Abstract
Zinc (Zn) is an essential microelement involved in various plant physiological processes. However, in excess, Zn becomes toxic and represents serious problem for plants resulting in Zn toxicity symptoms and decreasing biomass production. The effect of high Zn and its combination with silicon (Si) on ionome and expression level of ZmLsi genes was investigated in maize (Zea mays, L; hybrid Novania). Plants were cultivated hydroponically in different treatments: control (C), Zn (800 μM ZnSO4 · 7H2O), Si5 (5 mM of sodium silicate solution), and Si5 + Zn (combination of Zn and Si treatments). Growth of plants cultivated for 10 days was significantly inhibited in the presence of high Zn concentration and also by Zn and Si interaction in plants. Based on principal component analysis (PCA) and mineral element concentration in tissues, root ionome was significantly altered in both Zn and Si5 + Zn treatments in comparison to control. Mineral elements Mn, Fe, Ca, P, Mg, Ni, Co, and K significantly decreased, and Se increased in Zn and Si5 + Zn treatments. Shoot ionome was less affected than root ionome. Concentration of shoot Cu, Mn, and P decreased, and Mo increased in Zn and Si5 + Zn treatments. The PCA also revealed that the responsibility for ionome changes is mainly due to Zn exposure and also, but less, by Si application to Zn stressed plants. Expression level of Lsi1 and Lsi2 genes for the Si influx and efflux transporters was downregulated in roots after Si supply and even more downregulated by Zinc alone and also by Zn and Si interaction. Expression level of shoot Lsi6 gene was differently regulated in the first and second leaf. These results indicate negative effect of high Zn alone and also in interaction with Si on Lsi gene expression level and together with ionomic data, it was shown that homeostatic network of mineral elements was disrupted and caused negative alterations in mineral nutrition of young maize plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc is an essential microelement involved in various physiological processes. However, Zn excess in soil represents a serious problem for plants resulting in Zn toxicity symptoms including decreased biomass production (Marschner 1995). Zinc toxicity occurring in crops is far less widespread than the Zn deficiency (Broadley et al. 2007). The critical values for Zn toxicity in leaves is more than 300 mg Zn kg−1 of dry weight; however, some crops show zinc toxicity symptoms at the level of 100 mg Zn kg−1 of leaf dry weight (Marschner 1995; Broadley et al. 2007). Zinc in excess can cause increased level of reactive oxygen species (ROS) and also the cofactor metal ions such as Mn or Fe may be replaced from their sites in many proteins by Zn ions, because of their similar chemical properties with Zn (Remans et al. 2012; Gutierrez-Carbonell et al. 2013).
Zinc excess can alter the ionome of plants. The ionome represents inorganic component and includes all the mineral nutrients and trace elements found in cellular and organismal system (Lahner et al. 2003; Salt et al. 2008). In the study of Wang et al. (2009), the authors revealed that Zn excess disturbed the nutrient balance and concentration of various root mineral elements decreased with increased Zn exposure. Zinc may decrease mineral element uptake and thus inhibit some metabolic processes. Inhibition of uptake and decreased translocation of nutrient elements may be a symptom of Zn toxicity (Wang et al. 2009). Mineral element interactions can affect absorption and bioavailability of other nutrients. Mineral elements with chemical similarities (in size and charge) can compete for the same transport proteins or other uptake mechanisms (Singh et al. 2013). Metal transport proteins are usually highly specific for a certain ion, but its specificity is not absolute (Singh et al. 2013). In sugar beet (Beta vulgaris L.), Zn toxicity also altered concentration of some mineral elements (Sagardoy et al. 2009). Also, other metals, e.g., Cu, can cause alteration in element profile of plants. In Silene paradoxa, Cu exposure strongly disturbed mineral element homeostasis in sensitive population; however, in tolerant metallicolous population, element homeostasis was not disturbed or just barely. The authors suggested that it was due to higher capacity to maintain proper root functioning under Cu excess in plants of metallicolous population (Pignattelli et al. 2013). The uptake, radial transport, translocation, and accumulation of zinc and/or other metal are controlled by checkpoints at specific sites of the plant body (Martinka et al. 2014). These checkpoints consist of cells present at the root surface, the root cortex cells, the transition between the vascular systems of root and shoot, connecting tissues, and cells at the nodes of the segmented stem. The terminal checkpoint presents stem-flower/fruit junction. The control processes of the checkpoints are due to the structural and functional properties of specialized cells and tissues (Martinka et al. 2014).
Except certain algae (diatoms) and Equisetaceae (horsetails or scouring rushes), silicon (Si) is not considered and listed among essential elements; however, its beneficial effect is obvious (Marschner 1995; Epstein 1999; Ma and Yamaji 2006). Plants take up Si in the form of soluble H4SiO4 or Si(OH)3O− depending on the pH of the soil environment (Epstein 1994; Ma and Yamaji 2006; Currie and Perry 2007). Uptake of Si is an active process in graminaceous plants, especially in rice, maize, or barley (Ma et al. 2006; Mitani et al. 2009a,b). Recently, various Si transporters have been recognized in rice (OsLsi1, OsLsi2, and OsLsi6), maize (ZmLsi1, ZmLsi2, and ZmLsi6), barley (HvLsi1 and HvLsi2), soybean (Si transporter genes GmNIP2-1 and GmNIP2-2), wheat (TaLsi1), and pumpkin (CmLsi1) (Ma et al. 2006; Ma et al. 2007; Yamaji et al. 2008; Mitani et al. 2008; Mitani et al. 2009a,b; Chiba et al. 2009; Mitani et al. 2011; Montpetit et al. 2012; Deshmukh et al. 2013). Silicon influx transporters Lsi1 and Lsi6 belong to the nodulin 26-like major intrinsic protein III (NIP III) subgroup of aquaporins (Ma et al. 2006; Mitani et al. 2008; Mitani et al. 2009a). On the contrary, Lsi2 is an active efflux Si transporter that is energetically driven by a proton gradient and does not belong to aquaporins (Mitani et al. 2009b; Zangi and Filella 2012).
Some role of Si in mitigating toxicity of Cd (Vaculík et al. 2012; Lukačová et al. 2013), Zn (Kaya et al. 2009; Song et al. 2011), and Mn (Horiguchi and Morita 1987; Shi et al. 2005) has been observed; however, there is also evidence of negative effect of Si on growth and abiotic stress of plants. Root biomass production of Sorghum bicolor was decreased by higher Zn supply, whereby this toxicity symptom was not alleviated by Si (Masarovič et al. 2012). The activity of some antioxidant enzymes was also negatively affected by the combination of Si and Zn (Masarovič et al. 2012). Negative effect of Si on Zn toxicity has also been observed in maize (Bokor et al. 2014). The next study of Schaller et al. (2012) revealed reduced biomass production at high Si concentration (100 g of amorphous Si per pot) in Phragmites australis. The authors suggested a stress response and imperfect regulation of Si uptake and transport under experimental conditions. The generalization of alleviation effect of Si on metal toxicity should be therefore made with caution (Bokor et al. 2014).
The study of changes in mineral nutrition of crops cultivated in contaminated soils is very important for human nutrition and food safety. Therefore, the object of this study was to examine the effect of Zn and Si interaction on ionome and thus mineral element homeostasis in roots and shoots of young maize plants, hybrid Novania. The concentration of Zn used in this paper was based on screening of various Zn concentrations including 100, 200, 400, and 600 μM ZnSO4 · 7H2O. The results showed that hybrid Novania is resistant to these Zn concentrations because the differences between Zn treatments and control were only minor; therefore, we have chosen 800 μM Zn. Also, several Si concentrations were screened in combination with 800 μM Zn (Bokor et al. 2014). Silicon is a beneficial element for maize and improves yield production; thus, the expression levels of three genes for Si transporters ZmLsi1, ZmLsi2, and ZmLsi6 in maize treated by Zn alone or in the combination with Si were investigated to evaluate the possible correlation of Si uptake and accumulation in Zn-stressed plants. The study of expression level of genes for Si transporters is also crucial to overall understanding of ionome alteration under Zn toxicity in combination with Si application.
Material and methods
Plant material and growth conditions
Maize (Zea mays, hybrid Novania) corns were soaked in distilled water for 2–3 h and then germinated in rolls of wet filter paper for 3 days at 25 °C and then transferred to 3-l glass pots with Hoagland solution (Hoagland and Arnon 1950). Each pot contained 12–15 plants. Maize plants were grown for 10 days in a controlled environment chamber under a 12-h light/12-h dark regime, with a PAR (light intensity) of 200 μmol m−2 s−1, relative humidity of approximately 75 %, and day/night temperatures of 24/20 °C. Plants were grown in four different treatments—control (Hoagland solution), Si5 (Hoagland solution with 5 mM Si = 27 % SiO2 dissolved in 14 % NaOH/sodium silicate solution, Sigma/), Zn (Hoagland solution with 800 μM ZnSO4 · 7H2O), and combined Si5 + Zn (Hoagland solution with 5 mM Si + 800 μM ZnSO4 · 7H2O). The pH of each nutrient solution was adjusted to 6.2 using HCl. Solutions were aerated and changed every 3 days. Phreeqc Interactive software (version 2.18.3.5570) designed for aqueous geochemical modeling was used for detection of possible salt precipitations.
Root morphology measurements
Root architecture/morphology—total root surface area (TRS), total root length (TRL), root volume (RV), and number of root tips (RT)—was performed by scanning of fresh roots by Epson Perfection V700 Photo scanner. Roots were scanned in transparent box filled with distilled water and covered by a transparent plastic table to make the roots straight. After scanning, the roots were processed by WinRHIZO® Pro system.
Concentration of mineral elements in plant organs
Zinc and Si concentrations of root and shoot dry biomass were detected by atomic absorption spectrometry (AAS) in the Institute of Laboratory Research on Geomaterials, Faculty of Natural Sciences, Comenius University in Bratislava. Plant samples were dried at room temperature and grinded to small pieces (ca 1 mm). Digestions of plant tissues were carried out in the stainless steel-coated PTFE pressure vessels ZA-1 (Czech Republic). Plant sample of 0.1–0.5 g was weighted to the vessel and 5.0 ml of concentrated HNO3 was added for Zn detection or 5 ml of concentrated HNO3, 0.25 ml of concentrated HF, and 2 ml of 30 % H2O2 were added for Si detection. Vessels were closed and heated in the electric oven at 160 °C for 6 h. After digestion, the solution was diluted to 25 ml with redistilled water (for Zn detection) or 2 ml of saturated solution of H3BO3 was added and then the solution was diluted to 25 ml with redistilled water (for Si detection) and stored in a 100-ml polyethylene bottle. Zinc concentrations were determined by flame atomic absorption spectrometry (AAS Perkin Elmer Model 4100, wavelength: 213.9 nm, deuterium background correction, flame: acetylene-air). Silicon concentrations were determined by flame atomic absorption spectrometry (AAS Perkin Elmer Model 5000, wavelength: 251.6 nm, flame: acetylene-N2O). The rest of the other elements (Mo, Cu, Ni, Co, Mn, Fe, Ca, P, Mg, B, Na, K, S, Se) were determined by inductively coupled plasma mass spectrometry (ICP-MS) in the Geoanalytical laboratories ACME, Vancouver, Canada.
RNA extraction and real-time PCR
All the experiments from RNA extraction to real-time PCR were performed by available commercial kits according to manufacturer’s instructions. Total RNA amount was extracted from frozen root segments (the segments were 4 cm long, cut at the distance of 1 cm from apex) and from the first and the second leaf segments (1 cm from the base of the leaf blade) using Spectrum plant total RNA kit (Sigma-Aldrich). Thereafter, 1 μg of RNA of each sample was treated by DNase I (Fermentas), cleaned and concentrated by RNA Clean & Concentrator™-5 columns (Zymo research, Exbio). The concentration of total RNA was measured by Qubit® 2.0 Fluorometer (Invitrogen). Total RNA was converted to cDNA by ImProm-II Reverse Transcription System (Promega) using Oligo(dT)15 primers. Afterwards, the expression level of observed genes was performed by real-time PCR method. Specific cDNA of the samples were amplified by Maxima SYBR Green/ROX qPCR Master Mix (Fermentas) and real-time PCR (ABI Fast 7900 HT; Applied Biosystems), with the following primer sets (Lambda life).
β-Actin 5′–3 | CACAGGTATCGTGCTCGACTC | Forward |
CAGGTCAAGACGAAGGATGG | Reverse | |
Lsi1 5′–3 | GATCCAGGTCCCGTTCTACTG | Forward |
GACGAGCGAGTGCCAGTG | Reverse | |
Lsi2 5′–3 | ACGTGCCAACAGGTGCTTCTTATG | Forward |
TACGATCGAGGCATACAATTATG | Reverse | |
Lsi6 5′–3′ | TTCAGGTGCCCTTCTACTGG | Forward |
ACGACGATCTCGATGAGGAG | Reverse |
Housekeeping gene β-actin (GRMZM2G097426) was used as a reference control (internal control of expression level) similarly to Mitani et al. (2009a, b) and also Kim et al. 2014. Primers for Lsi1 (NIP2-1; GRMZM2G028325), Lsi6 (NIP2-2; GRMZM2G137108), and β-actin genes were designed by Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) from gene sequences available in plant genome database Ensembl Plants (http://plants.ensembl.org/index.html). Primers for the Lsi2 gene were used according to Mitani et al. (2009b). Gradient PCR was used to determine optimal primer annealing temperature (β-actin, Lsi1, Lsi6 = 61 °C, and Lsi2 = 62 °C). Specificity of PCR products were verified by Sanger sequencing at the Department of Molecular Biology (Faculty of Natural Sciences, Comenius University in Bratislava) using several commercial kits (ExoSAP-IT®-USB, Applied Biosystems and BigDye® Terminator v3.1 Cycle Sequencing Kit-Applied Biosystems) according to manufacturer’s instructions. Mastercycler Pro (Eppendorf) was used for sequencing PCR reaction and genetic analyzer ABI3500 (Applied Biosystems) for electrophoretic separation of sequencing PCR products. Afterwards, sequences were analyzed by Sequencing Analysis software (version 5.4, Applied Biosystems) and verified in Blast tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis
Statgraphics Centurion (version 15.2.05) software was used for statistical analysis of experimental data. Statistical significance of the means was compared at 0.05 probability level. Analysis of variance (ANOVA) and least significant difference (LSD) was performed on all experimental data sets. Principal component analysis (PCA) was applied to the ionomics study. Microsoft Excel 2007 and R version 3.0.2 software were used for PCA analysis.
Mineral element behavior in the different samples was analyzed by PCA. This method transformed the original set of potentially correlated variables into a set of uncorrelated principal components, basically the linear combinations of original variables accounting for the same variance without redundancy of information. Internal structure of the biological system shaped by unknown driving forces manifests itself through individual element concentrations. If only partially varying in groups, true internal structure will remain hidden. Construction of the principal components changes perspective by manipulation of axes defining variable space in 16 dimensions, in which original data are stored. First component is an axis in this space, along which the system varies at maximum. Second component is selection from all possible orthogonal axes with the first one. Continuation until the 16th one brings the variance to exhaustion and data seem relocated within a new, rotated set of dimensions. We focus on description of the system in the first three PCs, forming a 3D space. Before proceeding to PCA data were rescaled via standardization to Z scores to avoid scale bias.
Results
Root morphology
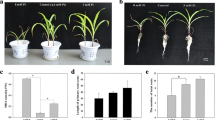
Negative effect of individually applied Zn or Zn in combination with Si in plants was evident in root morphology parameters at the end of the cultivation. The total root length (TRL) decreased more than 50 % in Zn and also Si5 + Zn treatment, when compared with control roots (Fig. 1a). Roots treated with Si (Si5 treatment) showed also decreased TRL (ca 15 %) in comparison to control treatment, but the difference was not significant.
Total root length (TRL) (a) and total root surface area (TRS) (b) of maize plants cultivated in four different treatments: control (C), Si5 (5 mM Si), Zn (800 μM ZnSO4 · 7H2O), and Si5 + Zn (combination of Zn and Si treatment). Values are means SE (n = 6), experiment was repeated three times. Different letters indicate significant differences between the treatments at 0.05 level
The total root surface area (TRS) also deceased in the presence of high Zn ca 40 %, when compared with control roots (Fig. 1b). The difference between Si5 and control was not significant in this parameter. The positive effect of Si was not shown in combined (Si5 + Zn) treatment; the decrease was similar to the Zn treatment.
The root volume (RV) of Zn treatment significantly decreased by 24.5 % (Fig. 2a) in comparison to control roots, and the addition of Si to Zn (Si5 + Zn treatment) was not positive, thus the decrease of root volume was similar (26.5 %) to the Zn treatment. Roots of Si5 treatment had lower RV (ca 15.5 %) in comparison to control, but the difference was not significant.
Root volume (RV) (a) and number of root tips (RT) (b) of maize plants cultivated in four different treatments: control (C), Si5 (5 mM Si), Zn (800 μM ZnSO4 · 7H2O), and Si5 + Zn (combination of Zn and Si treatment). Values are means SE (n = 6), experiment was repeated three times. Different letters indicate significant differences between the treatments at 0.05 level
The number of root tips (RT) dramatically changed due to high Zn in cultivation media (Fig. 2b). The number of tips was decreased more than 60 % in Zn treatment and Si5 + Zn treatment in comparison to control. Individually applied Si (Si5 treatment) caused insignificant decrease (ca 7 %) of RT parameter.
Root and shoot mineral element concentration of maize plants
Concentrations of many mineral elements in roots were affected to a large extent by Zn addition to cultivation media (Table 1). Concentration of mineral elements Mn, Fe, Ca, P, Mg, Ni, Co, and K significantly decreased in roots of Zn treatment, when compared with the concentration of these elements in control roots. Selenium (Se) is the only mineral element with increased concentration in roots of Zn treatment in comparison to control.
Similarly, Si interaction with Zn (Si5 + Zn treatment) negatively affected root concentration of Mn, Fe, Ca, P, Mg, Ni, Co, and K, i.e., it significantly decreased in comparison to control (Table 1). Concentration of Fe decreased significantly in roots of Si5 + Zn treatment in comparison to Zn treatment, but the concentration of other elements was statistically the same with Zn treatment.
Silicon present in Si5 treatment negatively affected concentration of only four elements in roots: Fe, Ca, P, and K which significantly decreased in comparison to control roots (Table 1). Root Mg concentration was significantly increased, and the other elements were not significantly influenced.
Interestingly, concentrations of root Mo, Cu, and B were not significantly changed in all tested treatments in comparison to control root (Table 1).
Shoot mineral element concentrations were not as much affected as in roots in the presence of high Zn concentration. Only concentrations of Cu, Mn, and P decreased in high Zn condition (Zn treatment), when compared to control shoots. On the contrary, Mo and Mg concentrations significantly increased in the shoot of Zn treatment (Table 2). Unaffected concentrations of Fe, Ca, Ni, Co, B, Se, and K were found out in Zn treatment in comparison to control.
In case of Si5 + Zn treatment, also Cu, Mn, P, and moreover K concentration of shoots was decreased in comparison to control (Table 2). Concentrations of Mo and Se significantly increased in Si5 + Zn treatment, and Mg concentration was statistically the same as in control treatment. Similarly to Zn treatment, concentrations of Fe, Ca, Ni, Co, and B were unaffected by Zn and Si interaction (Si5 + Zn treatment) in comparison to control. When Si5 + Zn and Zn treatment were compared, then concentrations of Cu and P increased and concentrations of Fe, Ca, and Mg decreased in Si5 + Zn treatment.
The shoot ionome of Si treatment was the least affected in comparison to Zn or Si5 + Zn treatment (Table 2). Increased concentration was evident only in case of P, and decreased concentration was detected in Mg, when compared with control shoots. The concentration of the other mineral elements was statistically the same as in control shoots.
PCA analysis of the ionome
The PCA was used to determine which parameter (root, shoot, or treatment) was mostly influenced by the variation of mineral composition of plants. All values of mineral element concentrations (ppm) were set as variables and subjected to a PCA method. The analysis revealed three main significant components, namely PC1, PC2, and PC3. The PCA graphs (Fig. 3a, b) capture relationships among the elements. Positive correlation is represented as an acute angle between the eigenvectors, and negative correlation is shown by an obtuse angle. Uncorrelated elements form a 90 °C angle of the eigenvectors. The first principal component PC1 explained 52 % of the total variance. Only P, K, and B positively correlated with this axis (Fig. 3a, b). The acute angle between the eigenvectors indicates strongly correlated elements. All other elements negatively correlated with the PC1 axis. The second principal component PC2 explains 23 % of the total variance. Elements Se and Zn had positive correlation with PC2 and exhibited strong correlation to each other (Fig. 3a). Also, Si, Na, S, and Cu positively correlated with PC2; however, the correlation was weak. The third principal component PC3 explains 11.5 % of the total variance. Positive correlation was involved in Na and Si (Fig. 3b). The other elements Mg, Mn, Co, and B were closely to 0 point of PC3 axis indicating positive but weak correlation.
Biplot graphs resulted from the principal component analysis (PCA) shows the correlation of measured variables (concentration of elements) to principal components PC1, PC2, and PC3. The length of arrows (eigenvectors) represents the strength of correlation with the PC. The angle between eigenvectors represents the correlation among various elements. Biplot of PC1/PC2—a; biplot of PC1/PC3—b
The responsibility of the organ (root or shoot) and treatment (Zn vs. Si) for changes in the ionome was also investigated by PCA. The scores scatter plots of PC1/PC2 and PC1/PC3 show that PC1 has positive correlation with the element data of shoots (all treatments) and negative correlation with the elements of the roots (of all treatments) (Fig. 4a, b). This indicates that difference between the shoot ionome and the root ionome is one of crucial importance in total set variation. The elements of the shoot irrespective of treatment are positively correlated with PC1, unlike to elements of roots (Fig. 4a, b). The PCA also show which treatment is more responsible for changes in the ionome of the roots—whether Zn or Si. The PC2 (Fig. 4a) shows positive correlation of Zn and Si5 + Zn treatment to the vertical axis in comparison to control and Si5 treatment which are negatively correlated. The correlation with PC2 indicates responsibility of Zn for the change of the ionome in the roots of Zn and Si5 + Zn treatments. The last scores scatter plot PC1/PC3 reveals that PC3 axis is positively correlated with Si5 treatment and Si5 + Zn treatment (Fig. 4b). This result explains responsibility of Si on the altered root ionome.
The principal component analysis (PCA) of the root and shoot element distribution data of all treatments. Biplots illustrate the correlation of measured variables (element concentration of roots and shoots) to principal components PC1/PC2—a, PC1/PC3—b. Squares represent root treatments: C—black, Si5—blue, Zn—red, and Si5 + Zn—purple color. Circles represent shoot treatments: C—black, Si5—blue, Zn—red, and Si5 + Zn—purple color
Silicon content and concentration
Concentration of root Si was statistically the same in Si5 and Si5 + Zn treatment (Bokor et al. 2014); however, the content of Si was significantly higher in Si5 treatment, when compared with Si5 + Zn treatment (Table 3). The same concentration, but increased Si content, is due to higher dry weight in Si5 treatment in comparison to Si5 + Zn treatment. Silicon content and also concentration did not significantly differ between control and Zn treatment. In the first leaf, concentration and also content of Si was significantly higher in Si5 treatment, when compared to Si5 + Zn treatment (Table 3). Silicon accumulation of the first leaf was significantly higher in control, when compared with Zn treatment. In the second leaf, Si accumulation significantly increased in Si5 treatment in comparison to Si5 + Zn treatment (Table 3). Also, Si content and concentration significantly increased in control, when compared with Zn treatment.
These data show that in roots, Zn application has no impact on Si concentration; however, in shoots, Zn exposure decreases Si concentration.
Expression level of ZmLsi genes
Expression level of ZmLsi1 (NIP2-1), ZmLsi6 (NIP2-2), and ZmLsi2 genes were determined in tissues of young maize plants. The expression level of ZmLsi1 and ZmLsi2 was determined in seminal roots and ZmLsi6 in the blades of the first and the second leaf.
For ZmLsi1 and ZmLsi2 genes, expression level was downregulated (3.7- and 3.5-fold, respectively) by continuous Si supplement (Si5 treatment) in cultivation media (Fig. 5a, b). Zinc addition to Si5 treatment (Si5 + Zn treatment) affected much more the expression level of ZmLsi1 gene, i.e., 6.7-fold decrease in comparison to Si5 and 25-fold decrease in comparison to control treatment. Similar pattern was observed in a case of expression level of ZmLsi2 gene. In Si5 + Zn treatment, ZmLsi2 expression level was decreased 25.6-fold in comparison to Si5 and 90.9-fold decrease in comparison to control treatment. Significant downregulation of ZmLsi1 and ZmLsi2 gene expression level was also observed in the case of Zn treatment, i.e., 28.6- and 8.5-fold, respectively, decrease in comparison to control treatment. The difference was not significant between Zn and Si5 + Zn treatment in case of ZmLsi1 expression level. Expression level of ZmLsi2 decreased 10.7-fold in Si5 + Zn, when compared to Zn treatment.
Expression level of ZmLsi1 (a) and ZmLsi2 (b) genes determined by relative real-time PCR in primary seminal roots. Treatments: control (C), Si5 (5 mM Si), Zn (800 μM ZnSO4 · 7H2O), and Si5 + Zn (combination of Zn and Si treatment). Values are means SE (n = 3). Different letters indicate significant differences between the treatments at 0.05 level
The expression of ZmLsi6 in the first leaf was not affected by Si addition in Si5 treatment, when compared with the control (Fig. 6a). The ZmLsi6 was significantly upregulated in Zn, i.e., 1.3-fold change and Si5 + Zn, i.e., 2.3-fold change, when compared with the control. In case of Si5 + Zn treatment, expression level increased 1.8-fold in comparison to Zn treatment.
Expression level of ZmLsi6 gene in the first leaf (a) and the second leaf (b) determined by relative real-time PCR. Treatments: control (C), Si5 (5 mM Si), Zn (800 μM ZnSO4 · 7H2O), and Si5 + Zn (combination of Zn and Si treatment). Values are means SE (n = 3). Different letters indicate significant differences between the treatments at 0.05 level
Expression level of ZmLsi6 gene in the second leaf was significantly upregulated in plants treated by Si (Si5 and Si5 + Zn treatment), i.e., 1.6- and 1.2-fold change in comparison to control (Fig. 6b). High Zn condition (Zn treatment) caused downregulation of ZmLsi6 expression level, i.e., 1.3-fold in comparison to control treatment. In Si5 + Zn treatment, ZmLsi6 expression increased 1.5-fold in comparison to Zn treatment.
Discussion
PCA analysis of the ionome
The PCA revealed three major factors affecting the ionome of maize plants—difference between root and shoot, treatment by Zn, and treatment by Si. The scatter plots showed that the values of roots of all treatments were discriminated and negatively correlated with PC1 and on the contrary, values of shoots of all treatments were overlapping and positively correlated with PC1. The overlapping of shoot values of all treatments shows that these values do not much differ relative to root values. This suggests that root ionome was affected more strongly than shoot ionome of maize plants. Also, PC1 explains the most of total variance; therefore, the difference between root and shoot is the most important influence considered. The other factor which affects ionome is captured by PC2. PC2 showed that Zn is responsible for ionome change mainly in root and less in shoot, because Zn and Si5 + Zn treatments positively correlated with PC2 axis. The third factor that participates on altered ionome is Si. This is explained by PC3, where both Si treatments (Si and Si5 + Zn) positively correlated with vertical PC3 axis. However, ionome of Si treatment in the root samples is affected more strongly in comparison to the shoot samples.
Results of recently published papers also revealed that high metal concentration affected element profiles, distribution, and correlation among elements (Chen et al. 2009; Vaculík et al. 2012; Pignattelli et al. 2013).
Regardless of organ (root or shoot), some elements can be clustered into several positively correlated groups revealed by the PCA method. The first group represents P, K, and B. Concentration of P and K decreased in roots and shoots of Zn and Si5 + Zn treatments, and B concentration was unaffected in any treatment. In the work of Pignattelli et al. (2013), correlation between P and K was found out in Cu tolerant plants, when compared with Cu sensitive population exposed to excess of Cu. Interaction of P and K is a part of cation-anion balance in plants and positive strong P and K correlation was found out in Lolium perenne (Dibb and Thompson 1985; Sárdi et al. 2012). Transport of B in plants is based on passive diffusion and also protein channels such as Si transporters (Lsi1) (Hove and Bhave 2011). The correlation of B with K and P has not been observed yet and is not easy to explain. The second group contains Se and Zn. In high Zn treatments (Zn and Si5 + Zn) with increasing of Zn, also Se is increased in roots and shoots, as well. This positive correlation cannot be explained by similarities in transport system, because Zn is transported by different transporters than Se. Selenium share chemical similarities with sulfur (S), and Se transport and assimilation follows the S pathway (Sors et al. 2005; Cabannes et al. 2011). Sulfur starvation increases Se accumulation and external sulfate inhibits Se accumulation, which may be due to competition of S and Se (Cabannes et al. 2011). In our case, Hoagland solution contains sufficient concentration of S for plants in each treatment, thus the S deficiency could not occur.
The PCA confirmed that the ionome was altered in plants of Zn and Si5 + Zn treatment. Taking plant organs into consideration, root ionome was more affected than the shoot ionome. Main factor responsible for root ionome changes is Zn and also, but less, Si. The PCA also revealed interesting positive correlations that occurred between some elements.
Root and shoot mineral element concentration of maize plants
The changes in root ionome were prominent upon high Zn effect and also Zn/Si interaction in plants. Decreased concentrations of P, Fe, Mg, Cu, and Mn in roots of Brassica napus seedlings were also observed in experiments with Zn excess (Wang et al. 2009). However, Cu concentration was not affected by Zn and/or Si5 + Zn in our work similarly to Sagardoy et al. (2009). We suggest that Cu uptake is not sensitive to Zn or Zn/Si stress in maize roots. Generally, Zn toxicity can cause reduced Fe uptake and shoot accumulation, which indicates competition between Zn and Fe represented by Fe transporters IRT1 and IRT2 that transport also Zn (Shanmugam et al. 2013). Hence, excess of Zn can be taken up, although it causes Fe deficiency (Shanmugam et al. 2011). In addition, transporters of divalent metal cations often exhibit broad substrate specificity. Some of recently investigated Zn transporters transport also other metals (for example ZIP transporters may transport also Fe, Mn, and Cu), thus Zn toxicity conditions may result in Cu, Fe, Mg, and other metal/nutrient deficiency in plants (Puig and Peñarrubia 2009; Sinclair and Krämer 2012; Milner et al. 2013). Similarly to our results, decreased root concentration of K and P was observed at different Zn supply levels (Päivöke 2003; Sagardoy et al. 2009). Zinc together with P can bind and form Zn-P complexes to protect the plant by decreasing bioavailability of high Zn concentration in maize plants (Jiang et al. 2007). Thus, decreased concentration of P induced by excess of Zn might enhance Zn toxicity in plants of Zn or Si5 + Zn treatments.
Shoot ionome was less affected by Zn toxicity and also by Zn/Si interaction in plants than the root ionome. In shoots of Zn and Si5 + Zn treatments, concentration of Cu, Mn, and P significantly decreased and Mo significantly increased in both treatments, when compared with control treatment. Concentration of Cu, Mn, and P was decreased in shoots of B. napus seedlings grown at high Zn (Wang et al. 2009). Here and also in our study, Cu accumulation was sensitive to high Zn concentration in shoots when compared to roots of Zn treatment. Considered together, high Zn concentration and also high Zn in combination with Si negatively affected uptake and translocation of many mineral elements and this may result in additional stress and also inhibition of some metabolic processes.
Taken together, Zn and also Zn and Si application in hydroponic media caused decrease in concentration of many elements in young maize plants. In case of Zn and Si5 + Zn roots, the decrease in mineral element concentration was much higher than in shoots of these treatments.
Expression level of ZmLsi genes
Gene expression of ZmLsi1 and ZmLsi2 was downregulated both by Zn and Si supply in the roots, while in the shoots, Lsi6 expression level showed different pattern (Figs. 5 and 6).
Correlation of Lsi1, Lsi2, and Lsi6 expression level can be also supported by Si content in roots and leaves. Increased levels of ZmLsi1 and ZmLsi2 genes correlated with higher Si content in Si5 treatment, when compared with Si5 + Zn treatment. However, expression level of Lsi1 gene was higher in control roots in comparison to other treatments. This may suggest higher demand of Si for plants, because Si was not added to the solution for control treatment. Silicon content and concentration of the first leaf is higher in Si5 treatment, when compared with Si5 + Zn treatment. This does not correlate with expression level of ZmLsi6, which was upregulated in Si5 + Zn treatment, when compared to Si5 treatment. We suggest that uncorrelated Si content with expression of ZmLsi6 in the first leaf is due to decreased Si content and expression of ZmLsi1 and ZmLsi2 in roots in Zn and Si5 + Zn treatments, thus there is higher demand for Si in the first leaf and this may lead to increased expression of ZmLsi6. In the second leaf, Si concentration and content was significantly higher in the Si5 treatment, when compared with Si5 + Zn treatment and this is well correlated with the upregulated expression level of ZmLsi6 in Si5 treatment. The recently revealed work of Yue et al. (2012) deals with the expression of NIP2–2 that is differently expressed in various parts of maize leaf.
Expression level of ZmLsi1 gene was not affected by continuous Si supply in comparison to control conditions in maize plants in Mitani et al. (2009a). In rice, expression level of OsLsi1 was decreased by Si supplement (Ma et al. 2006), which is similar to our results; however, Kim et al. (2014) found out increased expression of OsLsi1 in rice cultivated with Si. In barley, weak correlation between Si uptake and expression of HvLsi1 was observed, because HvLsi1 differs among the barley cultivars (Chiba et al. 2009). Thus, inter-cultivar/hybrid differences may result in different response of Lsi1 gene expression level. Effect of Si/metal interaction on expression level of Lsi1 is lacking to date; only Kim et al. (2014) recently revealed decreased OsLsi1 expression level in Cd-, Cd + Si-, Cu-, and Cu + Si-treated rice plants in comparison to control or Si treatment at the 10th day of cultivation. The possible mechanisms by which the expression levels of Si transporters may be affected by metals remain to be elucidate in the future; however, some analyses showed correlation between ZmNIPs and associated nutrient transporters of metals in maize (Yue et al. 2012).
The expression of maize, barley, and rice Lsi2 was downregulated in response to the Si supply (Mitani et al. 2009b; Ma et al. 2007). These results correspond with our experimental data. In case of metal effect, the expression level of OsLsi2 was downregulated and after Si exposure to metal treated plants, downregulated expression was also observed in comparison to Si or control treatment at the 10th day (Kim et al. 2014).
Mitani et al. (2009a) observed that Lsi6 expression level is not affected by Si supply in maize leaf blades. It is in agreement with our results, however, only in the first leaf of Si-treated plants. Again, inter-cultivar/hybrid differences may play a role in different results. In rice, Si supplement caused decreased expression level of OsLsi6 in leaf blades, however, not in leaf sheaths (Yamaji et al. 2008). To date, comparison of expression level of Lsi6 gene in heavy metal-treated plants are missing, thus discussion and comparisons with other authors are not possible.
Maize plants treated by Zn and also by Zn and Si showed downregulation of ZmLsi1 and ZmLsi2 genes in roots. This led to decreased Si content in roots and decreased translocation of Si into shoots. Therefore, we suggest that the upregulated expression level of ZmLsi6 in the first leaf is due to lack of Si in shoots and higher demand for Si in shoots. Expression of ZmLsi6 was differently regulated in the second leaf and decreased expression occurred in Zn and Si5 + Zn treatment.
Root morphology
High Zn concentration affected negatively various parameters of root morphology. The TRS, TRL, RV, and RT parameters significantly decreased in the presence of Zn in an excess. Similarly, other metals (Pb) also affect negatively various root morphology parameters in B. napus (Tian et al. 2014). The TRL, TRS, and RT parameters were also significantly reduced in high Zn conditions in the case of Zn-sensitive and also Zn-resistant cultivar of rice (Song et al. 2011). The authors noticed that the addition of Si (1.5 mM) to Zn treatment significantly increased these root morphology parameters regardless of cultivars used, compared with the corresponding Zn treatment alone (2 mM of Zn). In our study, application of 5 mM Si to Zn treatment (800 μM) did not affect positively root morphology parameters, thus there was no significant difference between Zn and Si5 + Zn treatments. This may be related with hybrid or cultivar specificity, and it is worthwhile mentioning here that hybrid Novania used in our experiments has low sensitivity to Zn excess (up to 600 μM of Zn). However, Zn concentration of 800 μM resulted in reduced biomass production (Bokor et al. 2014).
Conclusion
In conclusion, Zn alone or in interaction with Si caused inhibition of root growth and great imbalance of maize (Z. mays L., hybrid Novania) ionome. In roots, concentration of many elements decreased and thus the element homeostasis network disrupted what can lead to many metabolic alterations. The shoot ionome was less influenced by Zn effect or Zn and Si interaction than the root ionome. Root is directly in contact with soil environment (or hydroponic media) and it is involved in element uptake, accumulation, and translocation and therefore the root is more affected by negative effect of Zn excess than the shoot. Importance of Si in maize mineral nutrition is also supported by existence of Si transporters ZmLsi1, ZmLsi2, and ZmLsi6. Expression level of these transporter genes in roots (ZmLsi1, ZmLsi2) is negatively affected (downregulated) by high Zn concentration in media and also Zn and Si interaction in plants. This affects accumulation of Si in roots. In shoots, expression level of ZmLsi6 gene is differently regulated by Zn and also Zn and Si interaction in the first and second leaf. The PCA method revealed interesting correlations between P, B, and K, and between Se and Zn. It remains for future study why this correlation occurred between these elements and also study expression level of other transporter genes. This will help to better understand the changes in the ionome of maize as important crop plant in central Europe grown in various soils contaminated by heavy metals. Understanding of plant ionome of crops affected by excess of heavy metals is important for human nutrition and food safety.
Abbreviations
- PCA:
-
Principal component analysis
- RT:
-
Number of root tips
- RV:
-
Root volume
- TRL:
-
Total root length
- TRS:
-
Total root surface area
- ZmLsi :
-
Zea mays Si transporter gene
References
Bokor B, Vaculík M, Slováková Ľ, Masarovič D, Lux A (2014) Silicon does not always mitigate zinc toxicity in maize. Acta Physiol Plant 36:733–743
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cabannes E, Buchner P, Broadley MR, Hawkesford MJ (2011) A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol 157:2227–2239
Chiba Y, Mitani N, Yamaji N, Ma JF (2009) HvLsi1 is a silicon influx transporter in barley. Plant J 57:810–818
Chen Z, Shinano T, Ezawa T, Wasaki J, Kimura K, Osaki M, Zhu Y (2009) Elemental interconnections in Lotus japonicus: a systematic study of the effects of elements additions on different natural variants. Soil Sci Plant Nutr 55:91–101
Currie HA, Perry CC (2007) Silica in plants: biological, biochemical and chemical studies. Ann Bot 100:1383–1389
Dibb DW, Thompson WR (1985) Interaction of potassium with other nutrients. In: Munson RD (ed) Potassium in agriculture. Madison, Wisconsin, USA, pp 515–533
Deshmukh RK, Vivancos J, Guérin V, Sonah H, Labbé C, Belzile F, Bélanger RR (2013) Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol Biol 83:303–315
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci U S A 91:11–17
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Gutierrez-Carbonell E, Lattanzio G, Sagardoy R, Rodríguez-Celma J, Ruiz JJR, Matros A, Abadía A, Abadía J, López-Millán A-F (2013) Changes induced by zinc toxicity in the 2-DE protein profile of sugar beet roots. J Proteomics 94:149–161
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Soil Circ Univ Calif Agric Exp Station, Berkley, p 347
Horiguchi T, Morita S (1987) Mechanism of manganese toxicity and tolerance of plants. VI Effect of silicon on alleviation of manganese toxicity of barley J Plant Nutr 10:2299–2310
Hove RM, Bhave M (2011) Plant aquaporins with non-aqua functions: deciphering the signature sequences. Plant Mol Biol 75:413–430
Jiang HM, Yang JC, Zhang JF (2007) Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut 147:750–756
Kaya C, Tuna AL, Sonmez O, Ince F, Higgs D (2009) Mitigation effects of silicon on maize plants grown at high zinc. J Plant Nutr 32:1788–1798
Kim Y-H, Khan AL, Kim D-H, Lee S-Y, Kim K-M, Waqas M, Jung H-Y, Shin J-H, Kim J-G, Lee I-J (2014) Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Bio 14:1–13
Lahner B, Gong J, Mahmoudian M, Smith EL, Abid KB, Rogers EE, Guerinot ML, Harper JF, Ward JM, Mcintyre L, Schroeder JI, Salt DE (2003) Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotechno 21:1215–1221
Lukačová Z, Švubová R, Kohanová J, Lux A (2013) Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul 70:89–103
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448:209–211
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press Ltd., London, San Diego
Martinka M, Vaculík M, Lux A (2014) Plant cell responses to cadmium and zinc. In: Nick P, Opatrný Z (eds) Applied plant cell biology. Cellular Tools and Approaches for Plant Biotechnology. Heidelberg, New York, Dordrecht, London, Springer, pp 209–246
Masarovič D, Slováková Ľ, Bokor B, Bujdoš M, Lux A (2012) Effect of silicon application on Sorghum bicolor exposed to toxic concentration of zinc. Biologia 67:706–712
Milner MJ, Seamon J, Craft E, Kochian LV (2013) Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J Exp Bot 64:369–381
Mitani N, Yamaji N, Ma JF (2008) Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch 456:679–686
Mitani N, Yamaji N, Ago Y, Iwasaki K, Ma JF (2011) Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J 66:231–240
Mitani N, Yamaji N, Ma JF (2009a) Identification of maize silicon influx transporters. Plant Cell Physiol 50:5–12
Mitani N, Chiba Y, Yamaji N, Ma JF (2009b) Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 21:2133–2142
Montpetit J, Vivancos J, Mitani-Ueno N, Yamaji N, Rémus-Borel W, Belzile F, Ma JF, Bélanger RR (2012) Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol Biol 79:35–46
Päivöke AEA (2003) Mineral elements and phytase activity in Pisum sativum grown at different Zn supply levels in the greenhouse. Environ Exp Bot 49:285–294
Pignattelli S, Colzi I, Buccianti A, Cattani I, Beone GM, Schat H, Gonnelli G (2013) A multielement analysis of Cu induced changes in the mineral profiles of Cu sensitive and tolerant populations of Silene paradoxa L. Environ Exp Bot 96:20–27
Puig S, Peñarrubia L (2009) Placing metal micronutrients in context: transport and distribution in plants. Curr Opin Plant Biol 12:299–306
Remans T, Opdenakker K, Guisez Y, Carleer R, Schat H, Vangronsveld J, Cuypers A (2012) Exposure of Arabidopsis thaliana to excess Zn reveals a Zn-specific oxidative stress signature. Environ Exp Bot 84:61–71
Sagardoy R, Morales F, López-Millán AF, Abadía A, Abadía J (2009) Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biol 11:339–350
Salt DE, Baxter I, Lahner B (2008) Ionomics and the study of the plant ionome. Annu Rev Plant Biol 59:709–733
Sárdi K, Balázsy Á, Salamon B (2012) Interrelations in phosphorus and potassium accumulation characteristics of plants grown in different soil types. Commun Soil Sci Plant Anal 43:324–333
Sinclair SA, Krämer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta 1823:1553–1567
Singh UM, Sareen P, Sengar RS, Kumar A (2013) Plant ionomics: a newer approach to study mineral transport and its regulation. Acta Physiol Plant 35:2641–2653
Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Pickering IJ, Salt DE (2005) Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J 42:785–797
Schaller J, Brackhage C, Gessner MO, Bäuker E, Dudel EG (2012) Silicon supply modifies C:N:P stoichiometry and growth of Phragmites australis. Plant Biol 14:392–396
Shanmugam V, Lo J-C, Wu C-L, Wang S-L, Lai C-C, Connolly EL, Huang J-L, Yeh K-C (2011) Differential expression and regulation of iron-regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana—the role in zinc tolerance. New Phytol 190:125–137
Shanmugam V, Lo J-C, Yeh K-C (2013) Control of Zn uptake in Arabidopsis halleri: a balance between Zn and Fe. Front. Plant Sci 4:1–5
Shi Q, Bao Z, Zhu Z, He Y, Qian Q, Yu J (2005) Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66:1551–1559
Song A, Li P, Li P, Fan F, Nikolic M, Liang Y (2011) The alleviation of zinc toxicity by silicon is related to zinc transport and antioxidative reaction in rice. Plant Soil 344:319–333
Tian T, Ali B, Qin Y, Malik Z, Gill RA, Ali S, Zhou W (2014) Alleviation of lead toxicity by 5-aminolevulinic acid is related to elevated growth, photosynthesis, and suppressed ultrastructural damages in oilseed rape. Biomed Res Int 2014:1–11
Yamaji N, Mitatni N, Ma JF (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20:1381–1389
Yue X, Zhao XY, Fei YK, Zhang X (2012) Correlation of aquaporins and transmembrane solute transporters revealed by genome-wide analysis in developing maize leaf. Comp Funct Genomics 2012:1–14
Vaculík M, Landberg T, Greger M, Luxová M, Stoláriková M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot 110:433–443
Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75:1468–1476
Zangi R, Filella M (2012) Transport routes of metalloids into and out of the cell: a review of the current knowledge. Chem-Biol Interact 197:47–57
Acknowledgment
The work was supported by the Slovak Research and Development Agency under the contract Nr. APVV-0140-10 and also by the project implementation: Comenius University in Bratislava Science Park supported by the Research and Development Operational Programme funded by the ERDF Grant number: ITMS 26240220086.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bokor, B., Bokorová, S., Ondoš, S. et al. Ionome and expression level of Si transporter genes (Lsi1, Lsi2, and Lsi6) affected by Zn and Si interaction in maize. Environ Sci Pollut Res 22, 6800–6811 (2015). https://doi.org/10.1007/s11356-014-3876-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3876-6