S., Ondoš, S., et al. (2014a). Ionome and expression level of Si transporter genes (Lsi1, Lsi2, and Lsi6) affected by Zn and Si interaction in maize. Environmental Science and Pollution Research, 22(9), 6800–6811. doi:10.1007/s11356-014-3876-6.
Bokor, B., Vaculik, M., Slováková, L., et al. (2014b). Silicon does not always mitigate zinc toxicity in maize. Acta Physiologiae Plantarum, 36, 733–743.
Bolan, N., Kunhikrishnan, A., Thangarajan, R., et al. (2014). Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? Journal of Hazardous Materials, 266, 141–166.
Braud, A., Jezequel, K., Bazot, S., et al. (2009). Enhanced phytoextraction of an agricultural Cr and Pb contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere, 74, 280–286.
Braud, A., Jézéquel, K., Vieille, E., et al. (2006). Changes in extractability of Cr and Pb in a polycontaminated soil after bioaugmentation with microbial producers of biosurfactants, organic acids and siderophores. Water, Air, and Soil pollution, 6, 261–279.
Calvarro, L. M., de Santiago-Martín, A., Quirós Gómez, J., et al. (2014). Biological activity in metal-contaminated calcareous agricultural soils: The role of the organic matter composition and the particle size distribution. Environmental Science and Pollution Research, 21(9), 6176–6187.
Cao, X. D., Wahbi, A., Ma, L., et al. (2009). Immobilization of Zn, Cu, and Pb in contaminated soil using phosphate and phosphoric acid. Journal of Hazardous Materials, 164, 555–564.
Chander, K., & Joergensen, R. G. (2002). Decomposition of 14C labelled glucose in a Pb-contaminated soil remediated with synthetic zeolite and other amendments. Soil Biology & Biochemistry, 34, 643–649.
Chang, A. C., Warneke, J. E., Page, A. L., et al. (1984). Accumulation of heavy metals in sewage sludge-treated soils. Journal of Environmental Quality, 13, 87–91.
Cornu, J. Y., Elhabiri, M., Ferret, C., et al. (2014). Contrasting effects of pyoverdine on the phytoextraction of Cu and Cd in a calcareous soil. Chemosphere, 103, 212–219.
da Cunha, K. P. V., & do Nascimento, C. W. A. (2009). Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water, Air, and Soil pollution, 197, 323–330.
da Cunha, K. P. V., do Nascimento, C. W. A., & SilvaJ., A. (2008). Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on contaminated soil. Journal of Plant Nutrition and Soil Science, 171, 849–853.
Elliott, H. A., Dempsey, B. A., & Maille, P. J. (1990). Content and fractionation of heavy metals in water treatment sludges. Journal of Environmental Quality, 19, 330–334.
Gee, G. W., & Bauder, J. W. (1986). Particle Size Analysis. In A. Klute (Ed.), Methods of soil analysis: Part 1-physical and mineralogy methods (2nd ed., pp. 383–412). Madison, WI: SSSA.
Hamidpour, M., Khadivi, E., & Afyuni, M. (2016). Residual effects of biosolids and farm manure on speciation and plant uptake of heavy metals in a calcareous soil. Environmental Earth Sciences, 75(12), 1–9.
Herman, M. A. B., Nault, B. A., & Smart, C. D. (2008). Effects of plant growth promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Protection, 27, 996–1002.
Houba, V. J. G., Temminghoff, E. J. M., Gaikhorst, G. A., et al. (2000). Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Communications in Soil Science and Plant Analysis, 31(9–10), 1299–1396.
Huang, Q. Y., Chen, W. L., & Guo X. J. (2002). Sequential fractionation of Cu, Zn and Cd in soils in the absence and presence of rhizobia. In Proceedings of 17th WCSS, August 14e21, Thailand, 1453 pp.
Huang, Y., Hu, Y., & Liu, Y. (2009). Combined toxicity of copper and cadmium to six rice genotypes (oryza sativa L.). Journal of Environmental Sciences, 21, 647–653.
Inal, A., Pilbeam, D. J., & Gunes, A. (2009). Silicon increases tolerance to boron toxicity and reduces oxidative damage in Barley. Journal of Plant Nutrition, 32(1), 112–128.
Inglezakis, V. J., Loizidou, M. D., & Grigoropoulou, H. P. (2002). Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Research, 36(11), 2784–2792.
Issazadeh, K., Savaheli, H., & Momeni, N. (2014). Isolation and identification of heavy metal resistant bacteria from industrial wastewaters in Guilan Province. International Journal of Advanced Biological and Biomedical Research, 2(6), 2066–2071.
Janos, P., Vavrova, J., Herzogova, L., et al. (2010). Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma, 159, 335–341.
Jones, J., Wolf, B., & Mills, H. A. (1991). Plant analysis handbook: A practical sampling, preparation, analysis, and interpretation guide. Athens, GA: Micro-Macro Publishing.
Kamran, M. A., Eqani, S. A. M. A. S., Bibi, S., et al. (2016). Bioaccumulation of nickel by E. sativa and role of plant growth promoting rhizobacteria (PGPRs) under nickel stress. Ecotoxicology and Environmental Safety, 126, 256–263.
Khare, S., Ahmed, N., Pant, S., et al. (2010). Characterization and evaluation of heavy metal tolerance of bacterial species from soil of waste area near Riyan steel rolling mills, Muzaffarnagar, India. Journal of Applied and Natural Science, 2(1), 88–92.
Khurana, N., & Chatterjee, Ch. (2011). Influence of variable zinc on yield, oil content, and physiology of sunflower. Communications in Soil Science and Plant Analysis, 32(19–20), 3023–3030.
Kloepper, J. W., & Schroth, M. N. (1978). Plant growth-promoting rhizobacteria on radishes. In Station de pathologievegetale et phyto-bacteriologie, INRA, Angers, editors. Proceedings of the 4th international conference on plant pathogenic bacteria (Vol. 2, pp. 879–882). Tours: Gilbert-Clarey.
Kloke, A. (1980). Orientierungslaten fur tolerierbare Gesamtgehalteeiniger Elemente in Kulturboden. Mitteilungen der VDLUFA Heft, 1–3, 9–11.
Kumpiene, J., Lagerkvist, A., & Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Management, 28, 215–225.
Ledin, M., Krantz-Rulcker, C., & Allard, B. (1996). Soil Biology & Biochemistry, 28(6), 791–799.
Li, R. Y., Stroud, J. L., Ma, J. F., et al. (2009). Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environmental Science and Technology, 43(10), 3778–3783. doi:10.1021/es803643v.
Li, W. C., & Wong, M. H. (2010). Effects of bacteria on metal bioavailability, speciation, and mobility in different metal mine soils: A column study. Journal of Soils and Sediments, 10(2), 313–325.
Liang, Y., Zhang, W., Qin, C., et al. (2006). Effect of exogenous silicon (Si) on H + -ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environmental and Experimental Botany, 57, 212–219.
Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA test for zinc, iron, manganese, and copper. Soil Science Society of America Journal, 42, 421–428.
Ma, Y., Prasad, M., Rajkumar, M., et al. (2011). Plant growth promoting rhizobacteria and endophytes acceleratephytoremediation of metalliferous soils. Biotechnology Advances, 29, 248–258.
Mani, D., Kumar, C., & Patel, N. K. (2015). Integrated micro-biochemical approach for phytoremediation of cadmium and zinc contaminated soils. Ecotoxicology and Environmental Safety, 111, 86–95.
Marchiol, L., Fellet, G., Perosa, D., et al. (2007). Removal of trace metals by Sorghum bicolor and Helianthus annuus in a site polluted by industrial wastes: A field experience. Plant Physiology and Biochemistry, 45, 379–387.
Mench, M., Vangronsveld, J., Didier, V., & Clijsters, H. (1994). Evaluation of metal mobility, plant availability and immobilization by chemical agents in a limed-silty soil. Environmental Pollution, 86, 279–286.
Motesharezadeh, B., & Savaghebi-Firoozabadi, G. R. (2010). Bioaccumulation and phyto-translocation of nickel by medicago sativa in a calcareous soil of Iran. Desert, 15, 61–69.
Mousavi, S. M., Bahmanyar, M. A., Pirdashti, H., et al. (2010a). Trace metals distribution and uptake in soil and rice grown on a 3-year vermicompost amended soil. African Journal of Biotechnology. http://www.academicjournals.org/AJB.
Mousavi, S. M., Bahmanyar, M. A., & Pirdashti, H. (2010b). Lead and cadmium availability and uptake by rice plant in response to different biosolids and inorganic fertilizers. American Journal of Agricultural and Biological Sciences, 5(1), 25–31.
Mousavi, S. M., Bahmanyar, M. A., & Pirdashti, H. (2013). Phytoavailability of some micronutrients (Zn and Cu), heavy metals (Pb, Cd), and yield of rice affected by sewage sludge perennial application. Communications in Soil Science and Plant Analysis, 44, 3246–3258.
Mousavi, S. M., Bahmanyar, M. A., & Pirdashti, H. (2017). Nutritional (Fe, Mn, Ni, and Cr) and growth responses of rice plant affected by perennial application of two bio-solids. Environmental Monitoring and Assessment, 189(7), 340. doi:10.1007/s10661-017-6050-z.
Navel, A., & Martins, J. M. F. (2014). Effect of long term organic amendments and vegetation of vineyard soils on the microscale distribution and biogeochemistry of copper. Science of the Total Environment, 466–467, 681–689.
Ok, Y. S., Kim, S. C., Kim, D. K., et al. (2011). Ameliorants to immobilize Cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environmental Geochemistry and Health, 33, 23–30.
Park, J. H., Bolan, N., Megharaj, M., et al. (2011). Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. Journal of Hazardous Materials, 185, 829–836.
Patten, C. L., & Glick, B. R. (2002). Role of Pseudomonas putida indole acetic acid in development of the host plant root system. Applied and Environmental Microbiology, 68(8), 3795–3801.
Penrose, D. M., & Glick, B. R. (2001). Levels of ACC and related compounds in exudates and extracts of canola seeds treated with ACC-deaminase-containing plant growth promoting bacteria. Canadian Journal of Microbiology, 47, 368–372.
Perkins, L. B., Blank, R. R., Ferguson, S. C., et al. (2013). Quick start guide to soil methods for ecologists. Perspectives in Plant Ecology, Evolution and Systematics, 15, 237–244.
Putwattana, N., Kruatrachue, M., Pokethitiyook, P., et al. (2010). Immobilization of cadmium in soil by cow manure and silicate fertilizer, and reduced accumulation of cadmium in sweet basil (Ocimum basilicum). Science Asia, 36, 349–354.
Raval, A. A., & Desai, P. B. (2012). Rhizobacteria from rhizosphere of sunflower (Helianthus annuus L.) and their effect on plant growth. Research Journal of Recent Sciences, 1, 58–61.
Rogalla, H., & Romheld, V. (2002). Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumissativus L. Plant, Cell and Environment, 25, 549–555.
Saffari, M., Karimian, N., Ronaghi, A., et al. (2016). Stabilization of lead as affected by various amendments and incubation time in a calcareous soil. Archives of Agronomy and Soil Science, 62(3), 317–337.
Sanderson, P., Naidu, R., & Bolan, N. (2014). Ecotoxicity of chemically stabilized metal(loid)s in shooting range soils. Ecotoxicology and Environmental Safety, 100, 201–208.
Schalk, I. J., Hannauer, M., & Braud, A. (2011). New roles for bacterial siderophores in metal transport and tolerance. Environmental Microbiology, 13, 2844–2854.
Schwyn, B., & Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160, 47–56.
Shaheen, S. M., & Rinklebe, J. (2014). Geochemical fractions of chromium, copper, and zinc and their vertical distribution in soil profiles along the Central Elbe River, Germany. Geoderma, 228–229, 142–159.
Shi, X., Zhang, C., Wang, H., et al. (2005). Effect of Si on the distribution of Cd in rice seedlings. Plant and Soil, 272, 53–60.
Shim, J., Shea, P. J., & Oh, B. T. (2014). Stabilization of heavy metals in mining site soil with silica extracted from corn cob. Water, Air, and Soil pollution, 225, 2152.
Sparks, D. L. (1996). Methods of soil analysis part 3—Chemical methods. Madison, WI: Soil Science Society of America Inc., American Society of Agronomy Inc.
Syu, C. H., Huang, C. C., Jiang, P. Y., et al. (2016). Effects of foliar and soil application of sodium silicate on arsenic toxicity and accumulation in rice (Oryza sativa L.) seedling grown in As-contaminated paddy soils. Soil Science and Plant Nutrition, 62(4), 357–366.
Tessier, A., Campbell, P. G. C., & Blsson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 52, 45–53.
Weaver, R. W., Angle, S., Bottomley, P., et al. (1994). Methods of soil analysis: Part 2, microbiological and biochemical properties. Wisc: Soil Science Society of America Madison.
Wen, W. J., Yu, S., Yong-Xing, Z., et al. (2013). Mechanisms of enhanced heavy metal tolerance in plants by silicon: A review. Pedosphere, 23(6), 815–825.
Wu, S. C., Luo, Y. M., Cheung, K. C., et al. (2006). Influence of bacteria on Pb and Zn speciation, mobility and bioavailability in soil: A laboratory study. Environmental Pollution, 144, 765–773.
Wu, C., Yan, Sh, Zhang, H., et al. (2015). Chemical forms of cadmium in a calcareous soil treated with different levels of phosphorus-containing acidifying agents. Soil Research, 53, 105–111.
Xiu-Zhen, H., Dong-Mei, Z., Dan-Dan, L., et al. (2012). Growth, cadmium and zinc accumulation of ornamental sunflower (Helianthus annuus L.) in contaminated soil with different amendments. Pedosphere, 22(5), 631–639.
Xu, X., Huang, Q., Huang, Q., et al. (2012). Soil microbial augmentation by an EGFP-tagged Pseudomonas putida X4 to reduce phytoavailable cadmium. International Biodeterioration and Biodegradation, 71, 55–60.
Yao, A., Wang, Y., Ling, X., et al. (2017). Effects of an iron-silicon material, a synthetic zeolite and an alkaline clay on vegetable uptake of As and Cd from a polluted agricultural soil and proposed remediation mechanisms. Environmental Geochemistry and Health, 39(2), 353–367. doi:10.1007/s10653-016-9863-8.
Yasuda, H., Takama, K., Fukuda, T., et al. (1998). Effects of zeolite on water and salt control in soil. Bulletin of the Faculty of Agriculture, Tottori University, 51, 35–42.
Acknowledgements
This work was supported by the Iran National Science Foundation (Grant Number 96000777); and the study held under the auspices of the respected council; in this regard, the authors are truly grateful.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousavi, S.M., Motesharezadeh, B., Hosseini, H.M. et al. Geochemical fractions and phytoavailability of Zinc in a contaminated calcareous soil affected by biotic and abiotic amendments. Environ Geochem Health 40, 1221–1235 (2018). https://doi.org/10.1007/s10653-017-0038-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-0038-z