Abstract
A field experiment was conducted to investigate the effect of chicken manure compost on the fractionation of cadmium (Cd), soil biological properties and Cd uptake by wheat in a soil affected by mining activities in Hubei province, China. Compost was applied at five levels (0, 27, 54, 108, 216 t ha−1), and winter wheat (Triticum aestivum L.) was chosen as an indicator plant. Results showed that the application of compost increased soil pH and the content of total phosphorus and organic matter. Soil biological properties such as microbial biomass carbon, invertase, protease, urease and catalase activities were significantly enhanced by 0.24–3.47 times after compost application. Sequential extraction indicated that compost amendments decreased the acid-extractable Cd by 8.2–37.6 %, while increased the reducible and oxidisable Cd by 9.2–39.5 and 8.2–60.4 %, respectively. The addition of 27–54 t ha−1 compost reduced Cd content in wheat stems and seeds by 69.6–75.0 % and 10.3–18.4 %, respectively. However, only 25.5–26.5 % reductions in Cd content in wheat stems were observed in 108–216 t ha−1 compost amendments, and no significant decrease was detected for seeds. This study suggests that although compost is a suitable organic amendment to improve soil fertility and biological activities, the addition of compost should be moderated by an appropriate rate to optimize the use of compost for the reclamation of metal-contaminated soils at field scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a very toxic heavy metal for humans, animals, and plants, and its concentration in the soils varied from 0.005 to 2.40 mg kg−1. The pollution of agricultural soils by Cd is mainly resulted from different sources such as mining and smelting activities, sewage irrigation and application of industrial effluents and phosphate fertilizers (Adriano 2001; Huang et al. 2007). Plants can absorb Cd from soils and then transfer it to edible parts. The large presence of Cd in the soil can be responsible for many physiological and morphological harms in plants through decreasing their nutrients and water uptake, and diminishing growth of their roots and shoots (Arao et al. 2008; Wang et al. 2014). Moreover, Cd may cause reduction in the food quality and safety and then adversely affects human health via drinking of polluted water and entering food chain. Therefore, the remediation of cadmium-contaminated soils has become a matter of increasing agricultural focus and concerns (Brown et al. 2005).
The immobilization of heavy metals has been considered as an environmentally sustainable and less intrusive remediation method over the last decades (Lothenbach et al. 1998; Sneddon et al. 2006). Some amendments such as organic materials, phosphates, zeolite, lime and industrial by-products have been used to immobilize heavy metals in contaminated soils (Lombi et al. 2002; Kumpiene et al. 2008). Among immobilizing agents, compost is becoming increasingly widespread for its low cost and beneficial effect on soil fertility, permitting the re-establishment of vegetation on contaminated sites (Geebelen et al. 2002; Ruttens et al. 2006). A yard waste compost rich in humic and fulvic acid effectively reduced 0.01 M CaCl2-extractable Cu and Zn concentrations by 52.2–94.5 and 96.1–98.0 %, respectively, and diminished Cu and Zn uptake by B. carinatus in a Penn mine spoil in California, USA (O’Dell et al. 2007). In a greenhouse experiment, Liu et al. (2009) found that the addition of chicken manure compost in ferralsol soils treated with various levels of Cd (0–50 mg Cd kg−1 soil) decreased the KNO3-extractable Cd by 70 % in soil and Cd content by 50 % in wheat tissues.
Soil microbial and enzyme activities play important roles in organic matter turnover and nutrient cycling (Paul 2007). The measurement of soil microbial biomass and enzyme activity can be helpful to evaluate the efficiency of remediation processes and their influence on soil functional recovery (Pérez-de-Mora et al. 2005). Alvarenga et al. (2007) reported that sewage sludge amendment increased soil protease and urease activities, while the application of garden waste compost had no influence on their activities in an acid metal-contaminated soil affected by mining activities. Farrell et al. (2010) demonstrated that soil microbial biomass carbon (MBC) and basal respiration were improved after incorporation of green waste-derived compost into an acidic soil contaminated with Cu, Pb and Zn at Parys Mountain, Anglesey, North Wales.
There are many laboratory and pot experiments that have been conducted to demonstrate the influence of different types of compost on metals distribution in contaminated soils. However, the intensive effect of chicken manure compost on the remediation of acidic Cd-contaminated soils has never been evaluated under field condition. Therefore, the objectives of this work were to investigate the influences of various levels of compost on the fractionation of Cd, MBC, enzyme activity and the uptake of Cd by winter wheat (Triticum aestivum L.) in a Cd-contaminated soil.
Materials and methods
Site description and used compost
The field experiment was set up in Miaoyunao Village, Daye County (30°16′N and 114°93′E), Hubei province, China. The soil is contaminated with Cd due to mining activities. Soil texture is loamy with 38.7 % sand, 39.2 % silt and 22.1 % clay. The compost produced from poultry manure and chaff was obtained from the Biological Engineering Company of Wuhan, China. The total Cd content of the compost is 0.21 mg kg−1 dry weight. Some chemical characteristics of soil and compost are listed in Table 1.
Field experiment
The soil was treated with five levels of compost (0, 27, 54, 108, 216 t ha−1). The compost was applied to the surface of each plot and then mixed thoroughly with the soil layer (0–20 cm). All treatments were replicated four times in a randomized block design, and the area of each plot was 1.5 × 2.0 m2. Seeds of winter wheat (Triticum aestivum L.) were sown onto all plots and harvested after 6 months. Wheat seeds and stems were dried at 65 °C for 48 h and then ground by stainless steel mill. Composite (average) soil samples were collected from each plot at a depth of 0–15 cm after harvesting and divided into two subsamples. The first part was air-dried and ground to pass 2-mm sieve for further chemical analysis. The second part was sieved (2 mm) at field-moist and stored at 4 °C for determination of MBC and enzyme activities.
Chemical analysis
Soil particle-size distribution was determined by the pipet method (Gee and Bauder 1986). The pH of soil and compost was measured using water suspensions of 1:2.5 soil/water (w/v). Soil organic matter (OM) was analyzed by potassium dichromate oxidation and titration with ferrous ammonium sulfate (Bao 2000). The organic matter content of compost was obtained by ashing at 540 °C. Dissolved organic carbon (DOC) was determined by the total organic carbon analyzer (Multi N/C 2100, Germany) after shaking 1 g of soil in 10 ml deionized water for 24 h, centrifuging (4000 rpm) and filtering through a 0.45-μm cellulose nitrate membrane. Soil and compost samples were digested by H2SO4, and total nitrogen (N) and phosphorus (P) concentrations were determined by FIA-star 5000 analyzer (FOSS Tecator, Sweden). Available N was measured by NaOH pervasion method (Bao 2000). Available P was assayed by NaHCO3 method (Hesse 1972). Soil and compost were digested with aqua regia, and the concentration of Cd was analyzed by atomic adsorption spectrophotometer (Varian AA240FS, Australia). The plant materials were digested with concentrated nitric acid, and Cd was determined by graphite furnace atomic absorption spectrometry. The modified BCR sequential extraction procedure was applied to partition metals into fractions defined as acid-extractable (water-soluble, exchangeable and bound to carbonate), reducible (bound to Fe and Mn oxides), oxidisable (bound to organic matter and sulfide) and residual (bound to silicate) (Rauret et al. 1999). A brief summary of the procedure is presented in Table 2.
Biological and biochemical analysis
Soil MBC was estimated by the fumigation–extraction method (Vance et al. 1987). Urease activity was measured by the method of Nannipieri et al. (1978a, b). Protease activity was determined using ninhydrin colorimetry after the incubation of the soil with sodium caseinate (Shen et al. 2006). Invertase activity was analyzed as described by Schinner and von Mersi (1990) with modifications by Gander et al. (1994). Catalase activity was determined by measuring the O2 absorbed by KMnO4 after addition of H2O2 to the samples (Rodríguez-Kábana and Truelove 1982). Neutral phosphatase activity was assayed according to Alef and Nannipieri (1995).
Statistical analysis
All statistical analyses were carried out with the SAS system for Windows V8. The data were evaluated by one-way analysis of variance (ANOVA), and differences between means were determined using the least significant difference test. The statistical significance in this analysis was defined at p < 0.05.
Results and discussion
Soil chemical properties
The influences of compost application on soil chemical properties are listed in Table 3. Soil pH increased significantly from 6.55 in the control to 7.46 in the treatment with 216 t ha−1 compost. The content of soil total P was dramatically enhanced by 71.8–183.9 % due to the incorporation of compost. Soil OM content increased by 9.7–83.7 % after compost amendments. Furthermore, the increments in soil pH, total P and OM became more apparent with increasing amount of compost application. Obviously, compost addition was effective in increasing soil pH values and the content of total P and OM.
Cadmium fractions in soil and its absorption by wheat
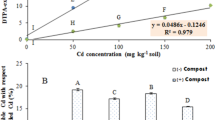
The percentages of various Cd fractions in the soils are presented in Fig. 1. In bulk soil, the acid-extractable and reducible Cd accounted for a large proportion of the total Cd (52.2 and 39.0 %, respectively), indicating these two fractions played important roles in Cd retention. After the incorporation of compost, the acid-extractable Cd decreased by 8.2–37.6 %, while reducible and oxidisable fractions were enhanced by 9.2–39.5 and 8.2–60.4 %, respectively. No significant difference was detected for residual Cd with compost application. Overall, with increasing rate of compost addition to soil, the proportion of acid-extractable Cd declined, whereas reducible and oxidisable fractions increased accordingly.
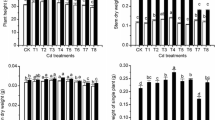
The concentration of Cd in wheat seeds and stems is given in Table 4. In the treatment without compost, Cd concentration in wheat seeds and stems was 0.87 and 2.04 mg kg−1, respectively. After the application of 27 and 54 t ha−1 compost, Cd content in seeds was diminished by 10.3 and 18.4 %, respectively. More significant decrease in Cd concentration was found in wheat stems. The concentration of Cd in stems was distinctly reduced by 69.6 % due to the addition of 27 t ha−1 compost. Furthermore, the application of 54 t ha−1 compost resulted in a 75.0 % reduction in Cd content in stems in comparison with the control group. However, at higher application levels (108 and 216 t ha−1), no significant changes in Cd concentration were noticed for seeds as compared to the control. Meanwhile, only 25.5–26.5 % reductions in Cd content in wheat stems were detected in 108–216 t ha−1 compost amendments. These data revealed that the application of 27–54 t ha−1 compost had better effect in decreasing the phytoavailability of Cd than the treatments with 108–216 t ha−1 compost, and the most effective treatment in decreasing Cd uptake by wheat was 54 t ha−1 compost.
Our results suggested that the acid-extractable Cd was converted to reducible and oxidisable forms. The conversion of Cd species was probably attributed to the high pH value, P and OM contents of compost which can immobilize soil Cd through adsorption, precipitation or formation of stable complexes (Shuman 1999; Guo et al. 2006). Similar results were also found in pot experiments. For example, the application of 40 t ha−1 hog manure compost reduced Cd accumulation in wheat by 35.1 % through decreasing aqueous and extractable species in a Cd-contaminated Tatan sandy soil (Lee et al. 2004). Poultry manure compost incorporation transformed the soluble/exchangeable Cd to organic-bound fraction and consequently decreased Cd uptake of pakchoi by 56.2–62.5 % in an artificially metal-contaminated soil (Chen et al. 2010). However, the results obtained by sequential extraction do not always correspond to plant uptake of metal because of the complexity interactions of contaminants with soil constituents and plant roots (McLaughlin et al. 2000; Menzies et al. 2007). In the present study, although the application of 108–216 t ha−1 compost induced a more pronounced transformation of Cd species, an increase of Cd content in wheat was observed in comparison with the 54 t ha−1 compost treatment. One possible explanation for this enhancement of Cd phytoavailability may be associated with dissolved organic matter, especially low molecular weight organic compounds, which would increase the mobility and bioavailability of metal contaminants through the formation of soluble metal–DOC complexes (Inaba and Takenaka 2005). The compost used in the experiment contained 14.49 g kg−1 DOC. Wang et al. (2010) demonstrated that the dissolved organic matter extracted from chicken manure with molecular weight less than 1 kDa increased the bioavailability of copper to lettuce. Sauvé et al. (2000) observed that the percentage of soluble metal–DOC complexes increased with increasing level of compost. The application of large quantities of compost may counteract the influence of immobilization by forming soluble Cd complexes and then decrease the alleviating effect on the toxicity of Cd in soils. A similar finding was reported by Narwal and Singh (1998) who showed that the application of 2 % cow manure to alum shale soils decreased Cd content in wheat grains by 23.0 %, whereas no significant reduction was detected at 8 % cow manure addition. It was reported by Almendro-Candel et al. (2014) that leachability and plant uptake of Cd and other heavy metals were highly affected by the mobility and availability of the metals in the soil in response to changes of soil pH and contents of organic C. Li et al. (2014) clearly indicated that water-soluble and exchangeable Cd (Cd mobility) was markedly reduced due to the addition of corn straw and then resulted in significant decrease for Cd concentration by about 77 % in vegetables grown on Cd-contaminated and calcareous soils. It was found by Rosen and Chen (2014) that using biosolid (sewage sludge) compost generally increased the proportion of Cu and Cd among the different studied fractions but reduced their phytoavailability. Moreover, they indicated that addition of the above-mentioned compost at doses of 28.8 and 72 Mg ha−1 was effective in decreasing Cd and Cu absorption by plants grown in sandy soil, respectively. Application of organic amendments such as active carbon significantly increased the dry biomass yield of rapeseed and showed high efficiency in decreasing soluble/exchangeable concentration of Cd by about 1.36 times and the accumulation of Cd in rapeseed tissues by 31 % compared to the control Cd (Shaheen and Rinklebe 2015). This might be resulted from the increase in soil pH and organic matter after incorporation of active carbon in the Cd/Pb polluted soil. Mohamed et al. (2015) indicated that the increase in pH after application of organic residues in heavy metal-polluted soils was considered as one of the most important factors in controlling both Cd immobilization and mobility in these soils by several ways, including adsorption of metals on active sites of organic materials or precipitation of metals in less mobile forms (oxides, hydroxides and carbonates). It was also reported by Shaheen and Rinklebe (2015) that organic matter had high ability to reduce both availability and mobility of heavy metals in the soils due to their redistribution from the soluble/exchangeable forms to other geochemical fractions bounded with carbonates, Fe/Mn oxides and organic matter. So, it is interesting that our finding could confirm that incorporation of the used compost declined the mobility of Cd through converting them to un-mobile forms or stable complexes, causing low Cd bioavailability for wheat plants grown on Cd-polluted soils.
Although compost amendment decreased the phytoavailability of Cd, when used at higher application rates, it led to increase the risk of metal uptake by plants. The recommended application rate of chicken manure compost in this field was 54 t ha−1. Different application rates had been recommended by other researchers based on various qualities of compost and soil properties. Schwab et al. (2007) stated that the application of 90 t ha−1 organic amendments is a commonly cited application rate for remediation of mine tailings sites in southeast Kansas. Santibáñez et al. (2008) found that the most appropriate rate of biosolids addition would be represented by 200 dry t ha−1 for stabilization of copper mine tailings.
Soil MBC and enzyme activities
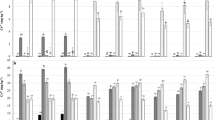
The content of MBC was conspicuously improved after compost addition (Fig. 2). The lowest concentration of MBC was recorded in the control sample (89.7 mg C kg−1), whereas the highest value was noticed in the soil amended with 216 t ha−1 compost (326.6 mg C kg−1). As illustrated in Fig. 2, the application of compost obviously enlarged urease and protease activities by 0.90–3.47 and 0.26–2.13 times, respectively. At the same time, the activities of catalase and invertase were enhanced by 0.24–1.32 and 0.62–1.61 times due to the incorporation of compost. Additionally, the increments in the enzymatic activities examined became more pronounced with increasing compost application except for neutral phosphatase. A decrease in neutral phosphatase activity was observed with continuous rise of compost level, which might be ascribed to the feedback inhibition by available P (Nannipieri et al. 1979). The improvement of soil MBC and enzymes activities was probably resulted from the stimulation of indigenous soil microorganisms and the incorporation of stable enzymes contained in the compost (Garcia-Gil et al. 2000). These results show obviously the beneficial effects of compost on soil MBC and enzymes activities in Cd-contaminated soils.
Conclusion
The results obtained in this study indicated that chicken manure compost was a good conditioner to improve soil fertility and biological status, and a suitable amendment to promote the redistribution of soil Cd fractions and reduce Cd uptake by wheat. The treatment with 54 t ha−1 manure compost resulted in the largest reduction in Cd content in wheat seeds and stems. However, a reactivation of Cd phytoavailability was observed with continuous increasing compost level to 108–216 t ha−1, probably due to the formation of soluble Cd complexes with dissolved organic carbon from the compost. These results suggest that the addition of compost should be moderated by an appropriate rate to optimize the use of compost for the remediation of metal-contaminated soils at field scale. In summary, compost is a suitable organic amendment for the restoration of heavy metal-contaminated soil, but the addition of compost should be moderated by an appropriate rate to optimize the remediation effectiveness. Furthermore, field experiments with plant tests must be performed before organic materials like compost are applied for the reclamation of metal-contaminated soils in extensive areas.
Change history
18 June 2019
The Editor-in-Chief has retracted this article (Li et al. 2016). An investigation by the Journal has not been able to confirm the identity and affiliation of the author David Raleve. This author was the corresponding author on submission but changed the corresponding authorship at the proof stage. The current corresponding author was not aware of the publication of this article. As the appropriate authorship for this article cannot be determined, the Editor-in-Chief no longer has confidence in this article. Ibrahim Mohamed disagrees with this retraction. Ming Li and Wenli Chen did not respond to any correspondence about this retraction. Qiaoyun Huang did not respond to any correspondence about this retraction notice.
References
Adriano, D. (2001). Trace elements in terrestrial environments: Biogeochemistry, bioavailability, and risks of metals (2nd ed.). Berlin: Springer.
Alef, K., & Nannipieri, P. (1995). Methods in applied soil microbiology and biochemistry. New York: Academic Press.
Almendro-Candel, M. B., Navarro-Pedreno, J., & Jordan, M. M. (2014). Use of municipal solid waste compost to reclaim limestone quarries mine spoils as soil amendments: Effects on Cd and Ni. Journal of Geochemical Exploration, 144, 363–366.
Alvarenga, P., Palma, P., Gonçalves, A. P., Fernandes, R. M., de Varennes, A., & Vallini, G. (2007). Organic residues as immobilizing agents in aided phytostabilization: (II) Effects on soil biochemical and ecotoxicological characteristics. Chemosphere, 74, 1301–1308.
Arao, T., Takeda, H., & Nishihara, E. (2008). Reduction of cadmium translocation from roots to shoots in eggplant (Solanummelongena) by grafting onto Solanum torvum rootstock. Soil Science and Plant Nutrition, 54, 555–559.
Bao, S. D. (2000). Soil and agricultural chemistry analysis. Beijing: China Agricultural Press. In Chinese.
Brown, S., Christensen, B., Lombi, E., McLaughlin, M., McGrath, S., & Colpaert, J. (2005). An inter-laboratory study to test the ability of amendments to reduce the availability of Cd, Pb, and Zn in situ. Environmental Pollution, 138, 34–45.
Chen, H. S., Huang, Q. Y., Liu, L. N., Cai, P., Liang, W., & Li, M. (2010). Poultry manure compost alleviates the phytotoxicity of soil cadmium: Influence on growth of pakchoi (Brassica chinensis L.). Pedosphere, 20, 63–70.
Farrell, M., Griffith, G., Hobbs, P., Perkins, W., & Jones, D. (2010). Microbial diversity and activity are increased by compost amendment of metal-contaminated soil. FEMS Microbiology Ecologly, 71, 94–105.
Gander, L. K., Hendricks, C. W., & Doyle, J. D. (1994). Interferences, limitations and an improvement in the extraction and assessment of cellulase activity in soil. Soil Biology & Biochemistry, 26, 65–73.
Garcia-Gil, J., Plaza, C., Soler-Rovira, P., & Polo, A. (2000). Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biology & Biochemistry, 32, 1907–1913.
Gee, G., & Bauder, J. (1986). Particle size analysis. In A. Klute (Ed.), Methods of soil analysis: Part I. Physical and minerological methods: Soil science society of America book series No. 5 (pp. 312–383). Madison, Wisconsin: Soil Science Society of America.
Geebelen, W., Vangronsveld, J., Adriano, D. C., Carleer, R., & Clijsters, H. (2002). Amendment-induced immobilization of lead in a lead-spiked soil: evidence from phytotoxicity studies. Water, Air, and Soil Pollution, 140, 261–277.
Guo, G., Zhou, Q., & Ma, L. Q. (2006). Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: A review. Environmental Monitoring and Assessment, 116, 513–528.
Hesse, P. (1972). A textbook of soil chemical analysis. New York: Chemical Publishing Co., Inc.
Huang, S. S., Liao, Q. L., Hua, M., Wu, X. M., Bi, K. S., & Yan, C. Y. (2007). Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere, 67, 2148–2155.
Inaba, S., & Takenaka, C. (2005). Effects of dissolved organic matter on toxicity and bioavailability of copper for lettuce sprouts. Environment International, 31, 603–608.
Kumpiene, J., Lagerkvist, A., & Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Management, 28, 215–225.
Lee, T. M., Lai, H. Y., & Chen, Z. S. (2004). Effect of chemical amendments on the concentration of cadmium and lead in long-term contaminated soils. Chemosphere, 57, 1459–1471.
Li, B., Yang, J., Wei, D., Chen, S., & Li, J. (2014). Field evidence of cadmium phytoavailability decreased effectively by rape straw and/or red mud with zinc sulphate in a Cd-contaminated calcareous soil. PLoS ONE, 9(10), e109967. doi:10.1371/journal.pone.0109967.
Liu, L., Chen, H. S., Cai, P., Liang, W., & Huang, Q. Y. (2009). Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. Journal of Hazardous Materials, 163, 563–570.
Lombi, E., Zhao, F. J., Zhang, G. Y., Sun, B., Fitz, W., & Zhang, H. (2002). In situ fixation of metals in soils using bauxite residue: Chemical assessment. Environmental Pollution, 118, 435–443.
Lothenbach, B., Krebs, R., Furrer, G., Gupta, S., & Schulin, R. (1998). Immobilization of cadmium and zinc in soil by Al-montmorillonite and gravel sludge. European Journal of Soil Science, 49, 141–148.
McLaughlin, M. J., Zarcinas, B. A., Stevens, D. P., & Cook, N. (2000). Soil testing for heavy metals. Communications in Soil Science and Plant Analysis, 31, 1661–1700.
Menzies, N. W., Donn, M. J., & Kopittke, P. M. (2007). Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environmental Pollution, 145, 121–130.
Mohamed, I., Zhang, G. S., Li, Z. G., Liu, Y., Chen, F., & Dai, K. (2015). Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecological Engineering, 84, 67–76.
Nannipieri, P., Johnson, R., & Paul, E. (1978a). Criteria for measurement of microbial growth and activity in soil. Soil Biology & Biochemistry, 10, 223–229.
Nannipieri, P., Johnson, R., & Paul, E. (1978b). Criteria for measurement of microbial growth and activity in soil. Soil Biology & Biochemistry, 10, 223–229.
Nannipieri, P., Pedrazzini, F., Arcara, P., & Piovanelli, C. (1979). Changes in amino acids, enzyme activities, and biomasses during soil microbial growth. Soil Science, 127, 26.
Narwal, R. P., & Singh, B. R. (1998). Effect of organic materials on partitioning, extractability and plant uptake of metals in an alum shale soil. Water, Air, and Soil Pollution, 103, 405–421.
O’Dell, R., Silk, W., Green, P., & Claassen, V. (2007). Compost amendment of Cu–Zn minespoil reduces toxic bioavailable heavy metal concentrations and promotes establishment and biomass production of Bromus carinatus (Hook and Arn.). Environmental Pollution, 148, 115–124.
Paul, E. (2007). Soil microbiology, ecology, and biochemistry (3rd ed.). New York: Academic Press.
Pérez-de-Mora, A., Ortega-Calvo, J. J., Cabrera, F., & Madejón, E. (2005). Changes in enzyme activities and microbial biomass after “in situ” remediation of a heavy metal-contaminated soil. Applied Soil Ecology, 28, 125–137.
Rauret, G., Lopez-Sanchez, J., Sahuquillo, A., Rubio, R., Davidson, C., & Ure, A. (1999). Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring, 1, 57–61.
Rodríguez-Kábana, R., & Truelove, B. (1982). Effects of crop rotation and fertilization on catalase activity in a soil of the southeastern United States. Plant and Soil, 69, 97–104.
Rosen, V., & Chen, Y. (2014). The influence of compost addition on heavy metal distribution between operationally defined geochemical fractions and on metal accumulation in plant. Journal of Soils and Sediments, 14, 713–720.
Ruttens, A., Mench, M., Colpaert, J. V., Boisson, J., Carleer, R., & Vangronsveld, J. (2006). Phytostabilization of a metal contaminated sandy soil. I: Influence of compost and/or inorganic metal immobilizing soil amendments on phytotoxicity and plant availability of metals. Environmental Pollution, 144, 524–532.
Santibáñez, C., Verdugo, C., & Ginocchio, R. (2008). Phytostabilization of copper mine tailings with biosolids: Implications for metal uptake and productivity of Lolium perenne. Science of the Total Environment, 395, 1–10.
Sauvé, S., Hendershot, W., & Allen, H. E. (2000). Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environmental Science and Technology, 34, 1125–1131.
Schinner, F., & von Mersi, W. (1990). Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biology & Biochemistry, 22, 511–515.
Schwab, P., Zhu, D., & Banks, M. K. (2007). Heavy metal leaching from mine tailings as affected by organic amendments. Bioresources and Technology, 98, 2935–2941.
Shaheen, S. M., & Rinklebe, J. (2015). Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecological Engineering, 74, 319–326.
Shen, R., Cai, H., & Gong, W. (2006). Transgenic Bt cotton has no apparent effect on enzymatic activities or functional diversity of microbial communities in rhizosphere soil. Plant and Soil, 285, 149–159.
Shuman, L. (1999). Organic waste amendments effect on zinc fractions of two soils. Journal of Environmental Qualtiy, 28, 1442–1447.
Sneddon, I. R., Orueetxebarria, M., Hodson, M. E., Schofield, P. F., & Valsami-Jones, E. (2006). Use of bone meal amendments to immobilise Pb, Zn and Cd in soil: A leaching column study. Environmental Pollution, 144, 816–825.
Vance, E., Brookes, P., & Jenkinson, D. (1987). An extraction method for measuring soil microbial biomass C. Soil Biology & Biochemistry, 19, 703–707.
Wang, X., Chen, X., Liu, S., & Ge, X. (2010). Effect of molecular weight of dissolved organic matter on toxicity and bioavailability of copper to lettuce. Journal of Environmental Sciences, 22, 1960–1965.
Wang, Y., Xiao, X., Zhang, T., Kang, H., Zeng, J., Fan, X., et al. (2014). Cadmium treatment alters the expression of five genes at the Cda1 locus in two soybean cultivars [Glycine Max (L.) Merr]. The Scientific World Journal,. doi:10.1155/2014/979750.
Acknowledgments
The authors are grateful to the National High Technology Research and Development Program of China (“863” Program) for the financial support of this work (2012AA101402-3). The second author is very thankful to the members of Soil Science Department, Faculty of Agriculture, Benha University, Egypt (http://www.bu.edu.eg and http://www.fagr.bu.edu.eg) for giving him the permission to carry out his postdoctoral research in China.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Editor-in-Chief has retracted this article. An investigation by the Journal has not been able to confirm the identity and affiliation of the author David Raleve. This author was the corresponding author on submission but changed the corresponding authorship at the proof stage. The current corresponding author was not aware of the publication of this article. As the appropriate authorship for this article cannot be determined the Editor-in-Chief no longer has confidence in this article. Ibrahim Mohamed disagrees with this retraction. Ming Li and Wenli Chen did not respond to any correspondence about this retraction. Qiaoyun Huang did not respond to any correspondence about this retraction notice.
About this article
Cite this article
Li, M., Mohamed, I., Raleve, D. et al. RETRACTED ARTICLE: Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environ Geochem Health 38, 1193–1201 (2016). https://doi.org/10.1007/s10653-015-9784-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9784-y